Abstract
While cytotoxic T lymphocyte antigen–4 (CTLA-4) negatively regulates T-cell receptor (TCR)–driven interleukin (IL)–2 production and proliferation, little is known regarding whether the coreceptor has the capacity to inhibit other events, such as Fas ligand (FasL) expression and antigen-induced cell death (AICD). In this study, it is shown that CTLA-4 expressed in a T-cell hybridoma can elicit a potent block of FasL expression and AICD. Inhibition occurred independently of CTLA-4 blockage of IL-2 production and was partially reversed by a single mutation in the cytoplasmic YVKM motif. These findings indicate that CTLA-4 can block TCR signaling prior to bifurcation of signals leading to IL-2 production and apoptosis.
Introduction
Cytotoxic T lymphocyte antigen-4 (CTLA-4) negatively regulates T-cell activation initiated by ligation of T-cell receptor (TCR)ζ/CD3 and CD28.1-3Blocking with soluble anti–CTLA-4 Fab fragments can augment T-cell responses,4,5 while co–cross-linking of CTLA-4 with either TCR or TCR × CD28 potently inhibits proliferation and interleukin (IL)-2 production.6-8 Consistent with this, CTLA-4−/− mice show extensive lymphadenopathy with T-cell infiltration of various tissues.9-13 This event requires CD28 since CTLA-4Ig blockage of CD28 binding to CD80/86 reverses the phenotype.11 Isolated CTLA-4−/− T-cells also exhibit a greatly enhanced response to antigen,14-16leading to the notion that the lowering of the signaling threshold to self-antigen and/or weakly antigenic foreign antigen is responsible for the lymphadenopathy. In this context, TCR transgenic mice on a Rag−/− × CTLA-4−/− background fail to develop disease.15,16 CTLA-4 has also been linked to anergy induction,17,18 while its absence enhances the development of TH2 cells.12
Despite its immunologic importance, the molecular basis for CTLA-4 function has yet to be established. Multiple intracellular proteins, including phosphatidylinositol 3-kinase (PI3-K),19,20 the tyrosine phosphatase SHP-2,21,22 as well as the clathrin-associated adaptor protein complexes AP-223-26 and AP-127 interact with the cytoplasmic domain. PI3-K, AP-1 and AP-2 bind directly to the GVYVKM motif, while the SHP-2 association appears to be indirect, possibly mediated through another protein such as PI3-K.28 PI3-K binds a tyrosyl-phosphorylated version of the motif,19 while AP-1/AP-2 complexes bind to an extended version of the nonphosphorylated sequence that includes additional N-terminal residues.23-26 The AP-2 tetramer regulates trafficking and endocytosis of the coreceptor from the cell surface.24,26 AP-1 acts to ensure steady-state levels of intracellular CTLA-4 by shuttling the receptor to lysosomes for degradation.27 Further downstream, CTLA-4 engagement has been reported to block extracellular signal-regulated kinase and Jun NH2-terminal kinase activity29 and the induction of IL-2 transcription.30-32
TCR/CD3 ligation induces antigen-induced cell death (AICD) as mediated by Fas (CD95/APO-1) and Fas ligand (CD95L) interactions and the activation of caspases, a process that ensures the elimination of self-reactive thymocytes and peripheral blood lymphocytes.33-36 Although AICD operates in CTLA-4−/− T cells,14 little is known regarding whether CTLA-4 has the capacity to block TCR-mediated signals leading to AICD. In this regard, T-cell hybridomas have served as a model for the study of AICD33,34; in particular, the hybridoma DC27.10 has been previously studied for the role of costimulation in the induction of apoptosis.37 In this report, we demonstrate that CTLA-4 can block AICD in transfectants of the murine hybridoma DC27.10 via inhibition of Fas ligand (FasL) surface expression. These findings support a model in which CTLA-4 interferes with an early TCR-signaling event linked to both IL-2 production and FasL expression.
Study design
Cells, reagents, and antibodies
Murine T-cell hybridoma DC27.10 (gift from Dr R. Zamoyska, Medical Research Council, London, United Kingdom) was cultured in RPMI-1640 containing fetal bovine serum 5% (vol/vol) (Sigma, St Louis, MO), L-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) (Life Technologies, Grand Island, NY) at 37°C, and 5% CO2. DC27.10 transfectants carrying different transfected forms of human CTLA-4 (wild-type human CTLA-4, Y201-2F) were generated as described.38Hamster anti–murine CD3 (145-2C11) was purchased from American Type Culture Collection (Manassas, VA), mouse anti–human CTLA-4 (BNI3.1) was purchased from Immunotech (Marseilles, France). Mouse immunoglobulin G2a (specific for 2,4,6-trinitrophenol [TNP]), hamster anti–mouse CD28 (37.10) purified rat anti–mouse IL-2 (JES6-1A12), biotinylated rat anti–mouse IL-2 (JES6-5H4), and biotinylated anti-FasL were purchased from Pharmingen (San Diego, CA). Avidin-peroxidase was purchased from Sigma. ABTS tablets and recombinant mouse IL-2 were purchased from Boehringer Mannheim (Indianapolis, IN). Mouse anti-hamster was purchased from Sigma. Phycoerythrin-labeled streptavidin was a gift from the laboratory of Dr Lee Nadler, Dana-Farber Cancer Institute (Boston, MA).
Stimulation assay
For all experiments, stimulation of T-cell hybridoma was performed by means of antibodies bound to plates. The antibodies were added at their designated concentration in a 96-well microtiter plate and left to adsorb overnight at 4°C. The wells were washed twice with complete media, followed by the addition of cells at a density of 1.5 × 105 cells per well and incubation for 24 hours at 37°C. The cells were then harvested and analyzed for apoptosis. The supernatant was frozen for later measurements of IL-2 by enzyme-linked immunosorbent assay (ELISA) (Pharmingen).
Apoptosis measurements
The samples were fixed with propidium iodide (PI) staining buffer (sodium citrate 0.1%, sodium dodecyl sulfate 1%, 50 μg/mL PI) overnight at 4°C. Apoptosis was analyzed on an Epics XL flow cytometry system (Coulter, Hialeah, FL) by gating on the subdiploid population below the G1 peak.
Results and discussion
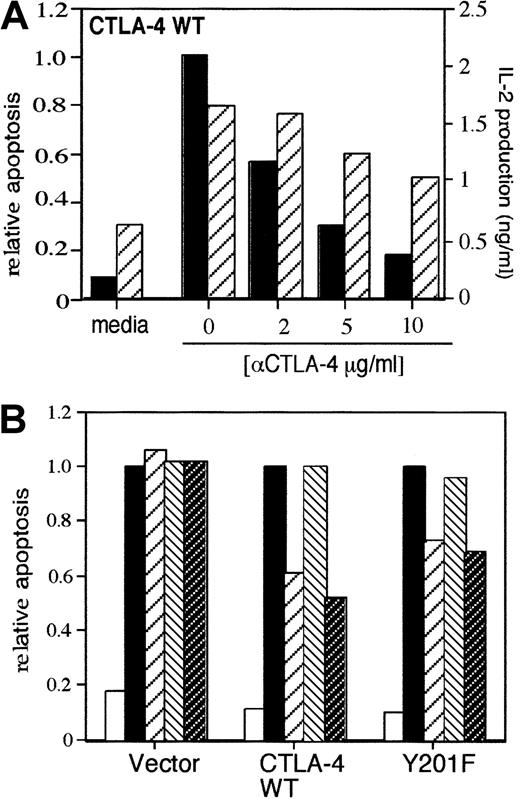
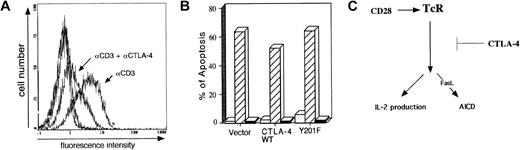
Stable transfectants of the T-cell hybridoma DC27.10 were initially generated that express either wild-type human CTLA-4 (CTLA-4 WT), or a mutant form with a substituted cytoplasmic tyrosine at position 201 in the YVKM motif (Y201-F).27Both CTLA-4 WT and Y201-F express the coreceptor at moderate levels with stable expression of TCR/CD3 and CD28 (Figure1A). To assess whether CTLA-4 could influence the ability of the TCRζ/CD3 to induce apoptosis, anti-CD3 was immobilized on plates at a concentration of 0.5 μg/mL in the presence or absence of anti–CTLA-4 at a concentration of 10 μg/mL. Cell death was assessed 24 hours after stimulation by examining the DNA content by PI staining as described in “Study design.” The results were confirmed by DNA laddering techniques (data not shown). Anti-CD3 induced AICD in transfected cells in the range of 50% to 65% as observed in 7 experiments (data not shown).33 34 Results were expressed as values relative to the anti-CD3 control. Coligation with anti–CTLA-4 caused a 60% reduction in the percentage of cells undergoing apoptosis in cells transfected with CTLA-4 WT (Figure 1B). In 7 experiments, anti–CTLA-4 blocked AICD within a range of 60% to 80%. No effect of anti–CTLA-4 was evident in vector-transfected control cells (Figure 1B). The efficacy of the AICD blocking was proportional to the concentration of anti–CTLA-4 in the range of 2 to 10 μg/mL (Figure 1C). Inhibition by CTLA-4 was also observed when anti-CD28 was coligated with anti-CD3 (Figure 1D). These findings indicate that coligation of CTLA-4 can attenuate anti-CD3–induced AICD.
CTLA-4 inhibition of AICD in T-cell hybridoma transfected with human CTLA-4.
(A) Fluorescent activated cell sorter (FACS) profile of TCRζ/CD3, human CTLA-4, and murine CD28 (mCD28) surface expression on transfectants. DC27.10 cells were stably transfected with a vector (denoted Vector), human CTLA-4 (denoted CTLA-4 WT), and CTLA-4 mutant (denoted Y201-F). (B) CTLA-4 inhibits AICD in CTLA-4 transfectants. Cells were stimulated on a 96-well plate coated with anti-CD3 (145-2C11) at a concentration of 0.5 μg/mL and with anti–CTLA-4 (10 μg/mL). Anti-TNP control antibody was added to keep a final concentration of 10.5 μg/mL with anti-CD3 alone. AICD was determined by PI as described in “Study design.” ■ indicates media; ▪, αCD3; and ▧, αCD3 + αCTLA-4. (C) CTLA-4 inhibits AICD in a concentration-dependent manner. Anti-CD3 was used at a concentration of 0.5 μg/mL, while anti–CTLA-4 was used at concentrations of 2, 5, and 10 μg/mL. Anti-TNP control antibody was added to keep the final concentrations equivalent. ▪ indicates vector; ▧, CTLA-4 WT; and ■, Y201F. (D) CTLA-4 inhibits AICD induced by anti-CD3 in conjunction with anti-CD28. Anti-CD3 was used at 0.5 μg/mL; anti-CD28 at 5 μg/mL; and anti–CTLA-4 at 5 μg/mL. Anti-TNP control antibody was added to keep the final concentrations equivalent. ■ indicates media; ▪, αCD3; ▨, αCD3 + αCD28; ▧, αCD3 + αCD28 + αCTLA-4.
CTLA-4 inhibition of AICD in T-cell hybridoma transfected with human CTLA-4.
(A) Fluorescent activated cell sorter (FACS) profile of TCRζ/CD3, human CTLA-4, and murine CD28 (mCD28) surface expression on transfectants. DC27.10 cells were stably transfected with a vector (denoted Vector), human CTLA-4 (denoted CTLA-4 WT), and CTLA-4 mutant (denoted Y201-F). (B) CTLA-4 inhibits AICD in CTLA-4 transfectants. Cells were stimulated on a 96-well plate coated with anti-CD3 (145-2C11) at a concentration of 0.5 μg/mL and with anti–CTLA-4 (10 μg/mL). Anti-TNP control antibody was added to keep a final concentration of 10.5 μg/mL with anti-CD3 alone. AICD was determined by PI as described in “Study design.” ■ indicates media; ▪, αCD3; and ▧, αCD3 + αCTLA-4. (C) CTLA-4 inhibits AICD in a concentration-dependent manner. Anti-CD3 was used at a concentration of 0.5 μg/mL, while anti–CTLA-4 was used at concentrations of 2, 5, and 10 μg/mL. Anti-TNP control antibody was added to keep the final concentrations equivalent. ▪ indicates vector; ▧, CTLA-4 WT; and ■, Y201F. (D) CTLA-4 inhibits AICD induced by anti-CD3 in conjunction with anti-CD28. Anti-CD3 was used at 0.5 μg/mL; anti-CD28 at 5 μg/mL; and anti–CTLA-4 at 5 μg/mL. Anti-TNP control antibody was added to keep the final concentrations equivalent. ■ indicates media; ▪, αCD3; ▨, αCD3 + αCD28; ▧, αCD3 + αCD28 + αCTLA-4.
The cytoplasmic tail of CTLA-4 has a tyrosine-based motif YVKM that binds several intracellular signaling proteins.19-22Mutation of the tyrosine within the YVKM sequence renders the motif unable to bind to these proteins. Disruption of the YVKM motif rendered CTLA-4 significantly less effective in blocking AICD (Figure 1B-C). A titration of anti–CTLA-4 showed that this partial blockage in Y201-F was observed only at the highest concentration of anti–CTLA-4 (Figure1C). This partial inhibition was also observed with anti-CD3 × CD28 cross-linking (Figure 1D). These observations indicate that the optimal blockage of AICD by CTLA-4 is dependent on an intact YVKM motif.
CTLA-4 inhibition of IL-2 production is a hallmark of the blockage effects on T-cell activation.6 7 Indeed, IL-2 production was inhibited by anti-CTLA-4 in a dose-dependent manner of 2 to 10 μg/ml of antibody on the CTLA-4 WT cells, albeit slightly less than observed for AICD (Figure 2A). To control for the possibility that the loss of IL-2 contributed to AICD, exogenous recombinant IL-2 was added in excess to cultures with anti-CD3 plus anti–CTLA-4 (Figure 2B). Under these conditions, IL-2 did not restore the induction of cell death, indicating that CTLA-4 blockage of AICD was not due to its blockage of IL-2.
CTLA-4 inhibition of AICD independent of a CTLA-4 effect on IL-2 production.
(A) CTLA-4 inhibits AICD and IL-2 production in CTLA-4 transfectants. Cells were stimulated with anti-CD3 at a concentration of 0.5 μg/mL and a range of anti–CTLA-4 concentrations from 2 to 10 μg/mL. Supernatants were collected 24 hours after stimulation, and IL-2 levels were determined by ELISA. Anti-TNP control antibody was added to keep the final concentrations equivalent. ▨ indicates IL-2 production; ▪, relative apoptosis. (B) Addition of exogenous IL-2 does not reverse anti–CTLA-4 blockage of AICD. Cells were stimulated with 0.5 μg/mL of anti-CD3 and 10 μg/mL of anti–CTLA-4 with or without recombinant mouse IL-2 (20 U/mL). ■ indicates media; ▪, αCD3; ▨, αCD3 + αCTLA-4; ▧, αCD3 + rIL-2; ▩, αCD3 + αCTLA-4 + rIL-2.
CTLA-4 inhibition of AICD independent of a CTLA-4 effect on IL-2 production.
(A) CTLA-4 inhibits AICD and IL-2 production in CTLA-4 transfectants. Cells were stimulated with anti-CD3 at a concentration of 0.5 μg/mL and a range of anti–CTLA-4 concentrations from 2 to 10 μg/mL. Supernatants were collected 24 hours after stimulation, and IL-2 levels were determined by ELISA. Anti-TNP control antibody was added to keep the final concentrations equivalent. ▨ indicates IL-2 production; ▪, relative apoptosis. (B) Addition of exogenous IL-2 does not reverse anti–CTLA-4 blockage of AICD. Cells were stimulated with 0.5 μg/mL of anti-CD3 and 10 μg/mL of anti–CTLA-4 with or without recombinant mouse IL-2 (20 U/mL). ■ indicates media; ▪, αCD3; ▨, αCD3 + αCTLA-4; ▧, αCD3 + rIL-2; ▩, αCD3 + αCTLA-4 + rIL-2.
AICD in T-cell hybridomas is mediated by the expression of Fas (CD95/APO-1), the expression of FasL (CD95L), and the activation of caspases.33 34 To investigate whether CTLA-4 blocks AICD by means of an inhibitory effect on FasL expression, cells were incubated with anti-CD3 in the presence or absence of anti–CTLA-4 followed by staining for FasL surface expression. Under these conditions, anti–CTLA-4 coligation caused a 4- to 5-fold reduction in the level of FasL expression (Figure 3A). This level of inhibition correlated well with the overall reduction of AICD (Figure 1A-D). Fas expression was also reduced, but to a lesser extent and less reproducibly (data not shown). The blockage of FasL expression was observed reproducibly in 4 experiments. To confirm that the AICD in our hybridomas was mediated by Fas/FasL binding, blocking anti-FasL antibodies were added to cultures and found to completely inhibit cell death (Figure 3B). CTLA-4 therefore impinges on TCR-induced apoptosis by blocking the expression of FasL.
Engagement of CTLA-4 accompanying reduced FasL surface expression.
(A) Anti–CTLA-4 reduced expression of FasL. Cells transfected with CTLA-4 WT were stimulated with the indicated antibodies bound to plates: anti–CTLA-4 (10 μg/mL), anti-TNP (10 μg/mL) control, and anti-CD3 (0.5 μg/mL). The cells were harvested after 6 hours, stained with biotinylated anti-FasL antibody followed by fluorescein isothiocyanate-labeled avidin. Subsequently, the cells were gated and analyzed by flow cytometry. A histogram of FasL staining on FL-1 is displayed. (B) Anti-FasL blocks anti-CD3–induced apoptosis in DC27.10 transfectants. CTLA-4 WT transfectants were stimulated with the indicated antibodies bound to plates: anti–CTLA-4 (10 μg/mL), anti-TNP (10 μg/mL) control, and anti-CD3 (0.5 μg/mL) with or without soluble blocking anti-FasL antibody (10 μg/mL). The cells were harvested 24 hours later and stained with PI. Apoptosis was measured as described in “Study design.” ■ indicates control; ▨, αCD3; ▪, αCD3 + αFasL. (C) Model of CTLA-4 blockage of IL-2 production and AICD. The coreceptor acts to dampen TCR/CD3 signaling at an early stage in T-cell activation prior to the bifurcation of signals leading to IL-2 production and FasL expression and AICD.
Engagement of CTLA-4 accompanying reduced FasL surface expression.
(A) Anti–CTLA-4 reduced expression of FasL. Cells transfected with CTLA-4 WT were stimulated with the indicated antibodies bound to plates: anti–CTLA-4 (10 μg/mL), anti-TNP (10 μg/mL) control, and anti-CD3 (0.5 μg/mL). The cells were harvested after 6 hours, stained with biotinylated anti-FasL antibody followed by fluorescein isothiocyanate-labeled avidin. Subsequently, the cells were gated and analyzed by flow cytometry. A histogram of FasL staining on FL-1 is displayed. (B) Anti-FasL blocks anti-CD3–induced apoptosis in DC27.10 transfectants. CTLA-4 WT transfectants were stimulated with the indicated antibodies bound to plates: anti–CTLA-4 (10 μg/mL), anti-TNP (10 μg/mL) control, and anti-CD3 (0.5 μg/mL) with or without soluble blocking anti-FasL antibody (10 μg/mL). The cells were harvested 24 hours later and stained with PI. Apoptosis was measured as described in “Study design.” ■ indicates control; ▨, αCD3; ▪, αCD3 + αFasL. (C) Model of CTLA-4 blockage of IL-2 production and AICD. The coreceptor acts to dampen TCR/CD3 signaling at an early stage in T-cell activation prior to the bifurcation of signals leading to IL-2 production and FasL expression and AICD.
Our findings provide the first evidence that CTLA-4 has the capacity to block TCR-signaling events that lead to FasL expression and AICD. Scheipers and Reiser44, however, have reported a role of CTLA-4 in the promotion of AICD. In their study, concanavalin A (ConA) is used as a stimulating agent instead of direct TCR cross-linking. It may be that stimulating with ConA and anti–CTLA-4 antibodies delivers different signals than using antibodies to directly cross-link the TCR with CTLA-4. Our study was not intended to address the physiological relevance of CTLA-4 on antigen-induced cell death, but rather to serve as a model to examine the point of blockage by CTLA-4 in the signaling cascade. With this perspective, our findings suggest that the coreceptor can block TCR signaling prior to the bifurcation of signals leading to FasL and IL-2 production (Figure 3C). Further CTLA-4 can block TCR signaling events that result in both positive (eg, IL-2 production) and negative outcomes (ie, apoptosis). Although the nature of signals that distinguish these events is unknown, p56lck and NF-AT have been reported to regulate both FasL and IL-2 expression.39Other transcription factors, c-Myc, Egr 2, and Egr 3, have been implicated in FasL expression.40-42 In our system, the inhibitory effect of FasL expression and AICD must be an active process since it depends on the integrity of the cytoplasmic tail of the receptor (ie, YVKM motif). This contrasts with previous studies that demonstrated anti–CTLA-4 rescue of thymocytes from cell death by blockage of negative signaling.43 In this context, our cellular assay could be used as an assay to distinguish between antibodies that operate by actively generating negative signals vs those that simply block engagement of the receptor with CD80/CD86. Further analysis will also be needed to define which of these associated proteins regulates the blockage of AICD and IL-2 production.
Supported by National Institutes of Health grant 5P01AI35297, funding from the Fundação para a Ciência e Technologia Praxis XXI/BD/9652/1996, and a doctoral fellowship (S.d.R.D.) from the Fundação para a Ciência e Technologia Praxis XXI/BD/9652/1996, Portugal.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christopher E. Rudd, Division of Tumor Immunology, Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail:christopher_rudd@dfci.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal