Abstract
The serpin antithrombin III (AT III) is reported to have hemostasis-regulating and anti-inflammatory properties. To determine its ability to influence thrombin-independent leukocyte responses, the direct effects of the AT III concentrate Kybernin P and a monoclonal antibody-purified AT III on neutrophil migration were studied. Chemotactic activity of human neutrophils isolated from the blood of healthy donors was determined in modified Boyden microchemotaxis chambers, and binding studies were performed according to standard experimental protocols. Preincubation in vitro of neutrophils with Kybernin P or immune-adsorbed AT III significantly deactivated migration toward fMet-Leu-Phe, or interleukin-8 (IL-8), in a concentration-dependent manner. In the absence of additional attractants, neutrophils exhibited a migratory response toward gradients of AT III preparations. True chemotaxis was confirmed in checkerboard assays. Analyses revealed that the AT III heparin-binding site interacts with neutrophil membrane–associated heparan sulfate proteoglycan receptors. Mechanisms of intracellular signaling differed; the deactivation of IL-8–induced chemotaxis resulted from tyrphostin-sensitive interactions of AT III-signaling with the IL-8 signal transduction pathway, whereas AT III–induced chemotaxis involved protein kinase C and phosphodiesterases. Signaling similarities between AT III and the proteoglycan syndecan-4 may suggest the binding of AT III to this novel type of membrane receptor. Under physiological conditions, AT III may prevent neutrophils from premature activation. Moreover, the systemic administration of AT III concentrate could have beneficial effects in combating systemic inflammation.
Introduction
Activation of neutrophils plays a crucial role in the development of tissue injury and is a hallmark of every inflammatory process. Several endogenous mediators have been described to prevent the organism from conditions of uncontrolled neutrophil migration and enzyme release.1 Disseminated intravascular coagulation is an early complication in patients with sepsis and may be a precipitating factor for complications such as acute respiratory distress syndrome.2 In this context, oxidants, cytokines, and proteinases play a major role in perpetuating inflammation.3 Not only cytokine-mediated activation of the coagulation system4 but also cytokine-induced leukocyte activation is an important step in the onset of the septic response and contributes to septic shock.5 In addition to exogenous bacterial-derived formyl-Met-Leu-Phe (fMLP), the chemokine interleukin-8 (IL-8) is one of the most potent endogenous proinflammatory mediators, and its role in leukocyte activation of sepsis is well established.6 7
Besides preventing hemorrhagic complications, antithrombin III (AT III) exerts anti-inflammatory effects by inhibiting thrombin and other activated clotting proteases.8,9 It has been shown to be efficacious in several animal models of sepsis.9-12 The hypothesis that AT III can reduce mortality in patients suffering from severe sepsis is being tested in an ongoing large-scale phase III clinical trial.13 Additionally, thrombin-independent effects exerted more directly on leukocytes and endothelial cells have also been reported.14-16 Previously, the direct action of serpins on leukocyte migration, which is independent of serpin-enzyme complex formation, has been proposed for heparin cofactor II, which is an AT III analogue.17 Recently, antiangiogenic properties of conformation-modified AT III have been described.18
Mechanisms by which AT III exerts its protective effect in systemic inflammation are thus far only partly understood. Because not only the anti-DIC disseminated intravascular coagulation activity of AT III in early sepsis but also thrombin-independent anti-inflammatory effects may involve neutrophils and could, therefore, be responsible for an increased survival in sepsis models, we investigated the impact of this serpin on human neutrophil function. Given that the effects of AT III on neutrophils occur at normal plasma concentrations below 1 U AT III, our findings could be of physiological relevance.
Materials and methods
Neutrophil isolation
Neutrophils were obtained from EDTA–anticoagulated peripheral blood of healthy donors after discontinuous density gradient centrifugation on Percoll by dextran sedimentation and centrifugation through a layer of Ficoll-Hypaque, followed by hypotonic lysis of contaminating erythrocytes using sodium chloride solution.19 Cell preparations yielded more than 95% neutrophils (by morphology in Giemsa stains) and more than 99% viability (by trypan dye exclusion). Experiments were performed in RPMI 1640 (Biological Industries, Kibbutz Beit Haemek, Israel) with 0.5% bovine serum albumin (BSA; Aventis Behring, Marburg, Germany). Except where needed, no reagent used contained heparin or heparin-like substances.
AT III preparation
To exclude the potential impact of plasmatic trace impurities, the AT III concentrate (Kybernin P; Aventis Behring GmbH) was run over a monoclonal antibody resin. The eluted immune-adsorbed AT III (AT III) was used in the migration and binding assays. The flow-through fraction was also collected and subjected to assays. One unit of AT III (FW 65 kd) equals 2.31 × 10−5 mol/L and is the activity present in 1 mL normal human pooled plasma tested in the presence of 0.1 U heparin.20
Thrombin–antithrombin III complex formation
Human α-thrombin (2000 U/mg, 1 mL) was incubated with AT III (10 U, 0.2 mL) (both Aventis Behring) at pH 8.2 for 40 minutes at room temperature. AT III inhibitory activity and thrombin activity were determined by the Berichrom AT III assay and a chromogenic assay using the substrate S 2238 (Chromogenix AB, Mölndal, Sweden). Inhibition of thrombin by AT III was confirmed by determination of the residual thrombin activity amounting to less than 0.1% of the starting material. Residual AT III activity (not complexed) in this preparation was less than 3% of the starting material. Thrombin–antithrombin III (TAT) complexes were visible on SDS-PAGE as well. TAT complexes were aliquoted and stored at −20°C.
Elastase-digested AT III
Five milliliters AT III (50 U/mL) was incubated with 175 μL human neutrophil elastase (100 μg/mL) for 20 hours at 37°C. The reaction was terminated with the addition of 35 μL elastatinal (1 mg/mL) (San Diego, CA) and with incubation for 30 minutes at 37°C. The mixture was subjected to gel filtration (SEC-HPLC) to remove the excess of the LMW-elastase inhibitor. Residual elastase activity was measured using the chromogenic substrate S 2484 (Chromogenix AB). AT III activity was determined as described above. Incubation with elastase led to a complete destruction of the AT III thrombin inhibitory activity. SEC-HPLC revealed 4 distinct peaks of the digest, reflecting monomeric (main peak) and oligomeric AT III fractions. The peaks were pooled for further analyses and for the chemotaxis assay. Neither elastase activity nor elastatinal inhibitory activity was detectable in the chromogenic test system. SDS-PAGE showed no significant difference in the starting material with respect to potential cleavage products if run under nonreducing conditions. As expected, after reduction of the sample, a 3- to 5-kd polypeptide became visible, representing the C-terminal peptide (linked to the residual protein by a disulfide bridge) separated by the distinct cleavage with elastase.
Neutrophil chemotaxis
Migration of neutrophils into cellulose nitrate to gradients of soluble attractants was measured using a 48-well microchemotaxis chamber (Neuroprobe, Bethesda, MD) in which a 5 μm pore-sized filter (Sartorius, Göttingen, Germany) separated the upper from the lower chamber.19 In various experiments neutrophils migrated toward AT III, Kybernin P, elastase-digested AT III (AT IIIED), TAT, or receptor-associated protein (RAP; American Research Products, Belmont, MA). Directed chemotaxis toward IL-8 served as a positive control. For deactivation, neutrophils were incubated for 20 minutes at various concentrations of AT III, Kybernin P, AT IIIED, or TAT. The preincubation period with RAP was 2 hours. After they were washed twice in phosphate-buffered saline (PBS), neutrophils migrated toward fMLP or IL-8 (both from Sigma Chemical, St Louis, MO) in the lower chambers for 35 minutes at 37°C in a humidified atmosphere (5% CO2). In some experiments, cells were treated with the intracellular enzyme blockers bisindolylmaleimide I GF 109203X (GFX; Böhringer Ingelheim KG, Ingelheim AM Rhein, Germany), staurosporine (from Streptomyces sp), tyrphostin, iso-butyl-methylxanthine (IBMX), or wortmannin for 30 minutes or with heparinase for a total time of 50 minutes (all from Sigma Chemical). Then the cells were washed before migration. Cells were incubated with human monoclonal antibodies (mAb) to sialyl Lewis(x), IL-8 receptors (CXCR1 and CXCR2) (Biomeda, Foster City, CA), or CD45 (Bra55) (Sigma Chemical) for 2 hours. To exclude thrombin contaminations or the effects of neutrophil-derived elastase, hirudin (Sigma Chemical) or a neutrophil elastase inhibitor (Zeneca Pharmaceuticals, Södertlje, Sweden) was present during cell migration in 2 sets of experiments. The assay was performed as recently described.19
Checkerboard analyses
To ensure that the effect observed was true chemotaxis, checkerboard analyses were performed. Neutrophils were resuspended in RPMI 1640–0.5% BSA containing various concentrations of AT III or Kybernin P just before they were transferred to the upper chamber. The same concentrations of the respective substances remained beneath the filter of the Boyden chamber; thus, distinct concentration gradients could be formed. Data are expressed as chemotaxis index within a matrix.
Receptor-binding analyses
Human neutrophils (107 cells/mL) were incubated with sodium iodide I 125–IL-8 (2000 Ci/mM) at concentrations of 10 fM/L plus or minus an excess of unlabeled IL-8, or AT III at various concentrations, in a total volume of 400 μL PBS–0.5% BSA, for 90 minutes at 4°C. After incubation, cells were washed twice with PBS–0.5% BSA containing 0.5 mol/L saline and centrifuged at 200g for 10 minutes. The bottom of the tube containing the neutrophil pellet was counted in a Beckman gamma counter. Results are expressed as counts per minute (cpm). Specific binding is defined as total binding minus nonspecific binding, which is the residual cpm bound in the presence of 100-fold excess unlabeled IL-8.21IL-8 receptor internalization (deactivation) was measured by the preincubation of cells with IL-8, fMLP, or AT III for 5 minutes according to previous time-course experiments. For homologous receptor desensitization experiments, IL-8 (0.1-10 nM/L) was used, and for heterologous desensitization, fMLP (10 nM/L-1 μM/L) or AT III (0.01-1 U/mL) was used. Thereafter, total 125I–IL-8 binding was quantified.
Statistical methods
Data are expressed as mean ± standard error of the mean (SEM). The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils into the nitrocellulose filters. Means were compared by Mann Whitney test and Kruskal-Wallis analysis of variance. P < .05 was considered significant.
Results
Induction of directed migration and deactivation of chemotaxis by AT III or Kybernin P
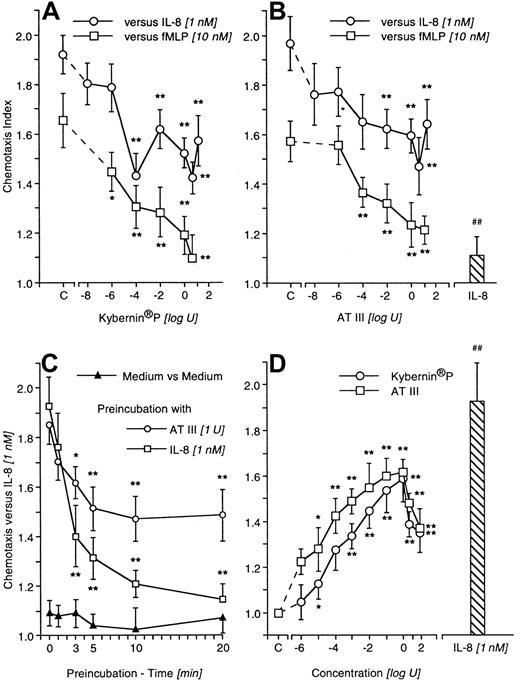
Preincubation of neutrophils with either immune-adsorbed AT III or Kybernin P at various concentrations decreased the migration of cells to fMLP and IL-8. Significant inhibition occurred at concentrations higher than 0.1 mU (Figure 1A-B). The flow-through fraction did not produce comparable results (not shown). Pretreatment with IL-8, which resulted in homologous deactivation, served as a positive control. The AT III–induced effect was strictly time dependent, with significant values appearing after 3 minutes of preincubation and reaching a plateau after 10 minutes (Figure 1C). AT III or Kybernin P put beneath the chemotaxis-filter was able to induce directed cell migration. Maximal stimulation was observed at doses between 10 mU and 1 U; further increasing the concentration up to 5 U diminished the dose-response (Figure 1D). In checkerboard analyses the migratory response was confirmed as true chemotaxis (Table1).
Deactivation of chemotaxis and directed migration induced by AT III or Kybernin P.
Neutrophils were pretreated for 20 minutes with Kybernin P or AT III before migration to fMLP or IL-8 was tested. Homologous deactivation by IL-8 served as a positive control (A,B). Time-course of pretreatment for AT III– or IL-8–induced deactivation of neutrophil chemotaxis to IL-8 (C). Directed migration of neutrophils to Kybernin P or AT III (D). Migration time into nitrocellulose filters was 30 minutes. After fixing and staining of filters, migration depth was quantified microscopically. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by the Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01. n = 13 (A,B); n = 5 (C); n = 9 (D).
Deactivation of chemotaxis and directed migration induced by AT III or Kybernin P.
Neutrophils were pretreated for 20 minutes with Kybernin P or AT III before migration to fMLP or IL-8 was tested. Homologous deactivation by IL-8 served as a positive control (A,B). Time-course of pretreatment for AT III– or IL-8–induced deactivation of neutrophil chemotaxis to IL-8 (C). Directed migration of neutrophils to Kybernin P or AT III (D). Migration time into nitrocellulose filters was 30 minutes. After fixing and staining of filters, migration depth was quantified microscopically. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by the Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01. n = 13 (A,B); n = 5 (C); n = 9 (D).
Effect of concentration gradients of Kybernin P or antithrombin III on neutrophil migration
| . | Upper chamber . | |||
|---|---|---|---|---|
| Medium . | 10−6 . | 10−3 . | 100 . | |
| Kybernin P (u) | ||||
| Lower chamber | ||||
| Medium | 1.000 ± 0.000 | 1.043 ± 0.064 | 1.056 ± 0.082 | 1.092 ± 0.048* |
| 10−6 | 1.171 ± 0.028 | 1.156 ± 0.034 | 1.124 ± 0.056 | 1.056 ± 0.048 |
| 10−3 | 1.400 ± 0.058† | 1.184 ± 0.028 | 1.131 ± 0.038 | 1.106 ± 0.034 |
| 100 | 1.522 ± 0.044‡ | 1.328 ± 0.046 | 1.207 ± 0.040 | 1.229 ± 0.030† |
| AT III (u) | ||||
| Lower chamber | ||||
| Medium | 1.000 ± 0.000 | 1.032 ± 0.058 | 0.995 ± 0.018 | 0.999 ± 0.068* |
| 10−6 | 1.224 ± 0.062 | 1.014 ± 0.056 | 1.054 ± 0.064 | 1.063 ± 0.039 |
| 10−3 | 1.323 ± 0.088† | 1.154 ± 0.068 | 1.009 ± 0.067 | 1.063 ± 0.044 |
| 100 | 1.489 ± 0.046‡ | 1.281 ± 0.088 | 1.255 ± 0.036 | 1.094 ± 0.072 |
| . | Upper chamber . | |||
|---|---|---|---|---|
| Medium . | 10−6 . | 10−3 . | 100 . | |
| Kybernin P (u) | ||||
| Lower chamber | ||||
| Medium | 1.000 ± 0.000 | 1.043 ± 0.064 | 1.056 ± 0.082 | 1.092 ± 0.048* |
| 10−6 | 1.171 ± 0.028 | 1.156 ± 0.034 | 1.124 ± 0.056 | 1.056 ± 0.048 |
| 10−3 | 1.400 ± 0.058† | 1.184 ± 0.028 | 1.131 ± 0.038 | 1.106 ± 0.034 |
| 100 | 1.522 ± 0.044‡ | 1.328 ± 0.046 | 1.207 ± 0.040 | 1.229 ± 0.030† |
| AT III (u) | ||||
| Lower chamber | ||||
| Medium | 1.000 ± 0.000 | 1.032 ± 0.058 | 0.995 ± 0.018 | 0.999 ± 0.068* |
| 10−6 | 1.224 ± 0.062 | 1.014 ± 0.056 | 1.054 ± 0.064 | 1.063 ± 0.039 |
| 10−3 | 1.323 ± 0.088† | 1.154 ± 0.068 | 1.009 ± 0.067 | 1.063 ± 0.044 |
| 100 | 1.489 ± 0.046‡ | 1.281 ± 0.088 | 1.255 ± 0.036 | 1.094 ± 0.072 |
Statistical analyses: Mann-Whitney test after Kruskal Wallis analysis of variance (P < .001); n = 5.
AT III indicates antithrombin III.
P < .01 vs. highest concentration of test substance exclusively in the lower chamber. Only those groups within the matrix being sufficient to distinguish between chemokinesis and chemotaxis were compared statistically by Mann-Whitney test.
P < .05.
P < .01 vs. medium in the upper and lower chamber.
Effects of AT IIIED or TAT complexes on neutrophil migration
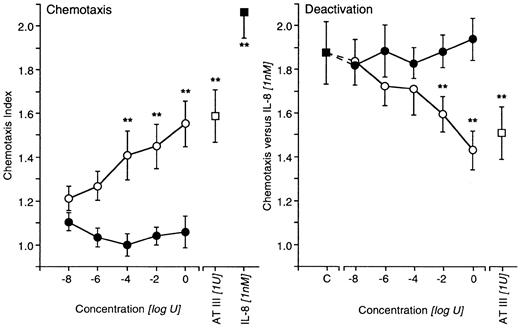
In contrast to TAT (10 nU-1 U), which was completely ineffective, AT IIIED (10 nU-1 U) mimicked the effects of AT III regarding the deactivation of IL-8–induced chemotaxis and the induction of directed migration at comparable effective dose ranges (Figure 2). The thrombin inhibitor hirudin, at various concentrations, also failed to affect AT III–induced chemotaxis; moreover, a neutrophil elastase inhibitor lacked such an effect when both substances were present during the migration of cells (Table 2).
Effects of AT IIIED or TAT complex on neutrophil migration.
Untreated neutrophils migrated to various concentrations of AT IIIED (○) or TAT complexes (●). AT III and IL-8 served as positive controls (left). For deactivation experiments, cells were pretreated with either AT IIIED or TAT complexes for 20 minutes before chemotaxis to IL-8 was run. Preincubation with AT III was the positive control (right). Cells migrated for 30 minutes into nitrocellulose filters, and, after fixing and staining of filters, migration depth was quantified microscopically. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). **P < .01. n = 5.
Effects of AT IIIED or TAT complex on neutrophil migration.
Untreated neutrophils migrated to various concentrations of AT IIIED (○) or TAT complexes (●). AT III and IL-8 served as positive controls (left). For deactivation experiments, cells were pretreated with either AT IIIED or TAT complexes for 20 minutes before chemotaxis to IL-8 was run. Preincubation with AT III was the positive control (right). Cells migrated for 30 minutes into nitrocellulose filters, and, after fixing and staining of filters, migration depth was quantified microscopically. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). **P < .01. n = 5.
Failure of hirudin or neutrophil elastase inhibitor to affect antithrombin III–induced chemotaxis or deactivation
| Treatment . | Neutrophil migration versus . | ||
|---|---|---|---|
| Medium . | IL-8 [1 nM] . | AT III [1 U] . | |
| Medium | 1.000 ± 0.00 | 2.147 ± 0.18 | — |
| AT III [1 U] | 1.045 ± 0.07 | 1.476 ± 0.06 | — |
| + EI [0.1 μM/L] | 1.105 ± 0.03 | 1.537 ± 0.12 ns | — |
| + EI [1 μM/L] | 0.978 ± 0.05 | 1.301 ± 0.14 ns | — |
| + EI [10 μM/L] | 1.006 ± 0.03 | 1.509 ± 0.15 ns | — |
| + EI [100 μM/L] | 1.104 ± 0.08 | 1.313 ± 0.13 ns | — |
| IL-8 [1 nM] | 1.201 ± 0.09 | 1.132 ± 0.11 | — |
| Medium | 1.000 ± 0.00 | — | 1.584 ± 0.07 |
| Hirudin [0.01 mg/mL] | 1.159 ± 0.03 | — | 1.484 ± 0.11 ns |
| Hirudin [0.1 mg/mL] | 1.150 ± 0.09 | — | 1.586 ± 0.10 ns |
| Hirudin [1 mg/mL] | 1.150 ± 0.08 | — | 1.428 ± 0.09 ns |
| Hirudin [5 mg/mL] | 1.037 ± 0.04 | — | 1.481 ± 0.08 ns |
| Treatment . | Neutrophil migration versus . | ||
|---|---|---|---|
| Medium . | IL-8 [1 nM] . | AT III [1 U] . | |
| Medium | 1.000 ± 0.00 | 2.147 ± 0.18 | — |
| AT III [1 U] | 1.045 ± 0.07 | 1.476 ± 0.06 | — |
| + EI [0.1 μM/L] | 1.105 ± 0.03 | 1.537 ± 0.12 ns | — |
| + EI [1 μM/L] | 0.978 ± 0.05 | 1.301 ± 0.14 ns | — |
| + EI [10 μM/L] | 1.006 ± 0.03 | 1.509 ± 0.15 ns | — |
| + EI [100 μM/L] | 1.104 ± 0.08 | 1.313 ± 0.13 ns | — |
| IL-8 [1 nM] | 1.201 ± 0.09 | 1.132 ± 0.11 | — |
| Medium | 1.000 ± 0.00 | — | 1.584 ± 0.07 |
| Hirudin [0.01 mg/mL] | 1.159 ± 0.03 | — | 1.484 ± 0.11 ns |
| Hirudin [0.1 mg/mL] | 1.150 ± 0.09 | — | 1.586 ± 0.10 ns |
| Hirudin [1 mg/mL] | 1.150 ± 0.08 | — | 1.428 ± 0.09 ns |
| Hirudin [5 mg/mL] | 1.037 ± 0.04 | — | 1.481 ± 0.08 ns |
IL-8 indicates interleukin-8; AT III, antithrombin III; ns, not significant.
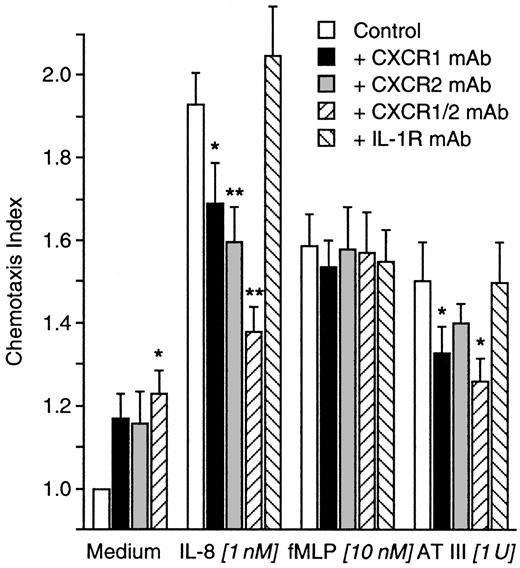
Inhibition of AT III–induced chemotaxis by anti–IL-8 receptor antibodies
Neutrophils were incubated for 2 hours with blocking antibodies to CXCR1, CXCR2, or both or with an anti–IL-1 receptor control mAb. Migration to fMLP (10 nM) was not disturbed by any of the mAbs used. Except the control mAb, all mAbs reduced migration to IL-8 (1 nM). AT III–induced chemotaxis was insensitive to the pretreatment of cells with control mAb or anti-CXCR2 mAb. In contrast, anti-CXCR1 or a combination of both anti–IL-8 receptor antibodies significantly diminished AT III–induced migration of neutrophils. However, the mAbs themselves slightly induced cell migration (Figure3).
Influence of IL-8 receptor blockade on AT III–induced chemotaxis.
Neutrophils were treated for 2 hours with blocking mAbs to both IL-8 receptors (CXCR1, CXCR2). An isotype-matched control mAb to IL-1 receptor served as the control. After washing, cells migrated to medium, IL-8, fMLP, or AT III for 30 minutes into nitrocellulose filters. Migration depth was measured by microscope. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01;n = 9.
Influence of IL-8 receptor blockade on AT III–induced chemotaxis.
Neutrophils were treated for 2 hours with blocking mAbs to both IL-8 receptors (CXCR1, CXCR2). An isotype-matched control mAb to IL-1 receptor served as the control. After washing, cells migrated to medium, IL-8, fMLP, or AT III for 30 minutes into nitrocellulose filters. Migration depth was measured by microscope. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01;n = 9.
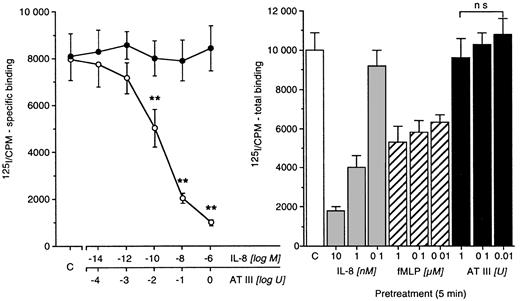
IL-8 receptor binding studies
Concomitant incubation of neutrophils with radioactive labeled and various concentrations of “cold” IL-8, but not with AT III, displaced 125I–IL-8 (Figure4). To investigate receptor internalization, we preincubated cells with IL-8 (0.1-10 nM), fMLP (0.01-1 nM), or AT III (0.01-1 U) before total binding was quantified. IL-8 or fMLP pretreatment dose-dependently down-regulated IL-8 receptor expression, whereas AT III treatment did not (Figure 4).
Receptor binding studies involving AT III and IL-8 receptors.
Human neutrophils were incubated with 125I–IL-8 (2000 Ci/mM) at a concentration of 10 μM plus or minus an excess of unlabeled IL-8 (○) or AT III (●) in PBS–0.5% BSA for 90 minutes at 4°C. After incubation, cells were washed twice with PBS–0.5% BSA, added to 0.5 mol/L saline, and centrifuged at 200g for 10 minutes. Activities were counted in a Beckman gamma counter. IL-8 receptor internalization (deactivation) was measured by the preincubation of cells with IL-8, fMLP, or AT-III for 5 minutes according to previous time-course experiments. Results are expressed as mean cpm, compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). **P < .01. ns, not significant; n = 6.
Receptor binding studies involving AT III and IL-8 receptors.
Human neutrophils were incubated with 125I–IL-8 (2000 Ci/mM) at a concentration of 10 μM plus or minus an excess of unlabeled IL-8 (○) or AT III (●) in PBS–0.5% BSA for 90 minutes at 4°C. After incubation, cells were washed twice with PBS–0.5% BSA, added to 0.5 mol/L saline, and centrifuged at 200g for 10 minutes. Activities were counted in a Beckman gamma counter. IL-8 receptor internalization (deactivation) was measured by the preincubation of cells with IL-8, fMLP, or AT-III for 5 minutes according to previous time-course experiments. Results are expressed as mean cpm, compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). **P < .01. ns, not significant; n = 6.
Effects of blocking sialyl Lewis(x)
Sialyl Lewis(x) (CD15) mAb binding to the neutrophil surface did not affect random migration. Incubation of untreated cells with AT III (1 U) for 20 minutes diminished chemotaxis to IL-8 (1 nM), as described above (IL-8, CI 1.997 ± .17; AT III + IL-8, CI 1.573, ± .06), but the blockade of CD15 could not abolish the AT III effect (anti-CD15 mAb + AT III + IL-8, CI 1.349 ± .10; not significant compared with AT III + IL-8).
Influence of leukocyte common antigen ligation
Ligation of leukocyte common antigen (CD45) by Bra55 significantly diminished the migration of neutrophils to fMLP (10 nM), IL-8 (1 nM), or AT III (1 U). These effects were tyrphostin sensitive only when IL-8 or AT III was used for chemo-attraction, whereas CD45-dependent deactivation of fMLP-induced chemotaxis was tyrphostin insensitive. The same was true of homologous deactivation by AT III of AT III–induced chemotaxis. In contrast, AT III deactivating properties of directed migration triggered by IL-8 and fMLP could be reversed by tyrosine kinase inhibition (Table 3).
Effects of CD45-ligation on antithrombin III–induced chemotaxis and deactivation
| . | Chemotaxis versus . | ||
|---|---|---|---|
| IL-8 [1 nM/L] . | fMLP [10 nM/L] . | AT III [1 U] . | |
| Medium | 2.080 ± 0.18 | 1.632 ± 0.20 | 1.374 ± 0.08 |
| CD45-Bra55 [5 μg/mL] | 1.469 ± 0.16 | 1.299 ± 0.11 | 1.103 ± 0.08 |
| CD45-Bra55 + Tyrphostin | 2.215 ± 0.123-150 | 1.367 ± 0.22 ns | 1.338 ± 0.043-150 |
| AT III [1 U] | 1.510 ± 0.07 | 1.119 ± 0.11 | 1.030 ± 0.04 |
| AT III + Tyrphostin | 1.904 ± 0.103-150 | 1.343 ± 0.133-150 | 1.194 ± 0.10 ns |
| CD45-Bra + AT III + Tyrphostin | 1.779 ± 0.14 | 1.367 ± 0.22 | 1.550 ± 0.26 |
| . | Chemotaxis versus . | ||
|---|---|---|---|
| IL-8 [1 nM/L] . | fMLP [10 nM/L] . | AT III [1 U] . | |
| Medium | 2.080 ± 0.18 | 1.632 ± 0.20 | 1.374 ± 0.08 |
| CD45-Bra55 [5 μg/mL] | 1.469 ± 0.16 | 1.299 ± 0.11 | 1.103 ± 0.08 |
| CD45-Bra55 + Tyrphostin | 2.215 ± 0.123-150 | 1.367 ± 0.22 ns | 1.338 ± 0.043-150 |
| AT III [1 U] | 1.510 ± 0.07 | 1.119 ± 0.11 | 1.030 ± 0.04 |
| AT III + Tyrphostin | 1.904 ± 0.103-150 | 1.343 ± 0.133-150 | 1.194 ± 0.10 ns |
| CD45-Bra + AT III + Tyrphostin | 1.779 ± 0.14 | 1.367 ± 0.22 | 1.550 ± 0.26 |
Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .01); n = 5.
For abbreviations, see Table 2.
P < .01.
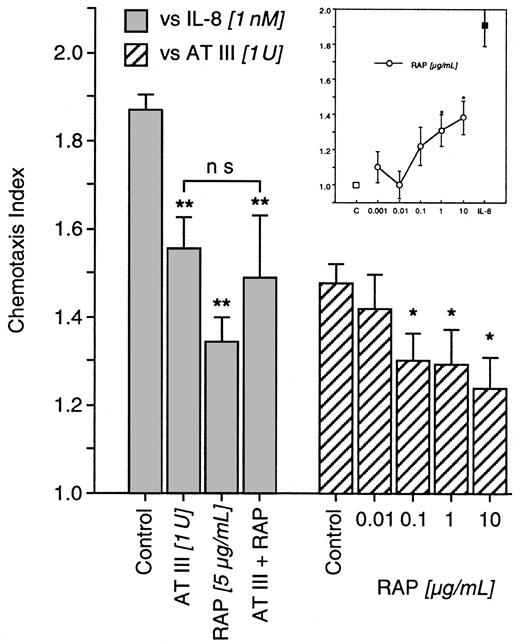
Blockade of LDL receptor–related protein by RAP
IL-8–induced chemotaxis was blocked by AT III (1 U) and by RAP (5 μg/mL). When neutrophils were exposed concomitantly to AT III and RAP before chemotaxis was tested, RAP failed to affect AT III; chemotaxis to IL-8 was not restored. However, RAP (0.001-10 μg/mL) was capable of diminishing directed migration to AT III (Figure5) and itself induced the chemotaxis of neutrophils (Figure 5, inset), suggesting cross-deactivation between AT III and RAP.
Impact of RAP on AT III effects in neutrophils.
In deactivation experiments, cells were pretreated for 20 minutes with AT III, RAP, or both concomitantly. Thereafter, IL-8–induced chemotaxis was tested (left). Chemotaxis to AT III was investigated after the treatment of cells with RAP at various concentrations (right). In addition, untreated neutrophils were allowed to migrate to various concentrations of RAP (inset). Migration time to nitrocellulose filters was 30 minutes. After fixing and staining of filters, migration depth was quantified microscopically. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01. ns, not significant; n = 6.
Impact of RAP on AT III effects in neutrophils.
In deactivation experiments, cells were pretreated for 20 minutes with AT III, RAP, or both concomitantly. Thereafter, IL-8–induced chemotaxis was tested (left). Chemotaxis to AT III was investigated after the treatment of cells with RAP at various concentrations (right). In addition, untreated neutrophils were allowed to migrate to various concentrations of RAP (inset). Migration time to nitrocellulose filters was 30 minutes. After fixing and staining of filters, migration depth was quantified microscopically. The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01. ns, not significant; n = 6.
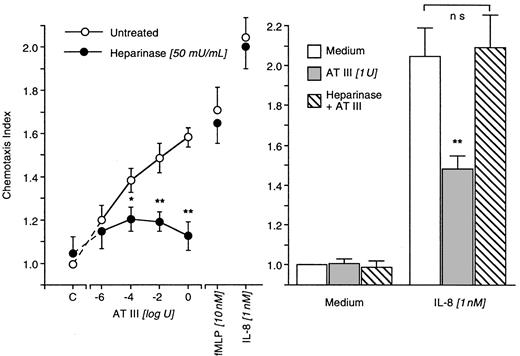
AT III effects after cleavage of pentasaccharide by heparinase
Neutrophil surface heparan sulfate proteoglycan pentasaccharide was cleaved by the treatment of cells with heparinase (50 mU/mL). This neither affected random migration nor chemotaxis of neutrophils to fMLP (10 nM) or IL-8 (1 nM). AT III (1 μU-1 U)–induced chemotaxis, however, was completely abolished. In addition, the deactivation properties of AT III disappeared and AT III was no longer able to diminish neutrophil chemotaxis to IL-8 (1 nM) (Figure6).
Impact of heparinase-induced cleavage on AT III effects in neutrophils.
Heparinase-treated (50 minutes) or -untreated neutrophils migrated for 30 minutes to AT III at various concentrations. Chemotaxis induced by fMLP or IL-8 served as positive controls (left). In deactivation experiments, heparinase-treated or -untreated neutrophils were exposed to AT III for 20 minutes; thereafter, migration to medium or IL-8 was run (right). The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01. ns, not significant; n = 8.
Impact of heparinase-induced cleavage on AT III effects in neutrophils.
Heparinase-treated (50 minutes) or -untreated neutrophils migrated for 30 minutes to AT III at various concentrations. Chemotaxis induced by fMLP or IL-8 served as positive controls (left). In deactivation experiments, heparinase-treated or -untreated neutrophils were exposed to AT III for 20 minutes; thereafter, migration to medium or IL-8 was run (right). The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils. Means of chemotaxis indices were compared by Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .001). *P < .05; **P < .01. ns, not significant; n = 8.
Signaling of AT III–induced neutrophil chemotaxis
When neutrophils were pretreated with intracellular enzyme blockers, the IL-8–induced chemotactic response was inhibited by GFX (500 nM), staurosporine (10 ng/mL), and IBMX (100 μM), whereas tyrphostin (10 ng/mL) and wortmannin (10 nM) lacked this effect. AT III (1 U)–induced neutrophil migration mimicked this signal transduction pattern. Random migration of cells remained unaffected by their pretreatment with enzyme blockers (Table4).
Antithrombin III–induced signaling mediating neutrophil chemotaxis
| Pretreatment . | Chemotaxis versus . | ||
|---|---|---|---|
| Medium . | AT III [1 U] . | IL-8 [1 nM] . | |
| Medium | 1.000 ± 0.00 | 1.491 ± 0.06 | 1.718 ± 0.16 |
| GFX [500 nM] | 1.054 ± 0.09 | 1.192 ± 0.084-150 | 1.387 ± 0.104-150 |
| Staurosporine [10 ng/mL] | 0.980 ± 0.12 | 1.053 ± 0.104-150 | 1.181 ± 0.164-150 |
| Tyrphostin [10 ng/mL] | 1.105 ± 0.08 | 1.354 ± 0.04 ns | 1.757 ± 0.11 ns |
| IBMX [100 μM] | 1.113 ± 0.10 | 1.124 ± 0.074-150 | 1.212 ± 0.044-150 |
| Wortmannin [10 nM] | 0.965 ± 0.09 | 1.349 ± 0.07 ns | 1.971 ± 0.17 ns |
| Pretreatment . | Chemotaxis versus . | ||
|---|---|---|---|
| Medium . | AT III [1 U] . | IL-8 [1 nM] . | |
| Medium | 1.000 ± 0.00 | 1.491 ± 0.06 | 1.718 ± 0.16 |
| GFX [500 nM] | 1.054 ± 0.09 | 1.192 ± 0.084-150 | 1.387 ± 0.104-150 |
| Staurosporine [10 ng/mL] | 0.980 ± 0.12 | 1.053 ± 0.104-150 | 1.181 ± 0.164-150 |
| Tyrphostin [10 ng/mL] | 1.105 ± 0.08 | 1.354 ± 0.04 ns | 1.757 ± 0.11 ns |
| IBMX [100 μM] | 1.113 ± 0.10 | 1.124 ± 0.074-150 | 1.212 ± 0.044-150 |
| Wortmannin [10 nM] | 0.965 ± 0.09 | 1.349 ± 0.07 ns | 1.971 ± 0.17 ns |
Mann-Whitney test after Kruskal-Wallis analysis of variance (P < .01); n = 6.
For abbreviations, see Table 2.
P < .05.
Discussion
Both AT III preparations, Kybernin P and the immune-adsorbed AT III of the concentrate, deactivated IL-8 and fMLP-induced neutrophil chemotaxis. A monoclonal antibody–column flow-through fraction had no comparable effect, thus excluding the effects of unidentified contaminations. Such inhibition of chemokine-triggered chemotaxis of neutrophils could also have occurred in the study by Ostrovsky et al,14 who showed that pretreatment and posttreatment in a feline model of mesentery ischemia–reperfusion with AT III reduced neutrophil rolling and adhesion to baseline levels. They explained the observed in vivo effect exclusively in terms of the thrombin-inhibiting properties of AT III.14 In our study, neutrophil chemotaxis to fMLP and IL-8 was diminished at physiological concentrations of purified AT III in the absence of thrombin because cells were isolated and experiments in the presence of the thrombin antagonist hirudin did not yield different results. In time-course experiments the AT III–induced process of chemotaxis deactivation occurred rapidly and the chemotactic effects of AT III and Kybernin P on neutrophils were confirmed in checkerboard analyses; this response pattern and the bell-shaped dose-response curve of neutrophil chemotaxis directly evoked by AT III clearly indicate a receptor-mediated process. Because interactions between the AT III molecule and a supposed receptor on neutrophils require binding sites on both, we further investigated this issue.
The molecule
AT III is a typical serpin with 432 residues, and it contains 9 helices and 3 β-sheets.22,23 The reactive site (thrombin binding) is located at Arg393-Ser394 in the COOH-terminal loop structure, whereas the heparin-binding site is part of the NH2-terminal region.24
Because TAT complexes completely failed to induce neutrophil migration and lacked deactivating IL-8–induced chemotaxis, one might assume that it is the thrombin-binding site that is mainly involved. This concept had to be rejected when elastase-digested AT III, in which absolutely no thrombin inhibitory action was left, completely mimicked the effects of the full functional AT III molecule, suggesting that conformational changes occurring in the course of complex formation23 25are responsible for the failure of TAT complexes to influence neutrophil function.
We could also demonstrate that neutrophil elastase, which plays an important role in the pathogenesis of acute inflammation,26 is not the driving force that makes AT III chemotactic given that the presence of an elastase inhibitor had no effect on neutrophil migration.
It may be the responsibility of the heparin-binding site to interact with cell surface receptors. The property of heparin binding is almost wholly owing to a small pentasaccharide fragment that binds to AT III, and the occurrence of a specific conformational change, together with the structural specificity of pentasaccharide, implies a well-defined binding site on the molecule.27
The receptor
In searching for a receptor that mediates both the deactivation of chemotaxis and the stimulation of directed migration, several experimental approaches were applied. Because of the initial results on AT III–induced deactivation of IL-8 chemotaxis, it was obvious that we should look for interactions with the 2 CXC receptors.28Surprisingly, we found inhibitory action of AT III–induced neutrophil chemotaxis by monoclonal antibodies to the IL-8 receptors, with CXCR1 mAb having a higher potency. Given that the antibodies themselves slightly induced cell migration, this effect might have resulted from weak stimulation of the IL-8 pathway and, therefore, might have mimicked the phenomenon described above. In subsequent binding assays, neither specific binding of AT III to IL-8 receptors nor AT III–induced receptor internalization29 could be shown.
Leukocyte common antigen (CD45) is a transmembrane protein tyrosine phosphatase expressed on all nucleated hematopoietic cells30 and leukocytes, the ligation of which has been reported to modulate IL-8 responsiveness.31Pretreatment of neutrophils with the anti-CD45 mAb Bra11 reduces cells surface expression of CXCR1 and CXCR2.32 This effect is completely tyrphostin reversible.32 We used Bra55, another anti-CD45 mAb that deactivates neutrophil chemotaxis without affecting receptor density,32 and found that the inhibition of AT III–induced directed migration by Bra55 was reversed by tyrphostin. Furthermore, the addition of tyrphostin abrogated AT III–induced deactivation of IL-8–triggered chemotaxis and thereby restored the migration of neutrophils to IL-8. In contrast, homologous deactivation of AT III chemotaxis was insensitive to the tyrosine kinase inhibitor. Insensitivity to tyrphostin was also observed for AT III–induced chemotaxis (see below). Thus, our results demonstrate that the so-called deactivating properties of AT III are related to the activation of CD45 PTPase-dependent, discrete signaling pathways that also interfere with IL-8 signaling, whereas its chemotactic action uses a distinct signal transduction that does not involve tyrosine kinases. Because an antibody to sialyl Lewis(x) did not disturb the interactions of AT III with neutrophils, any involvement of CD15 can be excluded, though structural homologies between the 2 may exist.
One candidate receptor protein for AT III on the neutrophil surface could be the serpin-enzyme complex receptor (SECR). Proteinase inhibitor (PI)–elastase complexes and the C-terminal peptide released from cleaved αPI have been reported to be chemotactic for neutrophils by interacting with the SECR,33 and binding can be competed for by several SECs, including TAT.34 TAT failed to mimic AT III–neutrophil interactions; hence, it appears unlikely that SECR is involved. Moreover, the C-terminal pentapeptide of AT III and other serpins are reported to bind SECR.35 Similar sequences were found in various bioactive peptides, such as bombesin and substance P; these peptides compete for SECR binding by SEC.36 Bombesin and carboxyterminal substance P are not chemotactic on neutrophils.37 Recently, low density lipoprotein (LDL) receptor–related protein (LRP) was found to be expressed on monocytes and on neutrophils.38 A defining characteristic of this receptor is that binding of all known ligands is blocked by RAP,39 but it failed to antagonize AT III–induced deactivation of neutrophil chemotaxis, indicating no involvement of LRP. Moreover, RAP itself was active in our experiments, probably, like AT III, through the ligation of another SEC binding entity, the proteoglycans. This concept would also be consistent with the deactivation of AT III–induced chemotaxis by RAP.
Syndecans are proteoglycans consisting of a core protein to which unbranched carbohydrate polymers (glycosaminoglycans) are covalently attached. All 4 known syndecans are transmembrane proteins with short but highly conserved C-terminal cytoplasmic domains.40 The ectodomain sequences show only limited amino acid sequence similarities, but it is notable that in heparan sulfate,O-sulfates may be found at C-3 of GlcNSO3-residues. Exactly this pentasaccharide41 is recognized by the heparin-binding site of AT III.27,42 Specific reagents, including heparinitase, nitrous acid, and heparinase, can degrade heparan sulfate. Heparinase is specific for N-sulfated disaccharides that contain IdoA,2S, and the susceptible linkages (GlcNSO3[α1-4]IdoA,2S) are located in the sulfated domains.43
Using the cleaving properties of heparinase, we treated neutrophils with the enzyme and found AT III–induced chemotaxis and deactivation completely abolished, whereas random migration and migration to fMLP and IL-8 remained intact. This clearly indicates a heparan sulfate proteoglycan–mediated action of AT III in neutrophils. Further, the results from signal transduction experiments may suggest involvement of syndecan-4. In contrast to other proteoglycans related to this family, the core protein of syndecan-4 can directly bind the catalytic domain of protein kinase C, and it activates this enzyme in the presence or absence of phospholipid mediators.44 45 This is consistent with the susceptibility of AT III–evoked chemotaxis to protein kinase C inhibitors (bisindolylmaleimide I, staurosporine) and a phosphodiesterase inhibitor (IBMX).
Conclusion
To summarize, our results revealed that AT III affects neutrophil functions by binding to heparan sulfate proteoglycans. Distinct signal transduction pathways are involved in the deactivation of IL-8–induced chemotaxis and in AT III–stimulated directed migration. As do CD45 ligation–related effects, syndecan-induced signaling can cross-react with IL-8–activated signal transduction, which, in turn, leads to deactivation.46 Neutrophil chemotaxis to AT III seems to be directly protein kinase C dependent. The overall finding is consistent with the anti-inflammatory effect of AT III administration in vivo. During circulation neutrophils are continuously exposed to AT III in plasma; therefore, this serpin may also protect neutrophils from premature activation under physiological conditions. Surprisingly, there are no reports of abnormalities in neutrophil function or recurrent infection in patients with congenital AT III deficiency. Additional studies are needed for clarifying the physiological role of AT III in leukocyte functions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christian J. Wiedermann, Department of Internal Medicine, Innsbruck University Hospital, Anichstrasse 35, A-6020 Innsbruck, Austria; e-mail: christian.wiedermann@uibk.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal