Abstract
Iron overload is highly prevalent, but its molecular pathogenesis is poorly understood. Recently, DMT1 was shown to be a major apical iron transporter in absorptive cells of the duodenum. In vivo, it is the only transporter known to be important for the uptake of dietary non-heme iron from the gut lumen. The expression and subcellular localization of DMT1 protein in 3 mouse models of iron overload were examined: hypotransferrinemic (Trfhpx) mice, Hfeknockout mice, and B2m knockout mice. Interestingly, in Trfhpx homozygotes, DMT1 expression was strongly induced in the villus brush border when compared to control animals. This suggests that DMT1 expression is increased in response to iron deficiency in the erythron, even in the setting of systemic iron overload. In contrast, no increase was seen in DMT1 expression in animals with iron overload resembling human hemochromatosis. Therefore, it does not appear that changes in DMT1 levels are primarily responsible for iron loading in hemochromatosis.
Introduction
Iron acquisition by the absorptive epithelium of the small intestine is a complex and carefully regulated process. DMT1 (formerly called Nramp2, DCT1) has been shown to be an apical transmembrane iron transporter that actively transports reduced dietary iron into intestinal enterocytes.1-3 Several other putative iron transport systems have been described but have not yet been shown to be involved in dietary absorption in animal models.4,5 Iron traverses the epithelial cell and is exported through the basolateral membrane by a process that is incompletely characterized but that probably involves a second transmembrane iron transporter, ferroportin (also known as IREG1, MTP1).6-8 A presumed ferroxidase, hephaestin, is also believed to be involved in basolateral iron export.9Although much progress has been made recently in understanding intestinal iron transport, its regulation remains poorly understood. At least 2 types of signals from the body to the intestine modify absorption. The first, termed the stores regulator (recently reviewed in10), regulates absorption in a manner that is inversely related to total body iron stores. Iron absorption is also modulated by the iron needs of the erythron, the erythroid regulator.10In the occasional patient with inherited atransferrinemia,11-14 the erythroid regulator predominates over the stores regulator. Such patients have profound iron deficiency anemia, which apparently stimulates intestinal iron absorption. Importantly, intestinal iron absorption continues to be increased even after massive tissue iron overload develops. Patients with the common iron loading disease, hereditary hemochromatosis, also have inappropriate absorption of increased amounts of iron despite expanded iron stores. However, they have no abnormalities in erythropoiesis. Although the recent identification of the gene associated with classical hemochromatosis,Hfe,15 has given an important clue, the molecular mechanisms regulating iron absorption have not yet been worked out.
Recent studies in mice have provided strong evidence that all or nearly all iron influx through the intestinal epithelium involves DMT1. Homozygous mk mutant mice, carrying a severe loss of function mutation in DMT1, are poorly viable because of generalized iron deficiency and anemia.2 We have previously shown thatmk mice have increased amounts of DMT1 protein in the duodenum,16 suggesting that DMT1 expression is regulated in response to the abnormal iron metabolism in these mice. However, it is not clear whether the induction of DMT1 is in response to the stores regulator, the erythroid regulator, or both, because mkhomozygotes have severe tissue iron deficiency and anemia. Whenmk/mk mice are bred to mice carrying a null mutation inHfe, the compound mutants are still iron deficient, indicating that iron loading in the mouse model of hemochromatosis requires DMT1.17 However, there are conflicting data as to whether DMT1 expression is increased in mice lacking Hfe. It has been published that DMT1 mRNA expression is increased several-fold,18 but we have been unable to reproduce this result in our own Hfe knockout animals (unpublished observations).
We hypothesized that different regulatory signals might act through different mechanisms to alter intestinal iron absorption. If so, some might involve changes in the level of DMT1 expression, whereas others might affect other iron transport steps, such as basolateral transfer. To begin to study the regulators of iron absorption and to determine their effects on the expression of DMT1, we examined DMT1 protein expression in 3 mouse models of iron overload. One, hypotransferrinemia (Trfhpx), results from a spontaneous mutation disrupting a splice donor site in the murine transferrin gene19 and is directly analogous to human atransferrinemia. The other 2 mutations, generated by targeted disruption of the murine Hfe20 and β-2 microglobulin21 genes, are murine models of hereditary hemochromatosis.
Study design
Animals
C57BL/6J (B6) and 129/SvEvfBRtac mice were purchased from Jackson Laboratory (Bar Harbor, ME) and Taconic Farms (NY) and used as wild-type controls for experiments with theB2m−/− and Hfe−/−mice, respectively. MK/ReJ-mk/+, MK/ReJ-mk/mk, and B2m−/− (B6 background) mice were derived from founders purchased from the Jackson Laboratory. Trfhpx/hpx (BALB/cJ background) mice were derived in Boston from founders generously provided by Jerry Kaplan (Salt Lake City, UT).Hfe−/− mice (129/SvEvfBRTac background) were generated in one of our laboratories.20 All strains have been maintained as inbred stocks fed identical diets in the animal facility at Children's Hospital (Boston, MA). HomozygousTrfhpx/hpx mice were kept alive by weekly intraperitoneal injection of 6 mg human transferrin during weeks 1, 2, and 3 of life as previously described.17Heterozygous Trfhpx/+ mice and wild-type Trf+/+ mice have been combined in a single control group referred to in the text asTrf?/+. We have previously shown thatTrfhpx/hpx, Hfe−/−,and B2m−/− mutants all have marked tissue iron overload19 20 (and data not shown).
Cell culture and transfection
The production of Chinese hamster ovary (CHO) cells stably expressing a c-Myc–tagged DMT1 expression plasmid was previously described.3
Crude membrane protein extracts
Production, purification, and use of anti-DMT1 antibody
The preparation of a specific polyclonal antiserum recognizing the N-terminal sequence (residues 1 to 73) of DMT1 protein was previously described.3 Its specificity was established by demonstrating DMT1-specific detection in transfected CHO cell membranes on Western blot3 and immunofluorescence22analyses. This antiserum, antisera against transferrin receptor (Trfr), and biliary glycoprotein 1 (Bgp1) were used for immunoblotting and immunohistochemistry as previously described.3
Results and discussion
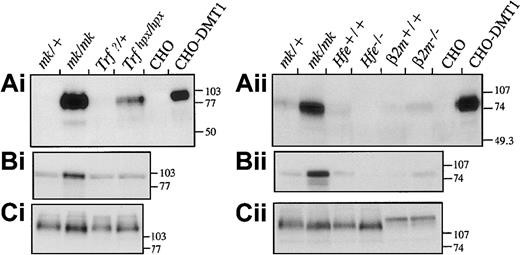
We first examined levels of expression of DMT1 by Western blotting of membrane fractions from mutant mice and controls (Figure1). Heterozygous mk/+ mice, with normal iron stores, and non-transfected CHO cells were used as negative controls. Microcytic mk/mk mice, previously shown to overexpress DMT1,16 and DMT1-transfected CHO cells were used as positive controls. The anti-DMT1 antiserum detects a mature 90- to 100-kd DMT1 polypeptide, expressed at high levels in duodenal samples from mk/mk mice and in CHO–DMT1 transfectants. Little or no DMT1 protein expression was detected in duodenum frommk/+ mice, Trf?/+ mice, or wild-type mice (of either 129Sv or B6 strains, designatedHfe+/+ and B2m+/+, respectively), consistent with earlier findings in wild-type mice.3 Thus, all control samples gave expected results.
Expression of DMT1 protein in the duodenum of
Trfhpx/hpx,Hfe−/−, andB2m−/− mice. Membrane proteins isolated from proximal duodenum of the different mice were fractionated on 10% acrylamide gels and transferred to polyvinylidene difluoride membranes. For comparison, microsomal fractions from mk/+and mk/mk mice were included in the analysis. To demonstrate the specificity of the anti-DMT1 antibody, membrane proteins from CHO cells or CHO cells expressing a c-Myc–tagged DMT1 protein (CHO-DMT1) were also included (Ai and Aii). Immunoblotting was performed with antibodies raised against DMT1 (Ai and ii), transferrin receptor (Bi and Bii), and Bgp1 proteins (Ci and ii). The positions and sizes (in kd) of molecular weight markers are indicated on the right.
Expression of DMT1 protein in the duodenum of
Trfhpx/hpx,Hfe−/−, andB2m−/− mice. Membrane proteins isolated from proximal duodenum of the different mice were fractionated on 10% acrylamide gels and transferred to polyvinylidene difluoride membranes. For comparison, microsomal fractions from mk/+and mk/mk mice were included in the analysis. To demonstrate the specificity of the anti-DMT1 antibody, membrane proteins from CHO cells or CHO cells expressing a c-Myc–tagged DMT1 protein (CHO-DMT1) were also included (Ai and Aii). Immunoblotting was performed with antibodies raised against DMT1 (Ai and ii), transferrin receptor (Bi and Bii), and Bgp1 proteins (Ci and ii). The positions and sizes (in kd) of molecular weight markers are indicated on the right.
We next examined DMT1 expression in the 3 mouse models of iron overload. Strong induction of DMT1 expression is noted by Western blotting of the duodenal sample fromTrfhpx/hpx mice (Figure 1Ai). There is no change in expression of either Trfr or Bgp1 controls in these mice (Figure 1Bi-Ci). This suggests that DMT1 levels respond to the erythroid regulator as the stores regulator must be inoperative. The induction of DMT1 was confirmed by immunohistochemistry (Figure2). No staining of DMT1 protein is detectable in the control, butTrfhpx/hpx mice have intense staining along the brush border. DMT1 is likely, therefore, to play a role in the iron overload that develops in these animals.
Immunohistochemical staining of DMT1 in the intestine of
Trfhpx/hpx andHfe−/− mice. Magnified (400×) tissue sections from the proximal duodenum ofTrf?/+,Trfhpx/hpx,Hfe+/+, and Hfe−/−mice were immunostained with polyclonal rabbit anti-mouse DMT1 N-terminus antibody (dilution 1:40). DMT1 detection produces dark brown staining.
Immunohistochemical staining of DMT1 in the intestine of
Trfhpx/hpx andHfe−/− mice. Magnified (400×) tissue sections from the proximal duodenum ofTrf?/+,Trfhpx/hpx,Hfe+/+, and Hfe−/−mice were immunostained with polyclonal rabbit anti-mouse DMT1 N-terminus antibody (dilution 1:40). DMT1 detection produces dark brown staining.
In contrast, neither Hfe−/− norB2m−/− mice had a measurable induction of DMT1 protein expression as shown by Western blotting (Figure 1) and by immunohistochemistry (Figure 2, forHfe−/−only; B2m−/− data not shown). Both of these mouse models of hemochromatosis gave results that were not appreciably different from those of controls. In these experiments, slight variations in the low level of basal DMT1 and Trfr expression were observed but were attributed to normal animal-to-animal variation or processing of otherwise identical samples. In contrast to the result seen inTrfhpx/hpx mice, the lack of induction in Hfe−/− andB2m−/− mice strongly suggests that an increase in DMT1 protein levels is not primarily responsible for accelerated iron absorption in hemochromatosis. Other possibilities, including increased activity of post-translationally modified DMT1 and variation in the reducing step23,24 or the basolateral transfer step,25 may also contribute to the increase of iron uptake in hereditary hemochromatosis.
Other groups have reported increases in DMT1 mRNA levels in a different Hfe knockout mouse18 and in human patients with hereditary hemochromatosis.26 There are several possible explanations for the discrepancy between their results and ours. First, we analyzed DMT1 protein expression, whereas the other groups examined DMT1 mRNA expression. It is possible that increased mRNA production does not result in increased protein production. We feel this is unlikely, however, because we also examined DMT1 mRNA expression in our Hfe knockout mice, and we could not detect a difference from wild-type mice (data not shown). We feel other explanations are more likely. The Hfe knockout mice studied by Sly et al27 did not have a homogeneous genetic background. We believe that their results may be attributable to strain-to-strain variations between mice, as have been reported in the literature. We controlled for this variability in the current study. Similarly, patients reported by Zoller et al26 did not have the same genetic background and had been treated with phlebotomy to varying extents. Phlebotomy may activate the erythroid regulator of iron absorption by inducing erythropoiesis. As shown by the analysis ofTrfhpx/hpx mice, the erythroid regulator can increase DMT1 expression even in the setting of tissue iron overload.
This study allows us to begin to distinguish between mechanisms of iron loading in 2 disorders of iron metabolism, atransferrinemia and hereditary hemochromatosis. Future work should give additional insight into the molecular regulators of iron balance.
Supported by National Institutes of Health grant AI35237 (P.G.) and partially supported by NIH grants DK53813 (N.C.A), HL03600 (M.D.F.), and HL03503 (J.E.L.). P.G. is an International Research Scholar of the Howard Hughes Medical Institute and a Senior Scientist of the Medical Research Council of Canada. N.C.A. is an Associate Investigator of the HHMI.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philippe Gros, Department of Biochemistry, McGill University, Rm 907, 3655 William Osler, Montreal, Quebec, Canada, H3G-1Y6; e-mail: gros@med.mcgill.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal