Abstract
This study was aimed at defining the histogenesis of the pathologic spectrum of lymphoma arising in the context of human immunodeficiency virus (HIV) infection. Toward this aim, 87 AIDS-related non-Hodgkin lymphomas (AIDS-NHL) and 16 Hodgkin lymphomas arising in HIV+ patients (HIV-HL) were comparatively analyzed for the expression pattern of several B-cell histogenetic markers, including BCL-6 (expressed by centroblasts and centrocytes), MUM1/IRF4 (expressed by late centrocytes and post–germinal center [GC] B cells), and CD138/syn-1 (expressed by post-GC B cells). Expression of MUM1, BCL-6, and syn-1 segregated 3 major phenotypic patterns among AIDS-NHL and HIV-HL: (1) the BCL-6+/MUM1−/syn-1− pattern, selectively clustering with a large fraction of AIDS-Burkitt lymphoma (17 of 19) and of systemic AIDS–diffuse large cell lymphoma (12 of 16); (2) the BCL-6−/MUM1+/syn-1−pattern, associated with a fraction of AIDS-immunoblastic lymphoma (8 of 24); and (3) the BCL-6−/MUM1+/syn-1+ pattern, associated with systemic and primary central nervous system immunoblastic lymphoma (14 of 24) and with primary effusion lymphoma (10 of 10), plasmablastic lymphoma of the oral cavity (7 of 7), and HIV-HL (15 of 16). Analysis of nonneoplastic lymph nodes showed that the 3 phenotypic patterns detected in AIDS-NHL and HIV-HL correspond to distinct stages of physiologic B-cell development—centroblasts (BCL-6+/MUM1−/syn-1−), late GC/early post-GC B cells (BCL-6−/MUM1+/syn-1−), and post-GC B cells (BCL-6−/MUM1+/syn-1+). Expression of the Epstein-Barr virus-encoded latent membrane protein-1 clustered with the BCL-6−/MUM1+/syn-1+profile throughout the clinicopathologic spectrum of AIDS-NHL and HIV-HL. Overall, these results define novel histogenetic subsets of AIDS-NHL and HIV-HL and may provide novel tools for refining the diagnosis of these disorders.
Introduction
Acquired immunodeficiency syndrome–related non-Hodgkin lymphoma (AIDS-NHL) represents a significant source of morbidity and mortality among persons infected with human immunodeficiency virus (HIV).1,2 The pathologic spectrum of AIDS-NHL includes systemic AIDS-NHL, primary central nervous system lymphoma (AIDS-PCNSL), primary effusion lymphoma (AIDS-PEL), and plasmablastic lymphoma (AIDS-PBL) of the oral cavity.1-7Systemic AIDS-NHL is histologically classified into AIDS-related Burkitt lymphoma (AIDS-BL), AIDS-related diffuse large cell lymphoma (AIDS-DLCL), and AIDS-related immunoblastic lymphoma (AIDS-IBL).4,5 AIDS-PCNSL is classified into AIDS-DLCL and AIDS-IBL.2,4,5,8 Hodgkin lymphoma (HL) has also been reported in HIV-infected patients, though this disease does not confer a diagnosis of AIDS.6,7 9
In recent times, the field of B-cell lymphoma histogenesis has progressed rapidly owing to the increasing availability of well-defined histogenetic markers. Genotypic markers of B-cell lymphoma histogenesis include mutations of immunoglobulin and BCL-6 genes somatically acquired at the time of B-cell transit throughout the germinal center (GC).10-13 Phenotypic markers are represented by expression of the BCL-6 protein, which is restricted to GC B cells, and, at least in the context of immunodeficiency-related lymphomas, expression of CD138/syndecan-1 (syn-1), a proteoglycan clustering with late stages of B-cell maturation.1 12-16
Recently, MUM1/IRF4 (for multiple myeloma-1/interferon regulatory factor-4) has been added to the panel of phenotypic markers available for the characterization of B-cell lymphoma histogenesis.12 MUM1 was discovered because of its involvement in the t(6;14)(p25;q32) translocation of multiple myeloma, which causes the juxtaposition of the MUM1 gene, mapping at 6p25, to the IgH locus on 14q32.17 MUM1 is a lymphocyte-specific member of the interferon regulatory factor (IRF) family of transcription factors, also known as ICSAT (for interferon consensus sequence binding protein for activated T cells) and Pip (for PU.1 interaction partner).18 Recent studies have shown that MUM1 expression may denote the final step of intra-GC B-cell differentiation and subsequent steps of B-cell maturation toward plasma cells.18-21 To refine the histogenesis of AIDS-NHL and HIV-HL, we compared the expression pattern of MUM1, BCL-6, and syn-1 protein throughout the pathologic spectrum of these lymphomas.
Patients, materials, and methods
Neoplastic samples
This study was conducted on 87 cases of AIDS-NHL and 16 cases of HIV-HL. The AIDS-NHL cases, all of B-cell origin, included 47 cases of systemic AIDS-NHL, 22 with AIDS-PCNSL, 11 with AIDS-PEL, and 7 with AIDS-PBL (Tables1-3). Reed-Sternberg cells of 7 cases of HIV-HL (3 mixed cellularity, 4 lymphocyte depletion) expressed a B-cell phenotype, whereas Reed-Sternberg cells of 9 cases of HIV-HL (2 nodular sclerosis, 3 mixed cellularity, 4 lymphocyte depletion) failed to express B-cell or T-cell antigens (Table 4).
Expression of MUM1, BCL-6, CD138/syn-1, and CD10 andBCL-6 gene mutations in systemic AIDS-related non-Hodgkin lymphomas
| Case . | Diagnosis . | Immunohistochemistry . | BCL-6 (mut) . | EBV . | ||||
|---|---|---|---|---|---|---|---|---|
| MUM1* . | BCL-6* . | Syn-1* . | CD10* . | EBER . | LMP1* . | |||
| 1 | BL | − | > 75 | − | > 75 | + | − | − |
| 2 | BL | − | 50-75 | − | > 75 | − | + | − |
| 3 | BL | − | < 10 | − | > 75 | nd | − | − |
| 4 | BL | − | < 10 | − | > 75 | nd | + | − |
| 5 | BL | − | > 75 | − | > 75 | nd | + | − |
| 6 | BL | − | 10-25 | − | > 75 | + | + | − |
| 7 | BL | − | 10-25 | − | > 75 | + | − | − |
| 8 | BL | − | > 75 | − | > 75 | + | + | − |
| 9 | BL | − | > 75 | − | > 75 | − | + | − |
| 10 | BL | − | > 75 | − | − | + | − | − |
| 11 | BL | − | > 75 | − | > 75 | − | − | − |
| 12 | BL | − | 50-75 | − | > 75 | + | − | − |
| 13 | BL | − | > 75 | − | > 75 | − | + | − |
| 14 | BL | − | > 75 | − | > 75 | + | − | − |
| 15 | BL | − | > 75 | − | > 75 | + | − | − |
| 16 | BL | − | > 75 | − | > 75 | + | + | − |
| 17 | BL | − | 50-75 | − | > 75 | nd | + | − |
| 18 | BL | +occ. | > 75 | − | > 75 | nd | + | − |
| 19 | BL | +occ. | 25-50 | − | nd | nd | − | − |
| 20 | DLCL | − | − | − | − | nd | − | − |
| 21 | DLCL | − | > 75 | − | > 75 | nd | + | − |
| 22 | DLCL | − | > 75 | − | − | nd | − | − |
| 23 | DLCL | − | 10-25 | − | − | + | − | − |
| 24 | DLCL | − | 50-75 | − | − | + | − | − |
| 25 | DLCL | − | > 75 | − | − | + | − | − |
| 26 | DLCL | − | > 75 | − | > 75 | nd | − | − |
| 27 | DLCL | − | 25-50 | − | − | nd | − | − |
| 28 | DLCL | − | 10-25 | − | − | nd | − | − |
| 29 | DLCL | > 75 | < 10 | − | − | nd | − | − |
| 30 | DLCL | 50-75 | 10-25 | − | − | nd | − | − |
| 31 | DLCL | − | 10-25 | − | − | + | + | − |
| 32 | DLCL | − | 50-75 | − | − | − | − | − |
| 33 | DLCL | − | 50-75 | − | > 75 | nd | − | − |
| 34 | DLCL | − | > 75 | − | − | nd | − | nd |
| 35 | DLCL | > 75 | − | − | nd | nd | + | < 10 |
| 36 | IBL | > 75 | − | > 75 | − | nd | − | − |
| 37 | IBL | < 10 | − | < 10 | − | + | + | < 10 |
| 38 | IBL | > 75 | − | 50-75 | − | − | + | 10-25 |
| 39 | IBL | 50-75 | − | − | − | nd | − | − |
| 40 | IBL | > 75 | − | < 10 | − | nd | + | +occ |
| 41 | IBL | 50-75 | − | < 10 | − | nd | + | 25-50 |
| 42 | IBL | > 75 | − | − | − | nd | + | − |
| 43 | IBL | 50-75 | − | < 10 | − | nd | + | 25-50 |
| 44 | IBL | 50-75 | − | − | − | nd | + | 10-25 |
| 45 | IBL | 25-50 | − | − | − | nd | + | − |
| 46 | IBL | 25-50 | − | 25-50 | > 75 | nd | + | − |
| 47 | IBL | 10-25 | − | − | − | nd | + | − |
| Case . | Diagnosis . | Immunohistochemistry . | BCL-6 (mut) . | EBV . | ||||
|---|---|---|---|---|---|---|---|---|
| MUM1* . | BCL-6* . | Syn-1* . | CD10* . | EBER . | LMP1* . | |||
| 1 | BL | − | > 75 | − | > 75 | + | − | − |
| 2 | BL | − | 50-75 | − | > 75 | − | + | − |
| 3 | BL | − | < 10 | − | > 75 | nd | − | − |
| 4 | BL | − | < 10 | − | > 75 | nd | + | − |
| 5 | BL | − | > 75 | − | > 75 | nd | + | − |
| 6 | BL | − | 10-25 | − | > 75 | + | + | − |
| 7 | BL | − | 10-25 | − | > 75 | + | − | − |
| 8 | BL | − | > 75 | − | > 75 | + | + | − |
| 9 | BL | − | > 75 | − | > 75 | − | + | − |
| 10 | BL | − | > 75 | − | − | + | − | − |
| 11 | BL | − | > 75 | − | > 75 | − | − | − |
| 12 | BL | − | 50-75 | − | > 75 | + | − | − |
| 13 | BL | − | > 75 | − | > 75 | − | + | − |
| 14 | BL | − | > 75 | − | > 75 | + | − | − |
| 15 | BL | − | > 75 | − | > 75 | + | − | − |
| 16 | BL | − | > 75 | − | > 75 | + | + | − |
| 17 | BL | − | 50-75 | − | > 75 | nd | + | − |
| 18 | BL | +occ. | > 75 | − | > 75 | nd | + | − |
| 19 | BL | +occ. | 25-50 | − | nd | nd | − | − |
| 20 | DLCL | − | − | − | − | nd | − | − |
| 21 | DLCL | − | > 75 | − | > 75 | nd | + | − |
| 22 | DLCL | − | > 75 | − | − | nd | − | − |
| 23 | DLCL | − | 10-25 | − | − | + | − | − |
| 24 | DLCL | − | 50-75 | − | − | + | − | − |
| 25 | DLCL | − | > 75 | − | − | + | − | − |
| 26 | DLCL | − | > 75 | − | > 75 | nd | − | − |
| 27 | DLCL | − | 25-50 | − | − | nd | − | − |
| 28 | DLCL | − | 10-25 | − | − | nd | − | − |
| 29 | DLCL | > 75 | < 10 | − | − | nd | − | − |
| 30 | DLCL | 50-75 | 10-25 | − | − | nd | − | − |
| 31 | DLCL | − | 10-25 | − | − | + | + | − |
| 32 | DLCL | − | 50-75 | − | − | − | − | − |
| 33 | DLCL | − | 50-75 | − | > 75 | nd | − | − |
| 34 | DLCL | − | > 75 | − | − | nd | − | nd |
| 35 | DLCL | > 75 | − | − | nd | nd | + | < 10 |
| 36 | IBL | > 75 | − | > 75 | − | nd | − | − |
| 37 | IBL | < 10 | − | < 10 | − | + | + | < 10 |
| 38 | IBL | > 75 | − | 50-75 | − | − | + | 10-25 |
| 39 | IBL | 50-75 | − | − | − | nd | − | − |
| 40 | IBL | > 75 | − | < 10 | − | nd | + | +occ |
| 41 | IBL | 50-75 | − | < 10 | − | nd | + | 25-50 |
| 42 | IBL | > 75 | − | − | − | nd | + | − |
| 43 | IBL | 50-75 | − | < 10 | − | nd | + | 25-50 |
| 44 | IBL | 50-75 | − | − | − | nd | + | 10-25 |
| 45 | IBL | 25-50 | − | − | − | nd | + | − |
| 46 | IBL | 25-50 | − | 25-50 | > 75 | nd | + | − |
| 47 | IBL | 10-25 | − | − | − | nd | + | − |
EBV indicates Epstein-Barr virus; mut, mutation; LMP1, latent membrane protein 1; BL, Burkitt lymphoma; −, negative; +, positive; nd, not done; occ, occasional positive cells; DLCL, diffuse large cell lymphoma; IBL, immunoblastic lymphoma.
Percentages of MUM1, BCL-6, syn-1, CD10, and LMP1+neoplastic cells were assigned to one of the following categories: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
Systemic AIDS-NHL was classified according to the revised European-American classification of lymphoid neoplasms.22All AIDS-PCNSL cases were classified as DLCL or IBL. AIDS-PCNSL cases contained a mixture of large noncleaved cells and large cells; immunoblastic plasmacytoid were classified separately as DLCL/IBL.23 AIDS-PEL, which morphologically bridges immunoblastic and anaplastic features,24-26 was classified on the basis of the peculiar clinicopathologic and virologic (eg, Kaposi sarcoma-associated herpesvirus [KSHV] positivity) characteristics of the lymphoma.24,25 AIDS-PBL was classified in accordance to the morphologic (plasmablastic appearance), immunophenotypic (expression of plasma cell–associated markers VS38c and syn-1 in the absence of common B-cell–associated surface antigens), and clinical features (presentation in the oral cavity and jaw) of the disease.27 The CD30+, CD45−, CD15+, EMA− phenotypic profile was required for the diagnosis of classic HL.22The Rye modification of the Lukes and Butler28-30classification was used to classify the histologic subtypes of classic HL.
Tissues were fixed in Bouin solution or neutral buffered formalin. In most cases, a portion of unfixed tissue was snap-frozen in liquid nitrogen and stored at −80°C. Cases of AIDS-PEL were collected under sterile conditions during standard diagnostic procedures and treated according to a standard regimen used at the Division of Pathology of the Centro di Riferimento Oncologico.25
Nonneoplastic samples
Nonneoplastic lymph node and tonsil samples from 10 HIV-infected cases of persistent generalized lymphadenopathy (PGL) were also included in the study. The histopathologic pattern of lymph nodes and tonsils was predominantly represented by hyperplastic changes of the lymphoid follicles.
Immunohistochemical studies
Immunohistochemistry was performed by the avidin-biotin-peroxidase complex or alkaline phosphatase antialkaline phosphatase (APAAP) method.31 32
The expression of MUM1 was investigated with an affinity-purified polyclonal goat antibody (ICSAT/M-17) specific for the MUM1 protein17 (Santa Cruz Biotechnology, Santa Cruz, CA). The M-17 antibody reacts with MUM1 of mouse, rat, and human origins, though it does not cross-react with other members of the IRF family proteins. Syn-1 expression was assessed using the B-B4 monoclonal antibody (mAb)33 (Serotec, Oxford, United Kingdom). The BCL-6 protein was detected by the PG-B6 mAb (Dakopatts A/S, Glostrup, Denmark).16 CD10 staining, detected by the 56C6 mAb (Novocastra Laboratories, Newcastle upon Tyne, United Kingdom), was also studied in cases of systemic AIDS-NHL and AIDS-PCNSL.
All antigens were tested on paraffin-embedded tissue sections or cytospin preparations. For MUM1, BCL-6, and CD10 assessment, paraffin-embedded sections were treated in a microwave oven at 250 W for 30 minutes in EGTA solution (1 mM, pH 8) (for MUM1 and BCL-6) or citrate buffer (pH 6) (for CD10). Immunostaining for MUM1, BCL-6, and CD10 was performed on an automated immunostainer (Nexes; Ventana Medical Systems, Tucson, AZ) according to a modified version of the manufacturer's protocols. Immunostaining for syn-1 was performed by using the APAAP method.32 The percentage of antigen-positive neoplastic cells was assigned to one of the following categories: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and more than 75%. Only definite and unambiguous staining on unequivocal malignant cells was accepted as positive. Negative control experiments were performed by pre-absorbing the anti-MUM1 antibody with a 5-fold weight excess of blocking peptide (Santa Cruz Biotechnology) overnight at 4°C and then carrying out immunostaining as described above. None of the negative control sections was immunostained. All the antibodies were also applied to frozen tissues from a representative subset of cases of AIDS-NHL for control purposes.
In selected cases, the reactivity pattern of the ICSAT/M-17 polyclonal antibody was compared with that of MUM1p,20 a mAb raised against the human MUM1/IRF4 protein (kindly provided by Prof B. Falini, Institute of Hematology and Internal Medicine, University of Perugia, Italy). The reactivity pattern of each antibody recognizing MUM1/IRF4 was generally superimposable.
2-color staining
Multiple color immunohistochemical stainings were performed to detect MUM1 plus BCL-6 in selected AIDS-NHL and PGL cases and to detect MUM1 plus syn-1 in PGL cases. Formalin-fixed, paraffin-embedded tissue sections were first immunostained with anti–BCL-6 or anti–syn-1 antibody by the APAAP method using naphthol AS-MX phosphate along with Fast Red (for BCL-6) and Fast Blue BB salt (for syn-1) for the development of alkaline phosphatase; subsequently, sections were treated twice for 5 minutes in citrate buffer (pH 6) in a microwave oven to denature bound antibody molecules and to inactivate the alkaline phosphatase. Then, sections were immunostained with anti-MUM1 by using a DAB or a Fast Red detection kit (Ventana Medical Systems).
Analysis of viral infection
All cases included in this study were subjected to determination of tumor infection by Epstein-Barr virus (EBV) and KSHV.25,34 35 In the case of EBER+ cases, immunostaining for LMP1 was performed with an LMP1-specific antibody (Dakopatts A/S, Glostrup, Denmark), as described above. The percentage of LMP1+ neoplastic cells was assigned to one of the following categories: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and more than 75%.
Genetic studies of BCL-6
The presence of BCL-6 mutations was tested in a 740-bp fragment containing 95% or more mutations detected in B-cell NHL of the immunocompetent host and AIDS-NHL.36
Results
Expression of MUM1, BCL-6, and syn-1 in nontransformed lymphoid tissue
A panel of 10 PGL cases was used to define the expression pattern of MUM1, BCL-6, and syn-1 in nonneoplastic lymph nodes and tonsils of HIV-infected cases. Two-color staining and serial sections were also used to compare the immunoreactivity of these antibodies (Figure1). Expression of BCL-6 selectively clustered with centroblasts and centrocytes within the follicular GC, whereas B cells of the mantle and paracortical zones scored negative (Figure 1A). Conversely, the expression of syn-1 clustered with plasma cells and was absent in all other cell populations (Figure 1 A-B). Syn-1–positive plasma cells were localized in the medullary cords, in the interfollicular areas, and, in variable proportion, also in follicular GC and their surrounding mantle zones.
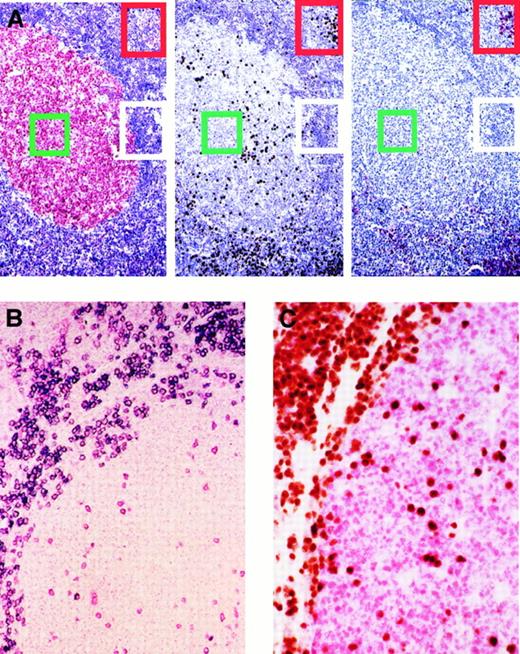
Expression of BCL-6, MUM1, and CD138/syn-1 in reactive B cells identifies 3 major phenotypic patterns that correspond to physiologic stages of B-cell development.
A subset of germinal center B cells express MUM1 and are negative for CD138/syn-1 and BCL-6. (A) Serial sections from hyperplastic lymph node from an HIV-infected patient with persistent generalized lymphadenopathy (PGL). (Left) Within a follicle numerous germinal center (GC) cells (centroblasts and centrocytes) exhibit nuclear staining (red) for BCL-6, whereas B cells of the mantle and perifollicular areas score negative. (Center) In the same follicle, expression of MUM1 is restricted to a subset of cells located in the GC. Conversely, most MUM1+ cells are located in the perifollicular and interfollicular areas; brown staining is nuclear. (Right) In the same field, large cells with plasma cell morphology show strong cytoplasmic and membrane staining (red) with the anti–syn-1 mAb. They are present within the perifollicular and interfollicular areas in which MUM1+ cells are more numerous (compare figure on the right for syn-1 and figure in the center for MUM1). Conversely, syn-1 is absent in intrafollicular or extrafollicular cell populations other than plasma cells (see also panel B). Thus, the coordinated expression of BCL-6, MUM1, and syn-1 in reactive B cells shows 3 major phenotypic patterns corresponding to physiologic stages of B-cell differentiation—(1) centroblasts (the BCL-6+/MUM1−/syn-1− profile, identified by green squares; (2) late GC/early post-GC B cells (the BCL-6−/MUM1+/syn-1− profile, identified by white rectangles); (3) post-GC B cells (the BCL-6−/MUM1+/syn-1+ profile, identified by red rectangles). Paraffin-embedded tissue sections, APAAP (left and right), immunoperoxidase (center), hematoxylin counterstain. Original magnification, × 250. (B) With the exception of plasma cells, MUM1+ cells in the GC and in the perifollicular areas are negative for CD138/syn-1. Hyperplastic lymph node from an HIV-infected patient with PGL. Two-color staining. The figure shows a follicle with a large GC and a thin mantle zone. Numerous perifollicular cells and some scattered GC cells exhibit nuclear staining (red) for MUM1. Moreover, in the same field, a fraction of large cells exhibiting plasma cell morphology coexpresses syn-1 with a strong cytoplasmic and membrane staining (blue). Cells coexpressing MUM1 and syn-1 are numerous within the perifollicular zone but are rare within the GC. Paraffin-embedded tissue section, no counterstain. Original magnification, × 250. (C) Expression of MUM1 by GC B cells is mutually exclusive with the expression of BCL-6 by the same cell. Reactive tonsil from an HIV-infected patient with persistent generalized lymphadenopathy with 2-color staining. Within a follicle, numerous GC cells exhibit nuclear staining (red) for BCL-6. In the same GC, several cells show nuclear staining (brown) with the anti-MUM1 antibody. MUM1+ cells are also present around the GC. No coexpression of BCL-6 or MUM1 markers by the same GC cell is detectable. Paraffin-embedded tissue section, no counterstain. Original magnification, × 250.
Expression of BCL-6, MUM1, and CD138/syn-1 in reactive B cells identifies 3 major phenotypic patterns that correspond to physiologic stages of B-cell development.
A subset of germinal center B cells express MUM1 and are negative for CD138/syn-1 and BCL-6. (A) Serial sections from hyperplastic lymph node from an HIV-infected patient with persistent generalized lymphadenopathy (PGL). (Left) Within a follicle numerous germinal center (GC) cells (centroblasts and centrocytes) exhibit nuclear staining (red) for BCL-6, whereas B cells of the mantle and perifollicular areas score negative. (Center) In the same follicle, expression of MUM1 is restricted to a subset of cells located in the GC. Conversely, most MUM1+ cells are located in the perifollicular and interfollicular areas; brown staining is nuclear. (Right) In the same field, large cells with plasma cell morphology show strong cytoplasmic and membrane staining (red) with the anti–syn-1 mAb. They are present within the perifollicular and interfollicular areas in which MUM1+ cells are more numerous (compare figure on the right for syn-1 and figure in the center for MUM1). Conversely, syn-1 is absent in intrafollicular or extrafollicular cell populations other than plasma cells (see also panel B). Thus, the coordinated expression of BCL-6, MUM1, and syn-1 in reactive B cells shows 3 major phenotypic patterns corresponding to physiologic stages of B-cell differentiation—(1) centroblasts (the BCL-6+/MUM1−/syn-1− profile, identified by green squares; (2) late GC/early post-GC B cells (the BCL-6−/MUM1+/syn-1− profile, identified by white rectangles); (3) post-GC B cells (the BCL-6−/MUM1+/syn-1+ profile, identified by red rectangles). Paraffin-embedded tissue sections, APAAP (left and right), immunoperoxidase (center), hematoxylin counterstain. Original magnification, × 250. (B) With the exception of plasma cells, MUM1+ cells in the GC and in the perifollicular areas are negative for CD138/syn-1. Hyperplastic lymph node from an HIV-infected patient with PGL. Two-color staining. The figure shows a follicle with a large GC and a thin mantle zone. Numerous perifollicular cells and some scattered GC cells exhibit nuclear staining (red) for MUM1. Moreover, in the same field, a fraction of large cells exhibiting plasma cell morphology coexpresses syn-1 with a strong cytoplasmic and membrane staining (blue). Cells coexpressing MUM1 and syn-1 are numerous within the perifollicular zone but are rare within the GC. Paraffin-embedded tissue section, no counterstain. Original magnification, × 250. (C) Expression of MUM1 by GC B cells is mutually exclusive with the expression of BCL-6 by the same cell. Reactive tonsil from an HIV-infected patient with persistent generalized lymphadenopathy with 2-color staining. Within a follicle, numerous GC cells exhibit nuclear staining (red) for BCL-6. In the same GC, several cells show nuclear staining (brown) with the anti-MUM1 antibody. MUM1+ cells are also present around the GC. No coexpression of BCL-6 or MUM1 markers by the same GC cell is detectable. Paraffin-embedded tissue section, no counterstain. Original magnification, × 250.
In all cases tested, reactivity for MUM1 was detectable in a fraction of GC cells and in all plasma cells (Figure 1A-C). Conversely, lymphoid cells of the mantle zone were usually MUM1−. Paracortical zones were mostly MUM1−, with the exception of some small lymphoid cells and rare isolated large cells (with blastic morphology) that were mainly represented by B cells. MUM1+ GC cells were preferentially localized in the light zone of the GC, exhibited moderate to strong nuclear staining, and displayed a small cleaved cell–centrocyte morphology. In these cells a weaker labeling of cytoplasm was also detectable. Expression of MUM1 by GC B cells was mutually exclusive with expression of BCL-6 by the same cell (Figure1C). In addition, MUM1+ GC cells scored consistently negative for syn-1 (Figure 1A). Plasma cells that scored positive for MUM1 expression were consistently present in the medullary cords and in the interfollicular areas. A small number of MUM1+ plasma cells was also found in follicular GCs (Figure 1B) and their surrounding mantle zones (Figure 1A). No reactivity for MUM1 in other cell populations was observed.
Systemic AIDS-NHL
The panel of 47 systemic AIDS-NHL cases included 19 AIDS-BL, 16 AIDS-DLCL, and 12 AIDS-IBL (Table 1). As expected,2 5infection of the tumor clone by EBV was detected in 10 of 19 cases of AIDS-BL, 3 of 16 cases of AIDS-DLCL, and 10 of 12 cases of AIDS-IBL (Table 1). All cases were devoid of KSHV infection (not shown).
Results of the expression pattern of MUM1, BCL-6, syn-1, LMP1, and CD10 are reported in Table 1. Representative examples are shown in Figure2. Expression of MUM1 was detected in 17 of 47 (36%) cases of systemic AIDS-NHL and clustered with AIDS-IBL (12 of 12 cases, 100%) (Figure 2A), whereas it was restricted to 3 of 16 (19%) cases of AIDS-DLCL (Figure 2B). Occasional MUM1+tumor cells could be detected in 2 of 19 (11%) cases of AIDS-BL (Table1). Expression of BCL-6 staining was detected in 33 of 47 (70%) cases of systemic AIDS-NHL (Table 1), clustered with AIDS-BL (19 of 19 cases, 100%) (Figure 2C) and AIDS-DLCL (14 of 16 cases, 87%), whereas it was consistently negative in cases of AIDS-IBL (Table 1). As previously observed,1 the expression of BCL-6 protein among cases of systemic AIDS-NHL occurred both in the presence and the absence of mutations of the BCL-6 gene (Table 1). Expression of syn-1 was detected in 7 of 47 (15%) cases of systemic AIDS-NHL (Table 1), selectively associated with AIDS-IBL (7 of 12 cases, 58%), and consistently negative in cases of AIDS-BL and AIDS-DLCL (Table 1). Expression of CD10 occurred in 21 of 45 (47%) cases of systemic AIDS-NHL. All but one case of CD10+ systemic AIDS-NHL (20 of 21 cases, 95%) expressed BCL-6 and stained negative for both MUM1 and syn-1. Expression of CD10 clustered with AIDS-BL (17 of 18 cases, 94%), whereas it was restricted to sporadic cases of AIDS-DLCL (3 of 15 cases, 20%) and was negative in all but one case of IBL.
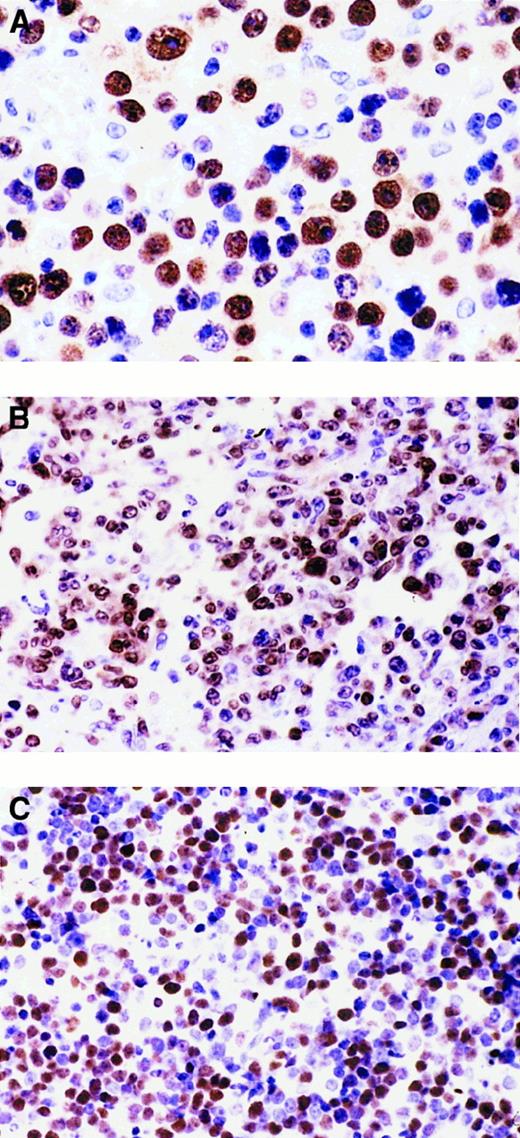
Heterogeneous expression pattern of BCL-6 and MUM1 proteins throughout the pathologic spectrum of systemic AIDS-NHL.
(A) Systemic AIDS-IBL displaying the BCL-6−, MUM1+, syn-1+, CD10−, LMP1+ phenotype (case 38, Table 1). In the figure, most immunoblastic-plasmacytoid tumor cells show nuclear immunoreactivity with the anti-MUM1 antibody. (B) Systemic AIDS-DLCL, displaying the BCL-6+, MUM1+, syn-1−, CD10−, LMP1− phenotype (case 29, Table 1). In the figure, large tumor cells show a nuclear staining pattern with anti-MUM1 antibody. (C) Systemic AIDS-BL displaying the BCL-6+, MUM1−, syn-1−, CD10+, LMP1− phenotype (case 6, Table 1). Neoplastic cells display strong nuclear immunoreactivity with the anti–BCL-6 mAb. Paraffin-embedded tissue sections, immunoperoxidase (A, B, C), hematoxylin counterstain. Original magnification, × 400 (A), × 250 (B, C).
Heterogeneous expression pattern of BCL-6 and MUM1 proteins throughout the pathologic spectrum of systemic AIDS-NHL.
(A) Systemic AIDS-IBL displaying the BCL-6−, MUM1+, syn-1+, CD10−, LMP1+ phenotype (case 38, Table 1). In the figure, most immunoblastic-plasmacytoid tumor cells show nuclear immunoreactivity with the anti-MUM1 antibody. (B) Systemic AIDS-DLCL, displaying the BCL-6+, MUM1+, syn-1−, CD10−, LMP1− phenotype (case 29, Table 1). In the figure, large tumor cells show a nuclear staining pattern with anti-MUM1 antibody. (C) Systemic AIDS-BL displaying the BCL-6+, MUM1−, syn-1−, CD10+, LMP1− phenotype (case 6, Table 1). Neoplastic cells display strong nuclear immunoreactivity with the anti–BCL-6 mAb. Paraffin-embedded tissue sections, immunoperoxidase (A, B, C), hematoxylin counterstain. Original magnification, × 400 (A), × 250 (B, C).
Among cases of systemic AIDS-NHL carrying EBV infection (23 cases), the expression of LMP1 was detected in 7 cases (Table 1). Most cases of LMP1+ systemic AIDS-NHL were morphologically classified as having AIDS-IBL (6 of 7 cases, 86%), expressed both MUM1 and syn-1 (5 of 7 cases, 71%), and stained negative for BCL-6 (7 of 7 cases, 100%) (Table 1). Conversely, the expression of LMP1 was consistently absent in all cases of EBV+ systemic AIDS-NHL expressing BCL-6 (Table 1).
AIDS-PCNSL
All cases of AIDS-PCNSL were EBV+ and KSHV− (not shown). The expression profile of MUM1, BCL-6, syn-1, and LMP1 in cases of AIDS-PCNSL is summarized in Table 2. Expression of MUM1 was detected in 15 of 22 (68%) cases of AIDS-PCNSL, including 10 of 12 (83%) cases of AIDS-IBL, 2 of 3 (67%) cases of AIDS-DLCL, and 3 of 7 (43%) cases of AIDS-DLCL/IBL (Table 2). Expression of BCL-6 was detected in 8 of 22 (36%) cases of AIDS-PCNSL (Table 2), including 3 of 3 cases of AIDS-DLCL and 5 of 7 cases of AIDS-DLCL/IBL. Conversely, the expression of BCL-6 was scored negative in all cases of AIDS-PCNSL classified as AIDS-IBL (Table 2). Expression of BCL-6 protein among cases of AIDS-PCNSL occurred both in the presence and in the absence of mutations of the BCL-6 gene (Table 2). Expression of syn-1 was detected in 12 of 22 (54%) cases of AIDS-PCNSL (Table 2), including 9 of 12 (75%) cases of AIDS-IBL and 3 of 7 (43%) cases of AIDS-DLCL/IBL (43%). Conversely, syn-1 scored negative in cases of AIDS-PCNSL classified as AIDS-DLCL (Table 2). The expression of CD10 scored negative in all 9 tested cases (1 with DLCL morphology, 5 with IBL morphology, and 3 with DLCL/IBL morphology).
Expression of MUM1, BCL-6, and CD138/syn-1 andBCL-6 gene mutations in AIDS-related primary central nervous system lymphomas
| Case . | Diagnosis . | Immunohistochemistry . | BCL-6 (mut) . | EBV . | |||
|---|---|---|---|---|---|---|---|
| MUM1* . | BCL-6* . | Syn-1* . | EBER . | LMP1* . | |||
| 1 | DLCL | − | 25-50 | − | − | + | − |
| 2 | DLCL | 10-25 | 50-75 | − | + | + | − |
| 3 | DLCL | > 75 | 50-75 | − | − | + | − |
| 4 | IBL | − | − | < 10 | − | + | − |
| 5 | IBL | − | − | > 75 | nd | + | 25-50 |
| 6 | IBL | 25-50 | − | − | − | + | 25-50 |
| 7 | IBL | 25-50 | − | < 10 | nd | + | 10-25 |
| 8 | IBL | 25-50 | − | 50-75 | nd | + | 50-75 |
| 9 | IBL | 50-75 | − | > 75 | nd | + | 50-75 |
| 10 | IBL | 50-75 | − | − | − | + | 25-50 |
| 11 | IBL | 50-75 | − | − | − | + | > 75 |
| 12 | IBL | > 75 | − | < 10 | − | + | 10-25 |
| 13 | IBL | > 75 | − | < 10 | nd | + | > 75 |
| 14 | IBL | > 75 | − | < 10 | nd | + | > 75 |
| 15 | IBL | > 75 | − | > 75 | nd | + | > 75 |
| 16 | DLCL/IBL | − | − | − | + | + | < 10 |
| 17 | DLCL/IBL | − | 10-25 | − | + | + | 10-25 |
| 18 | DLCL/IBL | − | < 10 | − | + | + | < 10 |
| 19 | DLCL/IBL | − | > 75 | 50-75 | nd | + | − |
| 20 | DLCL/IBL | 10-25 | 25-50 | 25-50 | nd | + | 25-50 |
| 21 | DLCL/IBL | 10-25 | 50-75 | − | − | + | 25-50 |
| 22 | DLCL/IBL | 50-75 | − | < 10 | nd | + | 10-25 |
| Case . | Diagnosis . | Immunohistochemistry . | BCL-6 (mut) . | EBV . | |||
|---|---|---|---|---|---|---|---|
| MUM1* . | BCL-6* . | Syn-1* . | EBER . | LMP1* . | |||
| 1 | DLCL | − | 25-50 | − | − | + | − |
| 2 | DLCL | 10-25 | 50-75 | − | + | + | − |
| 3 | DLCL | > 75 | 50-75 | − | − | + | − |
| 4 | IBL | − | − | < 10 | − | + | − |
| 5 | IBL | − | − | > 75 | nd | + | 25-50 |
| 6 | IBL | 25-50 | − | − | − | + | 25-50 |
| 7 | IBL | 25-50 | − | < 10 | nd | + | 10-25 |
| 8 | IBL | 25-50 | − | 50-75 | nd | + | 50-75 |
| 9 | IBL | 50-75 | − | > 75 | nd | + | 50-75 |
| 10 | IBL | 50-75 | − | − | − | + | 25-50 |
| 11 | IBL | 50-75 | − | − | − | + | > 75 |
| 12 | IBL | > 75 | − | < 10 | − | + | 10-25 |
| 13 | IBL | > 75 | − | < 10 | nd | + | > 75 |
| 14 | IBL | > 75 | − | < 10 | nd | + | > 75 |
| 15 | IBL | > 75 | − | > 75 | nd | + | > 75 |
| 16 | DLCL/IBL | − | − | − | + | + | < 10 |
| 17 | DLCL/IBL | − | 10-25 | − | + | + | 10-25 |
| 18 | DLCL/IBL | − | < 10 | − | + | + | < 10 |
| 19 | DLCL/IBL | − | > 75 | 50-75 | nd | + | − |
| 20 | DLCL/IBL | 10-25 | 25-50 | 25-50 | nd | + | 25-50 |
| 21 | DLCL/IBL | 10-25 | 50-75 | − | − | + | 25-50 |
| 22 | DLCL/IBL | 50-75 | − | < 10 | nd | + | 10-25 |
Diffuse large cell lymphoma/immunoblastic lymphoma, AIDS-related primary central nervous system lymphoma cases containing a mixture of large noncleaved cells and large cells, immunoblastic plasmacytoid (see “Patients, materials, and methods” and Larocca et al23).
See Table 1 for abbreviations.
Percentages of MUM1, BCL-6, syn-1, and LMP1+ neoplastic cells were assigned to one of the following categories: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
Expression of LMP1 was detected in 17 of 22 cases of AIDS-PCNSL. All cases of LMP1+ AIDS-PCNSL were morphologically classified as having AIDS-IBL or LDLCL/IBL. Most of these cases expressed MUM1 (13 of 17, 76%) or syn-1 (10 of 17, 59%) and stained negative for BCL-6 (13 of 17, 76%) (Table 2). The expression of LMP1 was negative in all cases of AIDS-PCNSL with AIDS-DLCL morphology.
AIDS-PEL
The panel of AIDS-PEL cases, all positive for KSHV, included 11 cases (Table 3). Seven cases of AIDS-PEL carried the EBV genome.
Expression of MUM1, BCL-6, and CD138/syn-1 andBCL-6 gene mutations in AIDS-related primary effusion lymphoma and AIDS-related plasmablastic lymphoma
| Case . | Diagnosis . | Immunohistochemistry . | BCL-6 (mut) . | EBV . | |||
|---|---|---|---|---|---|---|---|
| MUM13-150 . | BCL-63-150 . | Syn-13-150 . | EBER . | LMP13-150 . | |||
| 1 | PEL | 25-50 | ne | − | − | + | < 10 |
| 2 | PEL | > 75 | − | > 75 | − | + | < 10 |
| 3 | PEL | > 75 | − | > 75 | − | − | nd |
| 4 | PEL | 50-75 | − | > 75 | + | + | − |
| 5 | PEL | > 75 | − | > 75 | + | − | − |
| 6 | PEL | 50-75 | − | > 75 | + | + | < 10 |
| 7 | PEL | 25-50 | − | > 75 | + | − | − |
| 8 | PEL | 50-75 | − | 25-50 | − | + | − |
| 9 | PEL | > 75 | − | > 75 | − | + | − |
| 10 | PEL | > 75 | − | > 75 | + | + | − |
| 11 | PEL | > 75 | − | > 75 | − | − | − |
| 1 | PBL | > 75 | − | > 75 | nd | + | − |
| 2 | PBL | > 75 | − | 50-75 | nd | + | − |
| 3 | PBL | > 75 | − | > 75 | nd | + | − |
| 4 | PBL | > 75 | − | > 75 | nd | + | − |
| 5 | PBL | > 75 | − | > 75 | nd | − | − |
| 6 | PBL | > 75 | − | > 75 | nd | + | − |
| 7 | PBL | 10-25 | − | 25-50 | nd | + | − |
| Case . | Diagnosis . | Immunohistochemistry . | BCL-6 (mut) . | EBV . | |||
|---|---|---|---|---|---|---|---|
| MUM13-150 . | BCL-63-150 . | Syn-13-150 . | EBER . | LMP13-150 . | |||
| 1 | PEL | 25-50 | ne | − | − | + | < 10 |
| 2 | PEL | > 75 | − | > 75 | − | + | < 10 |
| 3 | PEL | > 75 | − | > 75 | − | − | nd |
| 4 | PEL | 50-75 | − | > 75 | + | + | − |
| 5 | PEL | > 75 | − | > 75 | + | − | − |
| 6 | PEL | 50-75 | − | > 75 | + | + | < 10 |
| 7 | PEL | 25-50 | − | > 75 | + | − | − |
| 8 | PEL | 50-75 | − | 25-50 | − | + | − |
| 9 | PEL | > 75 | − | > 75 | − | + | − |
| 10 | PEL | > 75 | − | > 75 | + | + | − |
| 11 | PEL | > 75 | − | > 75 | − | − | − |
| 1 | PBL | > 75 | − | > 75 | nd | + | − |
| 2 | PBL | > 75 | − | 50-75 | nd | + | − |
| 3 | PBL | > 75 | − | > 75 | nd | + | − |
| 4 | PBL | > 75 | − | > 75 | nd | + | − |
| 5 | PBL | > 75 | − | > 75 | nd | − | − |
| 6 | PBL | > 75 | − | > 75 | nd | + | − |
| 7 | PBL | 10-25 | − | 25-50 | nd | + | − |
PEL indicates primary effusion lymphoma; ne, not evaluable; PBL, plasmablastic lymphoma; see Table 1 for other abbreviations.
Percentages of MUM1, BCL-6, syn-1, and LMP1+ neoplastic cells were assigned to one of the following categories: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
The expression profile of MUM1, BCL-6, syn-1, and LMP1 in AIDS-PEL is summarized in Table 3. Cases of AIDS-PEL were found to express MUM1 (11 of 11, 100%) and syn-1 (10 of 11, 91%) but scored negative for BCL-6 protein expression (10 of 10, 100%) (Table 3). Mutations of theBCL-6 gene occurred in 5 of 11 (45%) tested cases. Of the 7 cases of AIDS-PEL carrying EBV infection, the expression of LMP1 was restricted to a small proportion of cells (Table 3).
AIDS-PBL
The panel of AIDS-PBL cases numbered 7 (Table 3). Six cases of AIDS-PBL carried the EBV genome. All cases were devoid of KSHV infection (not shown).
HIV-HL
The panel of HIV-HL numbered 16. Reed-Sternberg (RS) cell infection by EBV was scored positive in 13 of 16 (81%) cases. RS cells and their variants expressed MUM1 and syn-1 in all HIV-HL (16 of 16 cases, 100%), which were representative of the entire pathologic spectrum of classic HIV-HL (Table 4, Figure3). Conversely, the expression of BCL-6 by RS cells was negative in the overwhelming majority of HIV-HL cases (15 of 16 cases, 94%), whereas one case of HIV-HL displayed a low proportion of BCL-6+ RS cells (less than 10%) (Table 4). All HIV-HL cases carrying EBV infection scored positive for LMP1 (Table 4).
Expression of MUM1, BCL-6 and CD138/syn-1 in HIV-related Hodgkin lymphomas
| Case . | Diagnosis . | Immunohistochemistry . | EBV . | |||
|---|---|---|---|---|---|---|
| MUM14-150 . | BCL-64-150 . | syn-14-150 . | EBER . | LMP14-150 . | ||
| 1 | HL-NS | > 75 | − | > 75 | + | < 10 |
| 2 | HL-NS | > 75 | − | < 10 | − | − |
| 3 | HL-MC | > 75 | − | 25-50 | + | 25-50 |
| 4 | HL-MC | > 75 | − | 10-25 | − | − |
| 5 | HL-MC | > 75 | < 10 | 50-75 | + | > 75 |
| 6 | HL-MC | > 75 | − | > 75 | + | 25-50 |
| 7 | HL-MC | > 75 | − | > 75 | + | > 75 |
| 8 | HL-MC | > 75 | − | > 75 | + | 50-75 |
| 9 | HL-LD | > 75 | − | < 10 | + | > 75 |
| 10 | HL-LD | > 75 | − | < 10 | + | > 75 |
| 11 | HL-LD | > 75 | − | < 10 | + | 50-75 |
| 12 | HL-LD | > 75 | − | 25-50 | + | > 75 |
| 13 | HL-LD | > 75 | − | 25-50 | + | > 75 |
| 14 | HL-LD | > 75 | − | 50-75 | + | > 75 |
| 15 | HL-LD | > 75 | − | > 75 | − | − |
| 16 | HL-LD | > 75 | − | > 75 | + | > 75 |
| Case . | Diagnosis . | Immunohistochemistry . | EBV . | |||
|---|---|---|---|---|---|---|
| MUM14-150 . | BCL-64-150 . | syn-14-150 . | EBER . | LMP14-150 . | ||
| 1 | HL-NS | > 75 | − | > 75 | + | < 10 |
| 2 | HL-NS | > 75 | − | < 10 | − | − |
| 3 | HL-MC | > 75 | − | 25-50 | + | 25-50 |
| 4 | HL-MC | > 75 | − | 10-25 | − | − |
| 5 | HL-MC | > 75 | < 10 | 50-75 | + | > 75 |
| 6 | HL-MC | > 75 | − | > 75 | + | 25-50 |
| 7 | HL-MC | > 75 | − | > 75 | + | > 75 |
| 8 | HL-MC | > 75 | − | > 75 | + | 50-75 |
| 9 | HL-LD | > 75 | − | < 10 | + | > 75 |
| 10 | HL-LD | > 75 | − | < 10 | + | > 75 |
| 11 | HL-LD | > 75 | − | < 10 | + | 50-75 |
| 12 | HL-LD | > 75 | − | 25-50 | + | > 75 |
| 13 | HL-LD | > 75 | − | 25-50 | + | > 75 |
| 14 | HL-LD | > 75 | − | 50-75 | + | > 75 |
| 15 | HL-LD | > 75 | − | > 75 | − | − |
| 16 | HL-LD | > 75 | − | > 75 | + | > 75 |
HL indicates Hodgkin lymphoma; NS, nodular sclerosis; MC, mixed cellularity; LD, lymphocyte depletion.
Percentages of MUM1, BCL-6, syn-1, and LMP1+Reed-Sternberg cells were assigned to one of the following categories: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
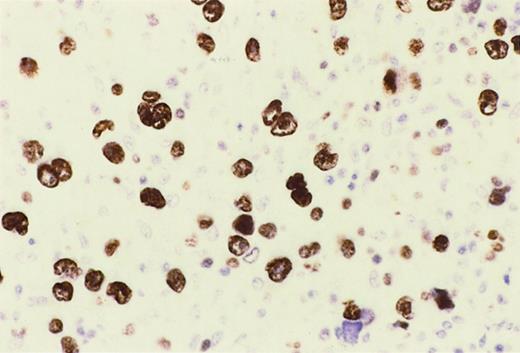
Reed-Sternberg cells of HIV-HL express MUM1.
In a lymph node involved by lymphocyte depletion HIV-HL (case 16, Table4), MUM1 is expressed by a large number of tumor cells corresponding to RS cells and their variants. The staining is nuclear and intense. Immunoperoxidase, hematoxylin counterstain. Original magnification, × 250.
Reed-Sternberg cells of HIV-HL express MUM1.
In a lymph node involved by lymphocyte depletion HIV-HL (case 16, Table4), MUM1 is expressed by a large number of tumor cells corresponding to RS cells and their variants. The staining is nuclear and intense. Immunoperoxidase, hematoxylin counterstain. Original magnification, × 250.
Comparison between the major phenotyic patterns and pathologic subtypes
The combination of MUM1, BCL-6, and syn-1 segregated 3 major phenotypic patterns among AIDS-NHL and HIV-HL (Table5, Figure4): (1) the BCL-6+/MUM1−/syn-1− pattern, which reflects GC centroblasts and MUM1− centrocytes and associates with CD10 expression in a subset of cases; (2) the BCL-6−/MUM1+/syn-1− pattern, which reflects late GC/early post-GC B cells; and (3) the BCL-6−/MUM1+/syn-1+ pattern, which reflects post-GC B cells. These major phenotypic patterns display preferential associations with specific clinicopathologic categories of AIDS-NHL and HIV-HL. Thus, the BCL-6+/MUM1−/syn-1− pattern associates with most cases of AIDS-BL (17 of 19) and systemic AIDS-DLCL (12 of 16). Conversely, the BCL-6−/MUM1+/syn-1+ pattern associates with most cases of AIDS-PEL (10 of 10), AIDS-PBL (7 of 7), HIV-HL (15 of 16), and AIDS-IBL (14 of 24) arising systemically or localized primarily to the central nervous system. Finally, the BCL-6−/MUM1+/syn-1− pattern associates with the remaining fraction of AIDS-IBL (8 of 24). An additional phenotypic pattern—the BCL-6+/MUM1+/syn-1− pattern—is occasionally detected in systemic AIDS-NHL and AIDS-PCNSL.
Expression patterns of BCL-6, MUM1, and CD138/syn-1 throughout the pathologic spectrum of HIV-related lymphomas
| . | Phenotypic patterns5-150 . | |||
|---|---|---|---|---|
| BCL6+/ MUM1−/ syn-1− . | BCL6−/ MUM1+/ syn-1+ . | BCL6−/ MUM1+/ syn-1− . | BCL6+/ MUM1+/ syn-1− . | |
| Systemic NHL | ||||
| BL (case 19) | 17 | 0 | 0 | 25-151 |
| DLCL (case 16) | 12 | 0 | 1 | 2 |
| IBL (case 12) | 0 | 7 | 5 | 0 |
| PCNSL | ||||
| DLCL (case 3) | 1 | 0 | 0 | 2 |
| IBL (case 12) | 0 | 7 | 3 | 0 |
| DLCL/IBL (case 7) | 2 | 1 | 0 | 1 |
| PBL (case 7) | 0 | 7 | 0 | 0 |
| PEL (case 105-152) | 0 | 10 | 0 | 0 |
| HL (case 16) | 0 | 15 | 0 | 0 |
| EBV+ | 14 of 32 | 38 of 47 | 8 of 9 | 4 of 7 |
| LMP1+ | 2 of 32 | 27 of 47 | 5 of 9 | 1 of 7 |
| . | Phenotypic patterns5-150 . | |||
|---|---|---|---|---|
| BCL6+/ MUM1−/ syn-1− . | BCL6−/ MUM1+/ syn-1+ . | BCL6−/ MUM1+/ syn-1− . | BCL6+/ MUM1+/ syn-1− . | |
| Systemic NHL | ||||
| BL (case 19) | 17 | 0 | 0 | 25-151 |
| DLCL (case 16) | 12 | 0 | 1 | 2 |
| IBL (case 12) | 0 | 7 | 5 | 0 |
| PCNSL | ||||
| DLCL (case 3) | 1 | 0 | 0 | 2 |
| IBL (case 12) | 0 | 7 | 3 | 0 |
| DLCL/IBL (case 7) | 2 | 1 | 0 | 1 |
| PBL (case 7) | 0 | 7 | 0 | 0 |
| PEL (case 105-152) | 0 | 10 | 0 | 0 |
| HL (case 16) | 0 | 15 | 0 | 0 |
| EBV+ | 14 of 32 | 38 of 47 | 8 of 9 | 4 of 7 |
| LMP1+ | 2 of 32 | 27 of 47 | 5 of 9 | 1 of 7 |
NHL indicates non-Hodgkin lymphoma; PCNSL, primary central nervous system lymphoma; see Table 1 for other abbreviations.
Other phenotypic patterns included: BCL6−/MUM1−/syn1− in 1 systemic NHL (with DLCL morphology) and in 1 PCNSL (with DLCL/IBL morphology); BCL6−/MUM1−/syn1+ in 2 PCNSL (with IBL morphology); BCL6+/MUM1+/syn1+ in 1 PCNSL (with DLCL/IBL morphology) and in 1 HL; and BCL6+/MUM1−/syn1+ in 1 PCNSL (with DLCL/IBL morphology).
In 2 cases occasional neoplastic cells expressed MUM1.
In 1 PEL case the expression of BCL-6 could not be assessed because the immunohistochemistry result was not evaluable for technical reasons.
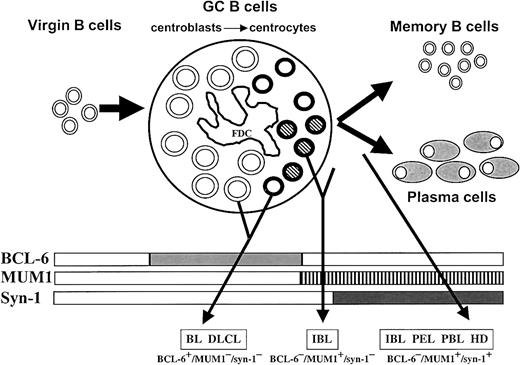
Proposed model for the histogenesis of HIV-related lymphomas.
This model is based on the physiologic stages of mature B-cell development identified by histogenetic markers, such as mutation pattern of immunoglobulin variable region genes (IgV) andBCL-6 gene and expression profile of BCL-6, MUM1, and CD138/syn-1 proteins (rectangles). Virgin B cells do not display immunoglobulin or BCL-6 mutations and lack protein expression of BCL-6, MUM1, and syn-1. At the time of B-cell transit through the GC, B cells acquire IgV and BCL-6 mutations, which are maintained during further differentiation, thus constituting genotypic markers of GC transit.10,11,13 B cells within the GC (ie, centroblasts and centrocytes) express BCL-6 but not syn-1.1 Post-GC B cells undergoing maturation toward the plasma cell stage switch off BCL-6 expression and stain positive for syn-1. Based on the results of this study, the histogenetic model presented in the figure has been enriched by the addition of the MUM1 marker, which is expressed by late stages of intra-GC differentiation (centrocytes) and by post-GC B cells undergoing plasma cell maturation. GC B cells are identified as follows: centroblasts, large open circles; MUM1− centrocytes, small open circles; MUM1+ centrocytes, small striped circles; FDC, follicular dendritic reticulum cell. The major HIV-lymphoma categories are indicated as BL (Burkitt lymphoma), DLCL, IBL, PEL, PBL, and HL. The putative histogenetic derivation of each lymphoma category is indicated by an arrow originating from the relevant B-cell compartment. The common phenotypic patterns exhibited by the different categories of HIV-lymphomas are indicated in the lower part of the figure. Some lymphoma categories, namely IBL, are characterized by histogenetic heterogeneity.
Proposed model for the histogenesis of HIV-related lymphomas.
This model is based on the physiologic stages of mature B-cell development identified by histogenetic markers, such as mutation pattern of immunoglobulin variable region genes (IgV) andBCL-6 gene and expression profile of BCL-6, MUM1, and CD138/syn-1 proteins (rectangles). Virgin B cells do not display immunoglobulin or BCL-6 mutations and lack protein expression of BCL-6, MUM1, and syn-1. At the time of B-cell transit through the GC, B cells acquire IgV and BCL-6 mutations, which are maintained during further differentiation, thus constituting genotypic markers of GC transit.10,11,13 B cells within the GC (ie, centroblasts and centrocytes) express BCL-6 but not syn-1.1 Post-GC B cells undergoing maturation toward the plasma cell stage switch off BCL-6 expression and stain positive for syn-1. Based on the results of this study, the histogenetic model presented in the figure has been enriched by the addition of the MUM1 marker, which is expressed by late stages of intra-GC differentiation (centrocytes) and by post-GC B cells undergoing plasma cell maturation. GC B cells are identified as follows: centroblasts, large open circles; MUM1− centrocytes, small open circles; MUM1+ centrocytes, small striped circles; FDC, follicular dendritic reticulum cell. The major HIV-lymphoma categories are indicated as BL (Burkitt lymphoma), DLCL, IBL, PEL, PBL, and HL. The putative histogenetic derivation of each lymphoma category is indicated by an arrow originating from the relevant B-cell compartment. The common phenotypic patterns exhibited by the different categories of HIV-lymphomas are indicated in the lower part of the figure. Some lymphoma categories, namely IBL, are characterized by histogenetic heterogeneity.
The phenotypic patterns identified above also displayed preferential associations with the virologic features of the tumors. In fact, among systemic AIDS-NHL and AIDS-PCNSL, expression of LMP1 preferentially associates with the BCL-6−/MUM1+/syn-1+ profile. A strict association between LMP1 expression and the BCL-6−/MUM1+/syn-1+ phenotype was also observed in the case of AIDS-HL.
Discussion
The aim of this study was to refine the histogenesis of the pathologic spectrum of AIDS-NHL and HIV-HL and to identify novel criteria for the classification and diagnosis of these disorders. By using a combination of several histogenetic markers, namely MUM1, BCL-6, and syn-1, we report 3 major phenotypic subsets of AIDS-NHL and HIV-HL—BCL-6+/MUM1−/syn-1−, BCL-6−/MUM1+/syn-1−, and BCL-6−/MUM1+/syn-1+ (Table 5, Figure 4). These phenotypic profiles correspond to distinct differentiation programs of normal B cells and preferentially associate with specific clinico-pathologic categories of AIDS-NHL and HIV-HL. On these bases, our findings bear implications for the histogenesis and the classification of these neoplasms.
In reactive lymphoid tissues, the phenotypic patterns identified by MUM1, BCL-6, and syn-1 map to lymph node areas that are populated by B cells at different stages of differentiation. Comparison of the topography of MUM1 and BCL-6 within the GC reveals that the expression of BCL-6 occurs immediately after a B cell enters the GC and is maintained until GC exit (Figure 4), whereas MUM1 positivity begins only at the centrocyte stage and is maintained during post-GC maturation (Figure 4).12,19 20 In this respect, most B cells within the GC, including all centroblasts and almost all centrocytes, express the BCL-6+/MUM1−/syn-1− phenotype (Figure 1). A small fraction of GC B cells, located in the light zone of the GC and morphologically identifiable as a subset of centrocytes, expresses the BCL-6−/MUM1+/syn-1−phenotype (Figure 1). As such, MUM1 expression by GC cells may denote the final step of intra-GC B-cell differentiation—late centrocytes (Figure 4). On GC exit, B cells retain MUM1 expression and start to express syn-1, as documented by the observation that post-GC B cells undergoing maturation toward the plasma cell stage predominantly display the BCL-6−/MUM1+/syn-1+phenotype (Figures 1, 4). Because many MUM1+ cells in the GC are negative for syn-1, MUM1 expression most likely precedes syn-1. Consequently, MUM1 provides a novel histogenetic marker denoting B-cell transition from BCL-6 positivity (GC B cells) to syn-1 expression (immunoblasts and plasma cells).
The phenotypic profiles displayed by AIDS-NHL and HIV-HL, combined with the distribution of MUM1, BCL-6, and syn-1 among normal B-cell subsets, point to a histogenetic model of these neoplasms. According to this model (Figure 4), AIDS-BL and systemic AIDS-DLCL express the BCL-6+/MUM1−/syn-1− phenotypic pattern and closely reflect B cells residing in the GC, namely centroblasts and early (ie, MUM1−) centrocytes. Intriguingly, AIDS-BL, but not AIDS-DLCL, expresses CD10, suggesting putative differences in the maturation or activation stage, or both, of these lymphomas. AIDS-IBL, either systemic or primarily localized to the central nervous system (AIDS-PCNSL), appears to be characterized by a certain degree of histogenetic heterogeneity because these lymphomas may reflect either classical post-GC B cells (BCL-6−/MUM1+/syn-1+) or late centrocytes/early post-GC B cells (BCL-6−/MUM1+/syn-1−). Finally, AIDS-PEL, AIDS-PBL, and HIV-HL consistently display the BCL-6−/MUM1+/syn-1+ phenotype and, therefore, reflect post-GC B cells in all cases.
Besides correlating with morphology, the phenotypic patterns identified by MUM1, BCL-6, and syn-1 correlate with the virologic features of AIDS-NHL and HIV-HL. In fact, expression of the LMP1 antigen among EBV-infected AIDS-NHL and HIV-HL associates preferentially with BCL-6–/MUM1+/syn-1+ cases that exhibit morphologic features consistent with an advanced stage of B-cell maturation (eg, IBL) (Tables 1, 2). Conversely, LMP1 expression is generally absent in BCL-6+/MUM1−/syn-1− AIDS-NHL. These findings suggest that the post-GC phenotype is the sole condition permissive for LMP1 expression in the context of AIDS-NHL and HIV-HL or, alternatively, that LMP1 expression forces post-GC maturation of these lymphomas. Although LMP1 expression is found preferentially in AIDS-NHL with the BCL-6−/MUM1+/syn-1+ phenotype, a subset of EBV+ lymphomas with a post-GC phenotype, namely AIDS-PEL and AIDS-PBL (Table 3), does not express LMP1, suggesting that the post-GC phenotype is not the sole requirement for LMP1 expression and that other factors may be involved. To date, the reason AIDS-PEL and AIDS-PBL do not express LMP1 is not understood and may be related to the tumor differentiation stage or to coinfection by other viruses.
The expression pattern of MUM1, BCL-6, and syn-1 bears implications for AIDS-NHL diagnosis. In fact, distinct pathologic and molecular categories of AIDS-NHL selectively associate with different expression patterns of the histogenetic markers used in this study. Remarkably, the association between phenotype and histology appears to be independent of the primary site of the disease, as exemplified by the fact that both systemic AIDS-IBL and AIDS-PCNSL with IBL morphology share the same BCL-6−/MUM1+ phenotype. Hence, it is conceivable that the expression pattern of MUM1, BCL-6, and syn-1 may contribute to the differential diagnosis of these lymphomas when the sole morphology is unable to provide a precise classification. In addition, the combination of MUM1, BCL-6, and syn-1 may offer advantages in refining the classification of AIDS-NHL categories thought to be nonhomogeneous in pathogenetic and histogenetic terms. In particular, the use of these histogenetic markers may be fruitful in subclassifying AIDS-DLCL (BCL-6+/MUM1−/syn-1− versus BCL-6−/MUM1+/syn-1−) and AIDS-IBL (BCL-6−/MUM1+/syn-1− versus BCL-6−/MUM1+/syn-1+). Our results also prompt large-scale investigation of AIDS-NHL gene expression by the micro-array DNA technology that has revealed histogenetic heterogeneity of DLCL of immunocompetent hosts.37 These studies are of potential clinical value because, in several B-cell disorders of immunocompetent hosts, histogenesis has been shown to influence prognosis.37 38
Acknowledgments
We thank Barbara Canal, Paola Ceolin, and Ivana Zanette for excellent technical assistance in immunohistochemistry experiments and in situ hybridization studies. We also thank Prof B. Falini (Institute of Hematology and Internal Medicine, University of Perugia, Italy) for the gift of the mAb MUM1p.
Supported in part by Istituto Superiore di Sanità, II Programma nazionale di ricerca sull'AIDS–Progetto Patologia clinica e terapia dell'AIDS, Rome; by Ministero della Sanita', RF 1999, Rome; by the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy; and by National Institutes of Health grant CA-37295.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Antonino Carbone, Division of Pathology, Centro di Riferimento Oncologico, Istituto Nazionale Tumori, IRCCS, via Pedemontana Occidentale, Aviano I-33081, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal