Abstract
Stem cell factor (SCF) or c-Kit ligand is a cytokine associated with the differentiation, survival, and activation of mast cells. Eosinophils have pleiotropic functions in several diseases and, together with mast cells, are key cells in allergy. Mast cell–eosinophil interactions can take place during the late and chronic phases of allergy. It was, therefore, investigated whether eosinophils can produce SCF and consequently influence mast cells. Human peripheral blood eosinophils variably expressed mRNA for the soluble and uncleaved forms of SCF (reverse transcription–polymerase chain reaction) and produced the 18.5-kd protein backbone of SCF (Western blot analysis). After overnight incubation in medium, eosinophils also produced SCF of higher molecular weight (42-45 kd) that might represent its glycosylated forms. Eosinophils expressed cytoplasmic SCF that colocalized with major basic protein (confocal laser microscopy). Freshly isolated eosinophils contained 8.9 ± 1.7 pg SCF/106 (mean ± SEM; enzyme-linked immunosorbent assay). Although overnight incubation of the eosinophils in either culture medium or in phorbol 12-myristate 13-acetate–calcium ionophore did not cause the secretion of SCF, the addition of chymase induced SCF release. In summary, it was demonstrated that human peripheral blood eosinophils are a source of SCF. These results may contribute to a better understanding of the interactions between eosinophils and mast cells in allergic inflammation.
Introduction
Stem cell factor (SCF) or c-Kit ligand is a cytokine produced by several cell types—including fibroblasts,1,2 bone marrow stromal cells,3endothelial cells,4,5 keratinocytes,6intestinal epithelial cells,7 Sertoli cells, and granulosa cells,8—and it is central to hematopoiesis.9,10 SCF is encoded by the Steel (Sl) locus in the mouse and is the ligand for the tyrosine kinase type receptor c-Kit.8 It exists in 2 different forms determined by alternative splicing: a longer one designated KL-1, a transmembrane protein of 248 amino acids that can be cleaved by proteolysis to release soluble SCF, and a shorter one designated KL-2, a transmembrane protein of 220 amino acids.2 It is clear that both the membrane-bound and the soluble forms of SCF have biologic activities, though they may be different on different target cells.11Cleavage can be carried out by serine proteases, and it has been found that the proteolytic cleavage machinery is activated by agents that increase the level of cytosolic calcium or that activate protein kinase C.12 Interestingly, Longley et al13showed that human mast cell chymase, a chymotrypsin-like protease specifically stored in cytoplasmic granules cleaves SCF at a novel site between 158-159 amino acids, in addition to the known cleavage site between 165-166 amino acids.13 Moreover, mast cells themselves, the key cells of allergic inflammation, have been found recently to produce, store, and release SCF.14-17Furthermore, human mast cell precursors and mature mast cells express the c-Kit receptor.18-20 Precursors differentiate and grow under the influence of SCF.18-20 SCF induces mature mast cell activation and can enhance IgE-dependent mast cell degranulation.21 It also induces mast cell adhesion to extracellular matrix proteins22 and their chemotaxis.23 Interestingly, eosinophils also express functional c-Kit receptors, and SCF binding induces VLA-4–mediated adhesion.24
We have been interested in mast cell–eosinophil interactions that take place in the late-phase reaction of allergy and in chronic allergic inflammatory diseases when the cells are in proximity within the tissues. We recently found that mast cell–derived tumor necrosis factor (TNF)-α enhances eosinophil survival, and that eosinophil basic preformed mediators cause histamine release from mast cells.25,26 Eosinophils have been shown to contain, preformed in their secondary granules, a number of pleiotropic cytokines, such as granulocyte macrophage–colony-stimulating factor,27 IL-6,28 IL-2,29RANTES,30 TNF-α,31 nerve growth factor,32 and IL-5.33 34 In this study we assessed whether eosinophils could be a source. We are reporting that eosinophils synthesize, store, and can release SCF.
Materials and methods
Isolation and purification of eosinophils and eosinophil cultures
Eosinophils were purified from the peripheral blood of mildly allergic patients (blood eosinophil levels, 5%-10%) as previously described.24 Written informed consent was obtained from all volunteers according to the guidelines established by the Hadassah-Hebrew University Human Experimentation Helsinki Committee. Briefly, venous blood (150 mL) was collected in heparinized syringes and left to sediment in 6% dextran (Sigma Chemicals, St Louis, MO). Leukocytes were centrifuged on Ficoll-Hypaque (density 1.077; Sigma Chemicals) for 25 minutes at 700g at 20°C. Neutrophils and lymphocytes were tagged in the granulocyte-enriched pellet with micromagnetic beads bound to anti-CD16 and anti-CD3 antibodies, respectively (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany). Eosinophils were purified by passing this cell suspension through a magnetic field and then were collected at a purity of 98% to 100% (Kimura staining) and at a viability of more than 98% (trypan blue staining).
Purified eosinophils were resuspended in 6-well plates (Corning, Corning, NY) or in 96-well plates (Nalge Nunc International, Roskilde, Denmark) in RPMI-1640 medium (Biological Industries, Beit Haemek, Israel) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 0.1 mM nonessential amino acids, and 5% heat-inactivated fetal calf serum (FCS; Biological Industries) (enriched medium). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% humidity for 4.5 hours and overnight. Eosinophil purity after overnight culture did not significantly change from that of the freshly isolated one.
HMC-1 and foreskin fibroblasts
The mast cell line HMC-1 was established from a patient with mast cell leukemia and was kindly donated by Dr J. H. Butterfield (Mayo Clinic, Rochester, MN). HMC-1 was cultured in Iscove medium (Biological Industries) supplemented with 10% FCS, Fe2+, 1.2 mM monothioglycerol, 100 U/mL penicillin, and 100 μg/mL streptomycin, and was maintained as described.35 Human foreskin fibroblasts were obtained from neonatal foreskins and cultured in supplemented Dulbecco modified Eagle medium as previously described.36
RNA isolation and reverse transcription–polymerase chain reaction amplification
Total RNA was extracted from 5 × 106 freshly isolated eosinophils or from eosinophils that were incubated overnight with enriched medium or from 5 × 106 human foreskin fibroblasts. RNA was extracted by using the commercial reagent Tri-Reagent (Sigma Chemicals) based on the acid guanidinium–thiocyanate RNA extraction technique.37First-strand cDNA synthesis reaction was catalyzed by Super Script II RNA–reverse transcriptase (Gibco BRL, Rockville, MD) and oligo(dT)12-18 primer, according to the manufacturer's instructions. The generated cDNA was amplified in a total volume of 25 μL using 2.5 U Taq DNA polymerase (Boehringer Mannheim, Mannheim, Germany), 0.2 mM dNTP mixture, 1 μM SCF primers, 10× polymerase chain reaction (PCR) buffer, and 10% glycerol as a specificity enhancement.
The primer sequences for SCF were 5′GTG GAT GAC CTT GTG GAG TG and 3′GG TTT TTA GGG GGA CCT CTG, generating a fragment of either 308 bp or 223 bp. This primer sequence was chosen to obtain the cDNA of the 2 SCF isoforms. SCF mRNA has been shown to be present in human foreskin fibroblasts.6 RNA of these fibroblasts was consequently used as a positive control. The thermocycler PTC-100 (MJ Research, Watertown, MA) was used for the PCR amplifications with the following settings: 40 cycles at 94°C for 30 seconds, 62°C for 1 minute, and 72°C for 1 minute. Before the first cycle, denaturation at 94°C for 3 minutes was performed. At the end of the cycles, a primer extension period of 10 minutes at 72°C was included. Primer sequences for G3PDH, used as a control to test the efficiency of cDNA synthesis, were 5′ ACC ACA GTC CAT GCC ATC ACT GCC and 3′ CAT GTG GGC CAT GAG GTC CAC CAC, generating a fragment of 468 bp. Amplified products were electrophoresed on 1.8% agarose gel stained with ethidium bromide and were photographed under ultraviolet light.
Western blot analysis
Cell lysates were prepared from 8 × 106 freshly isolated eosinophils or from eosinophils that were incubated for 4.5 hours and overnight with enriched medium. Lysis buffer (50 mM Tris, 25 mM KCl, 5 mM MgCl2, 1 mM EGTA, 1 mM Na3Vo4) containing a protease inhibitor cocktail (Sigma Chemicals) was added to cell pellets. This was followed by vortex mixing and sonication (six 10-second bursts at intervals of 10 seconds using a W-380 sonicator [Heat Systems Ultrasonics, Farmingdale, NY] at 50% duty cycles, output 5). Cell debris was removed from lysates by centrifugation (15 300g, 10 minutes). All the procedures were performed on ice or at 4°C. Samples were analyzed on 12.5% SDS-PAGE. Human recombinant SCF (75 ng/mL) (a kind gift from Dr K. Langley, Amgen, Thousand Oaks, CA) was run as a positive control.
The gel was electrotransferred (1 hour, 1 A) to nitrocellulose filter paper that was blocked in phosphate-buffered saline (PBS) containing 5% skimmed milk and 0.1% Brij (Sigma Chemicals), followed by incubation with goat anti-human SCF (1 μg/mL) (AB-255 NA antibodies; R&D System, Minneapolis, MN) overnight at 4°C. The filters were washed in PBS/Brij and incubated with secondary peroxidase-conjugated immunopure donkey anti-goat antibodies (1:5000) (Pierce, Rockford, IL) and then by incubation with the commercially available chemiluminescence system ECL detection method (Amersham, Pharmacia Biotec, Little Chalfont, Buckinghamshire, United Kingdom).
Confocal laser microscopy
Freshly isolated eosinophils (5 × 105/3 mL enriched medium containing 0.5% FCS) were added to 22-mm cover glasses (Deckglaser, Germany) precoated with plasma fibronectin (5 μg/mL in RPMI 1640 containing 0.5% FCS) in a 35-mm tissue culture dish (Falcon, Becton Dickinson, Oxford, United Kingdom) and incubated for 3 hours at 37°C. Control cells consisted of HMC-1 (4 × 105/3 mL) and foreskin fibroblasts (3.5 × 105/3 mL). Cells were then fixed with formalin (3.8% in PBS, 10 minutes), washed with PBS twice, and incubated with NH4Cl (50 mM in PBS, 1 minute). Cells were permeabilized by a 3-minute incubation with 0.2% Triton in PBS containing 1% BSA (Sigma Chemicals) and washed. Cells were then incubated for 3 minutes in 100% methanol followed by incubation at −20°C overnight in a blocking solution containing 5% horse serum in PBS (Biological Industries). Double labeling was performed by incubating the cells first with goat anti-human SCF (2 μg/mL in PBS) (R&D System) for 1 hour at 4°C. After washings (3×) with PBS, slides were incubated with CyTM5-conjugated donkey anti-goat IgG antibodies (1:200 in PBS) (Jackson Immunoresearch Laboratories, West Grove, PA) for 45 minutes at room temperature.
A second staining was performed by the addition of mouse anti-human c-Kit (2 μg/mL) (Pharmingen, San Diego, CA) or mouse anti-human major basic protein (MBP, 1:10; Monosan, Uden, The Netherlands). The detection of anti-c-Kit or anti-MBP antibody binding was performed by incubation of the slides for 30 minutes at 4°C, with fluorescein isothiocyanate FITC-conjugated goat anti-mouse IgG 1:60 (Alexis, Ancell, Bayport, MN). A drop of mounting solution (90% glycerol, 10% PBS, pH 8, 3% DABCO [Sigma Chemicals] 0.1% NaN3) was added to each slide before coverslips were added. Negative controls consisted of slides in which only the second antibody was added.
Slides were examined using a 63× objective under a Zeiss LSM 410 confocal laser scanning system attached to the Zeiss Axiovert 135 M inverted microscope with 63 × /1.2 C-Apochromat water immersion lens (Carl Zeiss, Thornwood, NY). The system was equipped with a 25-mW air-cooled argon laser (488-nm excitation line with 515-nm long pass barrier filter for the excitation of green fluorescence). Red fluorescence was excited with the 633-nm internal helium neon laser. Confocal images were converted to a TIF format and transferred to a Zeiss imaging workstation for pseudocolor representation. Brightness and contrast level were carried out using the Zeiss and Adobe Photoshop 3.0 programs (Adobe Systems, San Jose, CA).
ELISA assay
To determine SCF amounts in eosinophils and in their supernatants, freshly isolated eosinophils (2-8.4 × 106) were resuspended in 110 μL RPMI-1640. The cell suspension was frozen and kept at −80°C overnight. Thawed samples were sonicated by continuous sonication for 2 minutes in ice. The cell sonicate was centrifuged (15 300g, 12 minutes, 4°C) and the pellet was resuspended in 110 μL RPMI-1640 and kept at −80°C until assayed.
In some experiments eosinophils were cultured for 18 hours with enriched medium alone or with enriched medium containing 0.01 μM PMA (phorbol 12-myristate 13-acetate; Sigma Chemicals) and 0.01 μM calcium ionophore A23187 (Sigma Chemicals). At the end of the incubation, eosinophils were inspected for viability (trypan blue staining) and were found to be more than 99% viable. Supernatants were harvested, and cells were collected and sonicated as described above. In 2 experiments eosinophils from 2 different donors were incubated overnight with enriched medium alone or with enriched medium containing 0.1 μM human recombinant chymase (kindly donated by Dr R. Numerof, Axys Pharmaceuticals, San Francisco, CA), and samples were collected. Chymase activity was assessed by an enzymatic-colorimetric method.38
SCF was quantified by a commercial ELISA kit specific for human SCF (Quantikine; R&D Systems, Minneapolis, MN). The lowest limit of assay sensitivity was 9 pg/mL. Data were analyzed by the cubic curve fit, and results were expressed as picograms per milliliter.
Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis was performed with the paired Student t test. P< .05 was considered significant.
Results
Human peripheral blood eosinophils express mRNA for stem cell factor
To determine whether eosinophils can express mRNA for SCF, total RNA was extracted from freshly isolated eosinophils and from eosinophils cultured overnight with enriched medium. Reverse transcription (RT)-PCR for SCF was performed with specific primers whose flanks aligned from nucleotide 505 to 524 and from nucleotide 794 to 813 in the SCF mRNA sequence. As shown in Figure1, foreskin fibroblasts, known to constitutively produce SCF,6 expressed both mRNA isoforms consisting of 223 bp and 308 bp, respectively (according to the primers used) (Figure 1, FSK). Overnight incubated eosinophils from the patient displayed in this figure expressed the mRNA of both isoforms (Eos o.n.), whereas freshly isolated eosinophils did not (F. Eos). This pattern of mRNA expression was found in eosinophil preparations from 2 other patients. In addition, freshly isolated and cultured eosinophils from one patient were found to express only the shorter SCF isoform, and in 2 patients the 2 isoforms were present in both freshly isolated and cultured eosinophils.
Eosinophils express mRNA for SCF.
RNA was extracted from 5 × 106 fresh eosinophils (F. Eos) and eosinophils cultured overnight with enriched medium alone (Eos o.n.). As a positive control, total RNA from human foreskin fibroblasts (FSK) was used. As a negative control, a sample was obtained in the presence of water instead of reverse transcriptase (−RT). RNA was reverse-transcribed to cDNA and amplified with specific primers for SCF. The products were electrophoresed on a 1.8% agarose gel and then were stained with ethidium bromide. Molecular weight marker (D-15 marker NOVEX) was electrophoresed simultaneously with other PCR products (M).
Eosinophils express mRNA for SCF.
RNA was extracted from 5 × 106 fresh eosinophils (F. Eos) and eosinophils cultured overnight with enriched medium alone (Eos o.n.). As a positive control, total RNA from human foreskin fibroblasts (FSK) was used. As a negative control, a sample was obtained in the presence of water instead of reverse transcriptase (−RT). RNA was reverse-transcribed to cDNA and amplified with specific primers for SCF. The products were electrophoresed on a 1.8% agarose gel and then were stained with ethidium bromide. Molecular weight marker (D-15 marker NOVEX) was electrophoresed simultaneously with other PCR products (M).
Human peripheral blood eosinophils synthesize stem cell factor
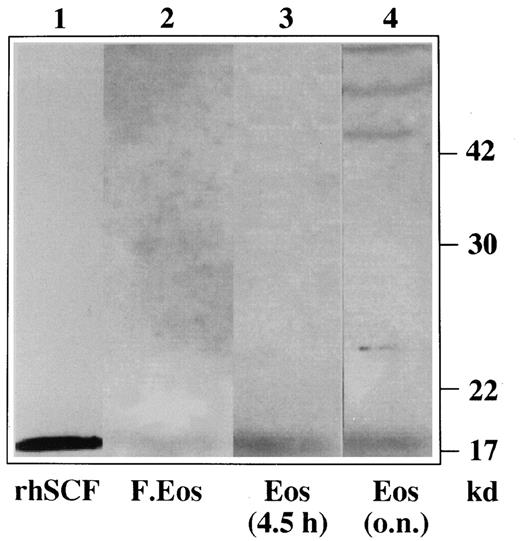
To analyze whether human peripheral blood eosinophils synthesize SCF, Western blot experiments with specific anti-SCF antibodies were carried out on eosinophil lysates obtained from freshly isolated and cultured eosinophils. As can be seen in Figure2, human peripheral blood eosinophils produce SCF. In fact, lysates from freshly isolated eosinophils expressed a weak band of 18.5 kd (lane 2), the backbone of the protein that corresponds to the positive control, the non-glycosylated backbone of SCF (lane 1).
Eosinophils synthesize SCF.
Lysates were obtained from freshly isolated eosinophils (lane 2) and from eosinophils cultured in enriched medium for 4.5 hours (lane 3) and overnight (lane 4). Recombinant SCF was loaded as a positive control (lane 1). The protein extracts were analyzed on a 12.5% SDS-PAGE gel and blotted. The blot was probed with goat anti-human SCF antibodies followed by incubation with peroxidase-conjugated donkey anti-goat antibodies. The detection was performed by enhanced chemiluminescence.
Eosinophils synthesize SCF.
Lysates were obtained from freshly isolated eosinophils (lane 2) and from eosinophils cultured in enriched medium for 4.5 hours (lane 3) and overnight (lane 4). Recombinant SCF was loaded as a positive control (lane 1). The protein extracts were analyzed on a 12.5% SDS-PAGE gel and blotted. The blot was probed with goat anti-human SCF antibodies followed by incubation with peroxidase-conjugated donkey anti-goat antibodies. The detection was performed by enhanced chemiluminescence.
The 18.5-kd band is more intense in eosinophils incubated with enriched medium for 4.5 hours (lane 3) and in eosinophils incubated overnight (lane 4). Eosinophils incubated overnight also expressed the mature proteins of 42 to 45 kd (lane 4) that might represent the translation and processing of SCF proteins progressively modified by glycosylation. Similar data were observed with eosinophil lysates obtained from the other patients.
Localization of SCF in human peripheral blood eosinophils
To localize the distribution of SCF present in the eosinophils, double-labeled staining laser confocal immunofluorescence microscopy was performed. As can be seen in Figure3, the eosinophils presented both a diffuse cytoplasmic staining and an additional cell membrane staining pattern for SCF (red fluorescence, Figure 3Ai). As expected, when the eosinophils were stained with anti-MBP antibodies, an intense cytoplasmic staining (green fluorescence, Figure 3Aii) was evident. Yellow regions that were present in combined fluorescent imaging, corresponding to overlapping green and red images, indicate that the 2 stained proteins, SCF and MBP, reside within the same intracellular compartments (Figure 3Aiii).
Eosinophils display cytoplasmic and cell membrane-staining patterns for SCF.
(A) Representative eosinophils with the Texas red channel corresponding to SCF (i), the FITC channel corresponding to MBP (ii), and combined fluorescence (iii). (B) Representative eosinophils with the Texas red channel corresponding to SCF (i), the FITC channel corresponding to c-Kit (ii), and combined fluorescence (iii). Isotype controls displayed minimal fluorescence background after subtraction of autofluorescence (Aiv and Biv). Original magnification, ×63. Control cells consisted of foreskin fibroblasts and HMC-1. Foreskin fibroblasts showed an intense membranal staining of SCF and were negative for c-Kit, whereas HMC-1 displayed intense membrane staining and weak, diffuse cytoplasmic staining for SCF that did not colocalize with the c-Kit receptors (not shown).
Eosinophils display cytoplasmic and cell membrane-staining patterns for SCF.
(A) Representative eosinophils with the Texas red channel corresponding to SCF (i), the FITC channel corresponding to MBP (ii), and combined fluorescence (iii). (B) Representative eosinophils with the Texas red channel corresponding to SCF (i), the FITC channel corresponding to c-Kit (ii), and combined fluorescence (iii). Isotype controls displayed minimal fluorescence background after subtraction of autofluorescence (Aiv and Biv). Original magnification, ×63. Control cells consisted of foreskin fibroblasts and HMC-1. Foreskin fibroblasts showed an intense membranal staining of SCF and were negative for c-Kit, whereas HMC-1 displayed intense membrane staining and weak, diffuse cytoplasmic staining for SCF that did not colocalize with the c-Kit receptors (not shown).
To further analyze the distribution of SCF in eosinophils, taking into account their surface expression of c-Kit receptors,34eosinophils were stained with anti-SCF antibodies (red fluorescence, Figure 3Bi) and with anti-c-Kit antibodies (green fluorescence, Figure3Bii). In Figure 3Biii the yellow regions that correspond to overlapping green and red images suggest that SCF and c-Kit only partially colocalize in some areas of the eosinophil membrane. Similar data were obtained in eosinophils isolated from normal, nonallergic patients (data not shown).
Human peripheral blood eosinophil stem cell factor content and release
Given that eosinophils were found to produce and store SCF, the next set of experiments were aimed at quantifying SCF and checking on whether it could also be released by the eosinophils. As depicted in Table 1, freshly isolated eosinophils obtained from 10 different patients contained 8.9 ± 1.7 pg/106 cells SCF (mean ± SEM). Incubation overnight in enriched medium did not significantly influence this value (9.6 ± 1.6 pg/106) and did not induce the appearance of SCF in the culture medium.
Stem cell factor content of human peripheral blood eosinophils
| Patient . | Eos no./sample . | SCF (pg/mL) in fresh Eos . | SCF (pg/106) in fresh Eos . | SCF (pg/106) in overnight cultured Eos . | Purity of Eos (%) . |
|---|---|---|---|---|---|
| 1 | 3.0.106 | 22.7 | 7.6 | 22.3 | 98.0 |
| 2 | 3.0.106 | 19.3 | 6.4 | 8.8 | 98.7 |
| 3 | 6.0.106 | 51.8 | 8.6 | 7.2 | 99.6 |
| 4 | 2.0.106 | 46.2 | 23.1 | 9.2 | 99.2 |
| 5 | 8.4.106 | 63.2 | 7.5 | 10.3 | 100.0 |
| 6 | 6.5.106 | 44.5 | 6.8 | 10.4 | 99.4 |
| 7 | 2.4.106 | 31.1 | 12.9 | 7.3 | 99.7 |
| 8 | 2.2.106 | 14.4 | 6.5 | 11.0 | 98.0 |
| 9 | 4.5.106 | 19.8 | 4.4 | 4.8 | 98.7 |
| 10 | 5.8.106 | 27.2 | 4.7 | 4.9 | 98.6 |
| Mean ± SEM | 4.4.106 ± 0.7 | 34.0 ± 5.2 | 8.9 ± 1.7 | 9.6 ± 1.6 | 98.9 ± 0.2 |
| Patient . | Eos no./sample . | SCF (pg/mL) in fresh Eos . | SCF (pg/106) in fresh Eos . | SCF (pg/106) in overnight cultured Eos . | Purity of Eos (%) . |
|---|---|---|---|---|---|
| 1 | 3.0.106 | 22.7 | 7.6 | 22.3 | 98.0 |
| 2 | 3.0.106 | 19.3 | 6.4 | 8.8 | 98.7 |
| 3 | 6.0.106 | 51.8 | 8.6 | 7.2 | 99.6 |
| 4 | 2.0.106 | 46.2 | 23.1 | 9.2 | 99.2 |
| 5 | 8.4.106 | 63.2 | 7.5 | 10.3 | 100.0 |
| 6 | 6.5.106 | 44.5 | 6.8 | 10.4 | 99.4 |
| 7 | 2.4.106 | 31.1 | 12.9 | 7.3 | 99.7 |
| 8 | 2.2.106 | 14.4 | 6.5 | 11.0 | 98.0 |
| 9 | 4.5.106 | 19.8 | 4.4 | 4.8 | 98.7 |
| 10 | 5.8.106 | 27.2 | 4.7 | 4.9 | 98.6 |
| Mean ± SEM | 4.4.106 ± 0.7 | 34.0 ± 5.2 | 8.9 ± 1.7 | 9.6 ± 1.6 | 98.9 ± 0.2 |
Peripheral blood eosinophils from 10 different patients were sonicated immediately after isolation (fresh Eos) or after overnight culture in enriched medium, and SCF content was evaluated with the use of a commercial ELISA kit.
Overnight activation of eosinophils with PMA and calcium ionophore did not induce measurable SCF release in the culture medium. However, the SCF content of these eosinophils was slightly less than that of eosinophils cultured in enriched medium alone (8.7 ± 0.9 pg/106 cells vs 11.3 ± 1.7 pg/106; n = 7), suggesting that some SCF might have been released. In one case in which 45 × 106 eosinophils were incubated with 0.1 μM chymase, 54.5 pg/mL SCF were detected in the supernatant (cell sonicate-associated SCF was 49.3 pg/mL). In the second case in which 26 × 106 eosinophils were incubated with chymase, 16.0 pg/mL SCF were released (cell sonicate-associated SCF was 237.0 pg/mL).
Discussion
In this study we have found that human peripheral blood eosinophils produce and can release SCF. This observation indicates a new function for eosinophils, both in hemopoiesis and in allergic disease. In fact SCF is a pleiotropic cytokine produced by several cell types, and it exerts activity on a variety of target cells.1,2 In allergy its importance relies on the fact that it is the main cytokine for the differentiation, maturation, and activation of the mast cells.21-23 We are interested in the reciprocal interactions between mast cells and eosinophils in allergic inflammation, and we have recently found that rat mast cells enhance eosinophil survival, cytokine production, and degranulation.25 Conversely, preformed eosinophil mediators can cause histamine release from rat mast cells.26 Eosinophils are a reservoir of basic mediators and of many preformed cytokines that can affect mast cell functions. Therefore, we investigated whether they could produce SCF.
First, we determined whether eosinophils can express mRNA for SCF. Messenger RNA for SCF exists in 2 isoforms. One is a full-length mRNA that encodes a 248-amino acid transmembrane protein that undergoes proteolytic cleavage to yield the soluble molecule. The other is the alternatively spliced SCF mRNA that lacks exon 6 and the proteolytic cleavage site and gives rise to a smaller 220-amino acid transmembrane form that produces soluble SCF less efficiently.1 2 We found that human peripheral blood eosinophils express mRNA for the 2 isoforms (223 and 308 bp) of SCF. However, variability in SCF mRNA expression exists—freshly isolated and cultured eosinophils from different patients differentially expressed either or both of the 2 isoforms. This might depend on their state of maturation, on the in vivo environment provided by different donors, or both. Similar variability of mRNA expression has been found for the mRNA of other eosinophil cytokines, after RT-PCR analysis.
For instance, though mRNA for GM-CSF, IL-6, and IL-8 has not usually been found in freshly isolated eosinophils but only detected after overnight culture, IL-4, IL-10, and TGF-β1 mRNA seem to be constitutively present.39 Although Zhang et al14 detected constitutive synthesis of SCF mRNA in mast cells isolated from human lung and skin by RT-PCR, interestingly Furitsu et al40 did not find it and Welker et al17 detected only a weak signal in unstimulated HMC-1. Welker et al17 also found that human skin-derived, cord blood-derived, and peripheral blood-derived mast cells and HMC-1 exhibited both SCF variants, though marked quantitative differences were observed among the various mast cell types.17 A time-dependent induction of both SCF forms was seen during culture and after stimulation.17 Studies of human bone marrow fibroblasts show that these cells express the 2 isoforms but predominantly contain mRNA for the larger form.3 It is interesting that the ratio, mRNA–transmembrane form of SCF/mRNA–soluble form of SCF, varies considerably in different tissues, ranging from 10:1 in the brain and 4:1 in the bone marrow to 0.4:1 in the testis.2 To date, mechanisms that control the tissue-specific and developmentally regulated production of SCF have not been well understood.11
Freshly isolated and cultured human peripheral blood eosinophils were also found to synthesize the protein backbone of SCF (18.5 kd) by Western blot analysis. Cultured eosinophils also expressed 42- to 47-kd proteins that might represent its glycosylated forms. The main data on the glycosylation of the mature forms were obtained from transfection experiments. Huang et al2 reported that in pulse-chase experiments with COS-1 cells transfected with the larger isoform of SCF, it was possible to detect specific protein products of 24, 35, 40, and 45 kd. When COS-1 cells were transfected with the shorter SCF isoform, they displayed specific protein products of 28 and 32 kd.2 In addition, Lu et al41 showed that COS-1 cells transfected with the larger SCF cDNA presented 3 glycosylated bands of 28, 35, and 40 kd. After complete deglycosylation, the molecular weight was 18 to 19 kd.
We then localized the distribution of SCF by double-labeled laser confocal immunofluorescence microscopy. SCF was found to colocalize with MBP, which typically resides in the eosinophil secondary granules. Other cytokines and chemokines such as IL-2, RANTES, GM-CSF, and IL-6 have been found by subcellular fractionation and confocal or electron microscopic analysis to reside in the eosinophil secretory granules.28,29,41,42 This observation suggests that these pre-stored molecules and, hence, SCF can be released together with MBP and other granule-associated mediators in a prompt fashion on eosinophil activation. Although incubation with PMA and calcium ionophore—potent stimuli for eosinophil degranulation43and SCF cleavage40—induced eosinophil peroxidase (EoP) release (data not shown), measurable SCF secretion from the eosinophils was not detected. Therefore, in spite of the colocalization, we cannot rule out a differential release of MBP or EoP and cytokines by eosinophils, as it has been described for eosinophil cationic protein and EoP.44 For example, the differential release of Th1-type cytokines (IL-2/IFN-γ) but not Th2 (IL-4, IL-5, IL-10) was detected in eosinophils stimulated by CD28 ligation.45 We also have shown in the past that nerve growth factor selectively induces the release of EoP, but not of IL-6, from eosinophils.32
In addition, in the confocal microscopy experiments, we could detect a small colocalization of SCF with c-Kit receptors on the eosinophil membrane.24 This slight membranal staining for SCF might be due to SCF produced by the eosinophils and transported to their membranes from the intracellular stores. Alternatively, the SCF in the patient serum can bind to the eosinophil c-Kit receptors before eosinophil isolation.
Freshly isolated human peripheral blood eosinophils contained preformed 8.9 + 1.7 pg SCF/106 cells, and their overnight incubation did not significantly change this value. In comparison, lysates of human lung mast cell were found to contain 59.3 pg SCF/106,16 whereas skin mast cell lysates contained 11 pg SCF/106.17 In one study, immunologically activated human lung mast cells did not show an enhancement in the secretion of SCF over baseline.14However, in other studies carried out in human lung and skin mast cells similarly activated, a rapid release of SCF has been detected.17 Interestingly, immunoreactive SCF detected in supernatants of challenged human lung mast cells rapidly declined, suggesting that the immunologic activation of these mast cells also releases enzymatic activity capable of degrading SCF or that prompt internalization of SCF bound to its cognate receptor c-Kit takes place.17 Indeed it has been found recently by Longley et al13 that chymase, the human-specific mast cell serine protease, cleaves SCF at a novel site (between amino acids 158 and 159). By incubating SCF with the supernatants of anti–IgE-activated human lung mast cells, de Paulis et al15 reconfirmed these data.
Therefore, eosinophils obtained from 2 patients were incubated with either chymase or culture medium overnight. Chymase treatment induced the release of SCF in both patients, possibly through the cleavage of SCF, as indicated by measurable amounts of SCF found in the respective supernatants.
These results point out a novel task for mast cell-derived chymase and an additional pathway for the interaction between mast cells and eosinophils. Even though SCF can be released by chymase, its effects can be produced by transmembrane and released-soluble forms. In preliminary experiments, the overnight incubation of HMC-1 with eosinophil sonicate resulted in a significant down-regulation of their c-Kit receptors, as detected by FACS analysis (81.2% ± 4.04% of control in untreated HMC-1 vs 36.4% ± 8.6% of control in HMC-1 incubated with eosinophil sonicate; n = 4). The down-regulation of the HMC-1 c-Kit receptor could be attributable to SCF, because it has been shown that SCF binding to c-Kit on HMC-1 can selectively down-regulate this receptor.46 However, eosinophil sonicates contain numerous protein and non-protein agonists that might also be able to down-regulate c-Kit.
Therefore, we speculate that eosinophils can affect the target cells either by soluble SCF, in the presence of chymase or other relevant proteolytic enzymes, or by the membrane-bound form. It is interesting that the soluble and transmembrane forms of SCF display somehow different effects and potency. For example, it has been shown that the expression of SCF in both isoforms by marrow microenvironmental cells may differentially influence the proliferation of erythroid and myeloid progenitor cells.47 The transmembrane SCF expressed by a fibroblast cell line was more efficient than the soluble one in supporting hematopoiesis.48
Other studies20 indicate that the transmembrane SCF expressed by fibroblasts is the responsible form for mast cell and fibroblast interactions. On the other hand, we have recently found that soluble SCF is necessary for cord blood-derived mast cell adherence to lung fibroblasts (Piliponsky AM, Levi-Schaffer F, unpublished data).
In summary, we have demonstrated that human peripheral blood eosinophils can produce, store, and release SCF. Eosinophils have c-Kit receptors that stimulate VLA-4–mediated cell adhesion to fibronectin and VCAM-1 and, therefore, contribute to their interactions with stromal SCF.34 Consequently, eosinophils have been viewed as target cells for SCF. In addition, our study shows that eosinophils through their SCF might contribute to hematopoiesis and, in allergic inflammation, to mast cell differentiation, maturation, activation, and adhesion, all processes in which SCF has a central function.
Acknowledgments
We thank Mark Tarshish for his excellent technical assistance with the confocal laser microscopy experiments, and we thank Madelyn Segev for her typing and editorial assistance.
Supported by a grant of the Aimwell Charitable Trust (United Kingdom). M.-L.H. is the recipient of a Golda Meir postdoctoral fellowship. F.L.-S. is affiliated with the David R. Bloom Center of Pharmacy at The Hebrew University of Jerusalem (Israel).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesca Levi-Schaffer, Department of Pharmacology, School of Pharmacy, Faculty of Medicine, The Hebrew University-Hadassah Medical School, PO Box 12065, Jerusalem 91120, Israel; e-mail: fls@cc.huji.ac.il.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal