Abstract
Nucleotides are emerging as an ubiquitous family of extracellular signaling molecules. It has been known for many years that adenosine diphosphate is a potent platelet aggregating factor, but it is now clear that virtually every circulating cell is responsive to nucleotides. Effects as different as proliferation or differentiation, chemotaxis, release of cytokines or lysosomal constituents, and generation of reactive oxygen or nitrogen species are elicited upon stimulation of blood cells with extracellular adenosine triphosphate (ATP). These effects are mediated through a specific class of plasma membrane receptors called purinergic P2 receptors that, according to the molecular structure, are further subdivided into 2 subfamilies: P2Y and P2X. ATP and possibly other nucleotides are released from damaged cells or secreted via nonlytic mechanisms. Thus, during inflammation or vascular damage, nucleotides may provide an important mechanism involved in the activation of leukocytes and platelets. However, the cell physiology of these receptors is still at its dawn, and the precise function of the multiple P2X and P2Y receptor subtypes remains to be understood.
Introduction
In 1978 the existence of plasma membrane receptors for extracellular nucleotides, the P2 purinergic receptors, was formally recognized.1 At that time, this identification was only based on pharmacologic and functional evidence and on the prophetic intuition of Geoff Burnstock. To date, 12 mammalian P2 receptors have been cloned, characterized, and recognized as responsible for the diverse cellular responses to stimulation with extracellular nucleotides.2,3 The P2 receptor family also includes receptors for extracellular pyrimidines. The new classification based on the molecular structure is rapidly replacing the previous one (P2Y, P2X, P2U, P2T, and P2Z) based on the pharmacologic profile,4 although doubts remain on whether functional responses of the native P2Z receptor of immune cells can be entirely explained by the cloned P2X7 subunit. A similar uncertainty also concerns the platelet P2T receptor, which is likely to arise from the combination of P2Y and P2X-dependent responses.2,5 Extracellular effects of nucleotides were initially recognized in smooth muscle contraction, neurotransmission, regulation of cardiac function, and platelet aggregation.6However, over the last 10 years it has become clear that the intercellular mediator role of these molecules is widespread, and blood cells have emerged as one of the most interesting targets.
Contrary to a widely held opinion, adenosine triphosphate (ATP) and possibly also uridine triphosphate (UTP) are often released into the extracellular environment via nonlytic mechanisms7-12 and also more frequently as a consequence of cell damage or acute cell death. Furthermore, platelet-dense granules are a relevant source of secreted ATP.13,14 Once in the pericellular environment, ATP can serve as a ligand for P2 receptors or be quickly hydrolyzed by powerful ubiquitous ecto-ATPases and ectonucleotidases.15-18 ATP can also be used as a phosphate donor by poorly characterized ectokinases.19Thus, ATP possesses all the properties of a bona fide fast-acting intercellular messenger: (a) it is released in a controlled fashion, (b) ligates specific plasma membrane receptors coupled to intracellular signal transduction, and (c) is quickly degraded to terminate its action.
Outside excitable tissues, P2 receptors have an obvious relevance in platelet aggregation, but immunity and inflammation are providing some of the most exciting developments in this evolving field. A few reviews covering different aspects of P2 receptor distribution and function in hemopoietic cells have appeared and have been an invaluable source of information for the present work.20-26
P2 receptors: what are they?
According to the International Union of Pharmacology (IUPHAR) Committee on Receptor Nomenclature and Drug Classification,27 receptors for extracellular nucleotides are termed P2 receptors (this nomenclature replaces the older “P2-purinoceptor”). P2 receptors are divided into 2 subfamilies: G protein–coupled (P2Y) and ligand-gated ion channels (P2X).3,28-30 Current P2Y/P2X nomenclature is based on the molecular structure and has replaced the previous one based on pharmacologic and functional criteria. In mammalian cells, 5 P2Y (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11) and 7 P2X (P2X1-7) receptors have been cloned and characterized pharmacologically2 (Table 1). P2Y5, P2Y7, P2Y9, and P2Y10 have been purged from this sequence because they are primarily non-nucleotide receptors (although they may also bind extracellular nucleotides). A p2y3 (lower case to indicate that it has not been cloned from mammals) receptor has been cloned from chick brain and suggested to be a homologue of the mammalian P2Y6.2 P2Y8 has so far only been cloned from Xenopus neural plate; thus it is not included in the list of mammalian receptors. The adenosine diphosphate (ADP)-activated, G protein–coupled receptor of platelets that triggers inhibition of stimulated adenylate cyclase has not yet been cloned; thus it is recommended that this receptor should be given in italics: P2Y ADP.2
P2Y and P2X receptor subtypes
| P2Y . | Amino acid number . | P2X . | Amino acid number . |
|---|---|---|---|
| P2Y1 | 362 | P2X1 | 399 |
| P2Y2 | 373 | P2X2† | 472, 401 |
| p2y3* | 328 | P2X3 | 397 |
| P2Y4 | 352 | P2X4† | 388, 329 |
| P2Y6 | 379 | P2X5 | 455 |
| P2Y11 | 371 | P2X6 | 379 |
| P2X7 | 595 |
| P2Y . | Amino acid number . | P2X . | Amino acid number . |
|---|---|---|---|
| P2Y1 | 362 | P2X1 | 399 |
| P2Y2 | 373 | P2X2† | 472, 401 |
| p2y3* | 328 | P2X3 | 397 |
| P2Y4 | 352 | P2X4† | 388, 329 |
| P2Y6 | 379 | P2X5 | 455 |
| P2Y11 | 371 | P2X6 | 379 |
| P2X7 | 595 |
p2y3 was cloned from chick brain and may be the chick homologue of the mammalian P2Y6.
P2X2 and P2X4 are present in two splice variants.
P2Y receptors
P2Y receptors are 7-membrane–spanning proteins, numbering from 328 to 379 amino acids, for a molecular mass of 41 to 53 kd after glycosylation.2,31,32 The aminoterminal domain faces the extracellular environment, and the carboxyterminal is on the cytoplasmic side of the plasma membrane (Figure1). Signal transduction occurs via the classical pathways triggered by most 7-membrane–spanning receptors: activation of phospholipase C and/or stimulation/inhibition of adenylate cyclase. All of the P2Y receptors are activated by ATP, but at 2 of them, P2Y4 and P2Y6, UTP is more potent,33-36 and at P2Y2 ATP and UTP are equipotent.31 At P2Y1, UTP is inactive and ADP is reported to be equipotent or even more potent than ATP37,38; at P2Y11 ATP is more potent than ADP and UTP is inactive.39 With respect to the signal transduction pathway, P2Y1 and P2Y2 are coupled to stimulation of phospholipase C-β and inhibition of adenylate cyclase via Gq/11 and Gi proteins, respectively.2 There are reports suggesting that P2Y2 can also trigger stimulation of phospholipase D and breakdown of phosphatidylcholine, but the mechanism is unclear.40 P2Y4 and P2Y6 seem to only couple to phosphoinositide breakdown, whereas P2Y11rather surprisingly stimulates activation of both the phosphoinositide and the adenylate cyclase pathways.
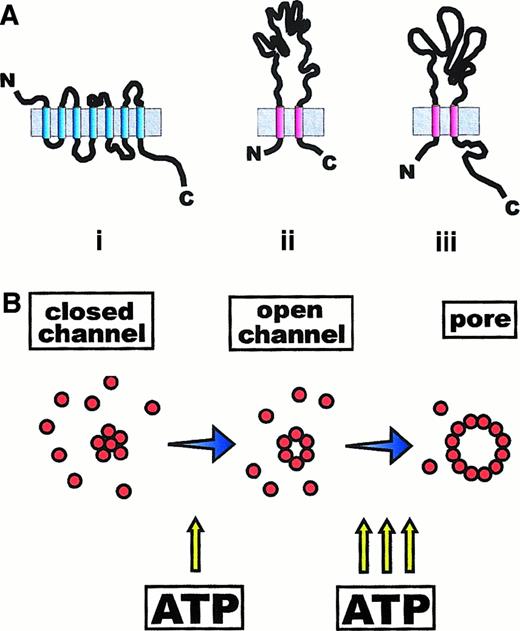
Membrane topology of P2Y and P2X receptor subunits.
(A) P2Y receptors (i) are typical 7-membrane–spanning receptors made of a single polypeptide chain, with the N- and C-termini on the external and cytoplasmic side of the plasma membrane, respectively. P2X receptors (ii) are formed by subunits that span the plasma membrane twice and have both the N- and C-termini on the cytoplasmic side. The P2X7 subunit differs from the other members of the P2X subfamily (P2X1-P2X6) in the extended carboxyterminal tail (iii). (B) It is hypothesized that P2X7 receptor is generated by the aggregation of an unknown number of subunits (maybe 6) to form an ATP-activated channel. Recruitment of additional subunits causes formation of a nonselective pore (also see Figure 2).
Membrane topology of P2Y and P2X receptor subunits.
(A) P2Y receptors (i) are typical 7-membrane–spanning receptors made of a single polypeptide chain, with the N- and C-termini on the external and cytoplasmic side of the plasma membrane, respectively. P2X receptors (ii) are formed by subunits that span the plasma membrane twice and have both the N- and C-termini on the cytoplasmic side. The P2X7 subunit differs from the other members of the P2X subfamily (P2X1-P2X6) in the extended carboxyterminal tail (iii). (B) It is hypothesized that P2X7 receptor is generated by the aggregation of an unknown number of subunits (maybe 6) to form an ATP-activated channel. Recruitment of additional subunits causes formation of a nonselective pore (also see Figure 2).
Investigation of P2Y receptors has been severely hindered by the lack of specific antibodies, whether polyclonal or monoclonal. Likewise, few selective agonists, besides naturally occurring nucleotides, or antagonists are available. A widely used P2Y antagonist is suramin,41 a drug originally developed for the treatment of tripanosomiasis. However, suramin does not discriminate between P2Y and P2X and has been reported to inhibit other receptors such as the nicotinic, glutamate, GABA, and 5-hydroxytryptamine receptors as well as the activity of diverse growth factors.2 Reactive blue 2, trypan blue, and reactive red have also been used as P2Y antagonists, but they also block P2X-dependent responses.2Recently Harden and coworkers have introduced a number of nucleotide analogues as competitive P2Y1antagonists.42 43 Pyridoxal phosphate (P5P) and pyridoxalphosphate-6-azophenyl 2′,4′-disulfonic acid (PPADS) are also sometimes used to inhibit P2Y-dependent responses, but they are more widely employed to block P2X receptors.
P2X receptors
P2X receptors are ATP-gated ion channels—originally cloned and characterized in excitable cells44,45 and then shown to be nearly ubiquitous24,46,47—that mediate fast permeability changes to monovalent and divalent cations (Na+, K+, and Ca++). One of the members of this subfamily, P2X7, has sparked vivid interest for its peculiar ability to undergo a progressive increase in size that leads to the generation of a nonselective membrane pore22,48-50(Figures 1 and 2). All P2X receptors are likely to be multimeric structures of which 7 basic subunits have been cloned. The subunit composition of the native receptors has been resolved only in one case: rat dorsal ganglia that express a P2X2/P2X3 heteromer.51 It is not known how P2X receptors assemble for most cell types. Subunit stoichiometry is likewise unknown but for P2X1 and P2X3, which have been shown to assemble as trimers or hexamers.52 Whether other P2X receptors also assemble according to the same stoichiometry is not known. P2X receptors range from 379 to 595 amino acids and are thought to have 2 transmembrane hydrophobic domains separated by a bulky extracellular region harbouring 10 cysteines and 2 to 6 N-linked glycosylation sites.2,30,44,45 The aminotermini and carboxytermini are both on the cytoplasmic side of the plasma membrane. This tertiary structure and membrane topology is reminiscent of that of other ion channels such as the epithelial amiloride-sensitive Na+channel (ENaC), the degenerins cloned from Caenorhabditis elegans, and the inward rectifying K+ channel (Kir).30 Signal transduction occurs via fast Na+ and Ca++ influx and K+ efflux, leading to depolarization of the plasma membrane and an increase in the concentration of cytosolic Ca++([Ca++]i). It is likely that the drastic upset in intracellular ion homeostasis caused by P2X receptor opening activates several additional intracellular messengers and enzyme pathways, but few studies are available on this novel and exciting field of P2X receptor biochemistry. Electrophysiologic investigation of recombinant P2X receptor subunits transfected into mammalian recipient cells has allowed identification of fast desensitizing and slowly desensitizing (or nondesensitizing) P2X receptors.53
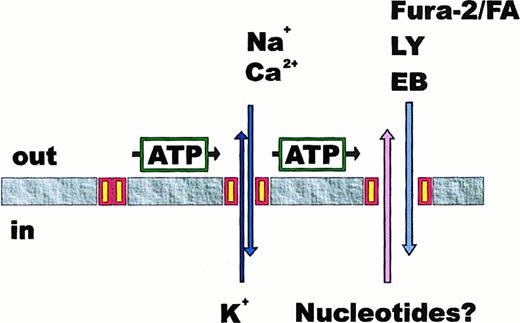
Permeability transition of P2X7 receptor.
A transient stimulation with ATP causes the opening of the P2X7 channel and the concomitant Ca++ and Na+ influx and K+ efflux. However, upon sustained stimulation with ATP, the P2X7 receptor undergoes a transition that generates a reversible membrane pore permeable to several low-molecular-weight hydrophylic solutes (Fura-2/FA, Fura-2 free acid; LY, lucifer yellow; EB, ethidium bromide) and to nucleotides.
Permeability transition of P2X7 receptor.
A transient stimulation with ATP causes the opening of the P2X7 channel and the concomitant Ca++ and Na+ influx and K+ efflux. However, upon sustained stimulation with ATP, the P2X7 receptor undergoes a transition that generates a reversible membrane pore permeable to several low-molecular-weight hydrophylic solutes (Fura-2/FA, Fura-2 free acid; LY, lucifer yellow; EB, ethidium bromide) and to nucleotides.
Although still on a limited basis, a few anti-P2X antibodies were made available over the last 2 years by single laboratories or commercial sources. Polyclonal antibodies against P2X1, P2X4, and P2X7 can be obtained from at least 2 companies; in a few laboratories sera against all the members of the subfamily have been raised.53,46,49,54 One monoclonal antibody selective for the human P2X7 receptor has been produced and characterized by Buell and colleagues.55 Interestingly, this monoclonal antibody, which recognizes an as yet to be identified epitope on the extracellular domain, inhibits activation of human macrophages by 3′-O-(4-benzoyl)benzoyl-ATP (BzATP), a P2X7agonist.55
The unique naturally occurring agonist of P2X receptors is ATP, albeit diadenosine polyphosphates, such as P,1P4-diadenosine tetraphosphate (Ap4A) and P,1P6-diadenosine hexaphosphate (Ap6A), are active at P2X1 2, and UTP has been reported to be an agonist at P2X3 as well as P2X1.56,57 There is an ongoing debate, initiated by the pioneeristic experiments of Cockcroft and Gomperts in mast cells,58,59 on whether P2X receptors recognize the bianionic (ATP2−) or tetra-anionic (ATP4−) form of the nucleotide. In physiologic solutions, the free acid ATP4− is complexed by Mg++, Ca++, or H+ to yield various mixtures of MgATP2−, CaATP2−, and HATP3−, whereas a small amount (1%-10%, depending on the divalent cation concentration and the pH) is present as the fully dissociated tetra-anion. Removal of Mg++ and Ca++ and alkalinization of the medium increases the apparent affinity of ATP and BzATP for the native P2Z receptor of mast cells and other cell types58-61 and for the recombinant P2X7 receptor,48,62 but addition of Mg++ quickly terminates stimulation. Interpretation of the effects of divalent cation removal is complicated by the concomitant inhibition of ecto-ATPase/ectonucleotidase activity, because these enzymes require the presence of divalent cations. Slowing of hydrolytic activity prolongs the half-life of the nucleotide, thus increasing its apparent potency. This may explain the finding that the potency of stable ATP analogues, such as α, β-methylene ATP (α, β MeATP), is unaffected by Ca++ and Mg++removal.63 This ATP analogue is used to pharmacologically discriminate P2X receptor subtypes: P2X1 and P2X3 are sensitive to low (0.5-5 μM) concentrations, and P2X2 and P2X4-7 are activated by high (>100 μM) doses.2 An often neglected finding is the high potency of BzATP at all P2X—not just P2X7—receptors and its agonist activity at P2Y receptors, a feature that makes this ATP analogue one of the most useful tools for the study of native and recombinant P2X receptors.53
Better antagonists, with better-characterized activity, are available at P2X than at P2Y receptors. PPADS is a noncompetitive inhibitor of most P2X receptors53 and, depending on the experimental conditions, may act irreversibly. Oxidized ATP (oATP) was introduced 7 years ago as a selective P2Z (P2X7) inhibitor,64 but it is likely to show the same P2X antagonist selectivity of PPADS, although no detailed investigation has been carried out. At the effective concentrations (100-300 μM), oATP shows little or no inhibitory activity at P2Y receptors and at ectonucleotidases.64 Action of oATP on ectokinases has not been tested in depth; thus it cannot be excluded that some effects of this compound may be due to inhibition of ectophosphorylation.65 PPADS and oATP likely share the same mechanism of action. Both compounds have aldehyde groups (1 PPADS, 2 oATPs) that can react with unprotonated lysines to form Schiff's bases. It is assumed that they preferentially modify lysine residues in the vicinity of the ATP binding site, but this assumption is yet to be proved. Although PPADS has been used as a P2 blocker for some time, it was only after the introduction of oATP that attention has been paid to the time-dependent and irreversible inhibitory effect of this P5P derivative. Time-dependent and irreversible block is extensively documented for oATP at the P2X7 receptor: A 1- to 2-hour preincubation with this inhibitor, even if followed by extensive rinsing, makes all cells so far investigated fully refractory to ATP stimulation via the P2X7receptor.64 66-69 Refractoriness lasts several hours, until new receptors are inserted into the plasma membrane.
More recently, Wiley and colleagues have introduced another powerful blocker of P2X7, compound 1-[N,0-bis(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine (KN-62).70 This molecule was originally used as an inhibitor of the calcium calmodulin–dependent kinase71and made its first appearance in the purinergic field in a study by Blanchard et al72 aimed at investigating the role of the P2Z receptor in cell-mediated cytotoxicity. KN-62 acts as a competitive inhibitor at nanomolar concentrations and shows a striking species specificity: It is active only at the human and not at the rat or mouse P2X7 receptor.73 KN-62 is a very useful tool for short-term studies, but modification of long-term responses should be interpreted with caution because of concomitant inhibition of calcium calmodulin kinase. Surprenant and colleagues74have recently shown that Coomassie Brilliant Blue G selectively blocks rat P2X7 with nanomolar affinity.
P2 receptors in monocyte/macrophages
Early studies by Steinberg and Silverstein showed that the J774 mouse macrophage cell line expressed a plasma membrane receptor selectively activated by ATP and a few analogues.60,75Stimulation of this receptor triggered the same reversible increase in plasma membrane permeability to low-molecular-mass solutes originally described by Cockcroft and Gomperts in rat mast cells.58,59 An intriguing finding of these studies was that stimulation of the ATP-permeabilizing receptor eventually led to cell death.75 This incidental observation stirred interest in the possible physiologic meaning of ATP-dependent cytotoxicity and fostered subsequent studies on the role of P2 receptors in the immune system. At about the same time, Greenberg et al demonstrated that J774 macrophages also expressed P2Y-like receptors coupled to Ca++ mobilization via a mechanism other than the ATP-permeabilizing receptor.76 This was made possible by the selection by Steinberg and colleagues75 of ATP-resistant J774 macrophage clones later shown to lack the P2X7 receptor.77 78
According to the nomenclature proposed by Gordon,4 the macrophage-permeabilizing receptor was named P2Z, analogously to the mast cell and lymphocyte ATP receptor. The receptor responsible for ATP-dependent permeabilization has been referred to as P2Z until very recently and, even after the cloning of P2X7 and the demonstration that its transfection confers susceptibility to ATP-dependent permeabilization, some investigators prefer the P2Z nomenclature to indicate the native ATP-permeabilizing receptor, because it is not clear whether P2X7 is the only constitutive subunit or, rather, the native P2Z receptor is formed by the assembly of P2X7 in association with other P2X subtypes. However, because P2X7 reproduces all known effects of the native P2Z and cells resisting ATP-mediated permeabilization lack P2X7, we will assume heretofore that the macrophage P2Z and P2X7 receptors are the same molecule. As seen below, the picture is more complex in lymphocytes and other cells that do not undergo the typical ATP-dependent permeabilization, although they may express P2X7.
P2Y and P2X subtype expression by macrophages
All murine macrophage lines so far investigated express P2Y receptors coupled to release of Ca++ from intracellular stores and IP3 generation, but the individual subtypes have not been investigated in detail. Functional and molecular expression of P2X7 has been shown in some murine cell lines and in mouse and rat peritoneal macrophages.60,75,79-83Monocyte-derived human macrophages are susceptible to ATP-mediated permeabilization and express P2X7.66,84,85Among human macrophage lines, THP-1 and U937 cells express P2Y receptors (P2Y2, P2Y4, and P2Y6),5,86-88 but only the THP-1 monocytic cell line has been reported to express P2X7 to a significant level.88 However, P2X7 receptor expression can differ significantly among cell batches propagated in different laboratories. Monocytes freshly isolated from peripheral blood express P2Y receptors but lack P2X7, whether investigated at the molecular or at the functional level. Although a few studies are available, it is generally agreed that, at the most, 15% to 17% of human monocytes undergo the plasma membrane permeability transitions diagnostic of P2X7 expression when stimulated with ATP.66,84 There appears to be an inverse correlation between P2Y2 and P2X7 expression during monocyte to macrophage maturation: P2Y2 messenger RNA (mRNA) declines while P2X7 mRNA increases.89 Up-regulation of P2X7 and acquisition of P2X7-dependent responses are detectable within 24 hours of seeding human monocytes on plastic dishes. Up-regulation of P2X7 and down-regulation of P2Y2 by the inflammatory mediators interferon-γ and tumor necrosis factor (TNF)-α and by lipopolysaccharide (LPS) have been reported.66,89 In addition, phorbol myristate acetate causes a decrease in P2Y2 mRNA in THP-1 cells.90
Role of P2 receptors in monocyte/macrophage physiology
The first report on the effect of exogenous nucleotides on macrophage function was a paper by Cohn and Parks.91 In this study the authors showed that addition of adenine nucleotides to a mouse macrophage culture resulted in a dramatic increase in pinocytic vesicle formation. After this early study, exogenous nucleotides as a stimulant for macrophages were basically neglected for several years and resurrected only in 1985 by Silverstein and coworkers,92 who reported that extracellular ATP inhibited Fc receptor–mediated phagocytosis and at the same time caused influx of Na+, efflux of K+, and an increase in [Ca++]i. In this study it was also for the first time suggested that macrophages expressed receptors specific for ATP. The possibility that these ATP effects could be due to ATP hydrolysis by plasma membrane ecto-ATPase was ruled out by subsequent papers by Steinberg and Silverstein60,75,76 that reported an in-depth characterization of the macrophage-permeabilizing ATP receptor. It was also soon clear that the ATP receptor coupled to release of Ca++ from intracellular stores (P2Y) and the ATP-permeabilizing (P2Z/P2X7) receptor were 2 separate entities with widely different nucleotide selectivity and affinity and likely involved in different responses.76 In J774 macrophages, the concentration of ATP giving one half of the maximal response (EC50) for Ca++ release from intracellular stores (and which therefore reflects activation of P2Y) is in the range of 50 to 70 μM. In microelectrode impalement experiments, the ATP EC50 for depolarization, presumably reflecting opening of P2X7, was reported to be between 250 and 400 μM,93 but a lower EC50 was reported for P2X7-triggered Ca++ rise in thioglycollate-elicited mouse peritoneal macrophages.94However, determinations based on the measurement of uptake of fluorescent markers give higher EC50 (1.0-1.5 mM ATP) for the activation of the native mouse P2X7receptor.76,95 The UTP EC50 for Ca++ release from intracellular stores is between 300 and 500 nM 76 and thus much lower than the ATP EC50.76 94 This suggests that macrophages express P2Y4 or P2Y6 or an endogenous yet to be identified uridine nucleotide–specific receptor. Therefore, it is clear that should ATP release occur in a tissue, macrophage P2Y receptors are likely to be activated more easily and more frequently than P2X7.
An early and, with hindsight, obvious proposal was that macrophages and, in general, inflammatory cells, could use P2Y receptors as very sensitive sensors of cell and tissue damage.76 After all, mammalian cells contain huge amounts (5-10 mM) of ATP in their cytosol; thus, any event that causes even a transient break in the plasma membrane will cause release of ATP into the pericellular environment. Furthermore, it is becoming apparent that frank cell injury or death might not even be necessary for ATP release because shear stress forces and stretching are also powerful stimuli for ATP leakage.8-12 J774 macrophages chemotact in response to micromolar concentrations of ADP but, rather intriguingly, not of UTP.96 Human macrophages in the vicinity of dying K562 cells have been shown in vitro to undergo an increase in [Ca++]i that can be closely mimicked by the addition of cell lysate or of ATP at micromolar doses.97Precedent treatment with the cell lysate made the macrophages refractory to the subsequent application of ATP, suggesting, although not proving, that a substance contained in the lysate and ATP might converge on the same receptor. Thus it can be hypothesized that ATP and other intracellular nucleotides function as early alarm signals that alert macrophages of even minor cell and tissue damage (a response could be elicited with as little as 100 nM ATP) (Figure3).
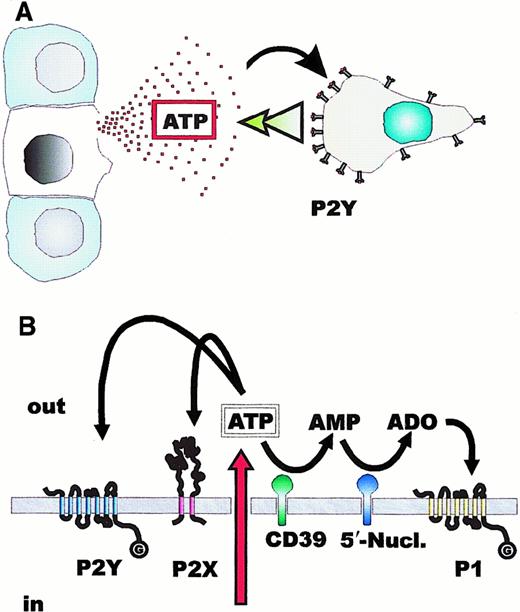
Fate of released ATP: possible role in leukocyte chemotaxis.
(A) The intracellular ATP concentration is in the 5 to 10 mM range; thus, an ATP gradient capable of driving leukocyte chemotaxis by acting at P2Y receptors is likely to occur at sites of cell or tissue damage. (B) ATP released into the pericellular milieu can either ligate P2Y or P2X receptors or be hydrolyzed by plasma membrane ecto-ATPases or ecto-ATP diphosphohydrolase (CD39). Hydrolysis of AMP by 5′-nucleotidase generates adenosine that activates P1 purinergic receptors.
Fate of released ATP: possible role in leukocyte chemotaxis.
(A) The intracellular ATP concentration is in the 5 to 10 mM range; thus, an ATP gradient capable of driving leukocyte chemotaxis by acting at P2Y receptors is likely to occur at sites of cell or tissue damage. (B) ATP released into the pericellular milieu can either ligate P2Y or P2X receptors or be hydrolyzed by plasma membrane ecto-ATPases or ecto-ATP diphosphohydrolase (CD39). Hydrolysis of AMP by 5′-nucleotidase generates adenosine that activates P1 purinergic receptors.
The [Ca++]i rise could also be exploited by the macrophages for the potentiation of antimicrobial defense mechanisms. Nucleotides by themselves are unable to activate the macrophage NADPH oxidase but enhance superoxide generation stimulated by phagocytosable particles.98 It is conceivable that P2 receptors could also be used as an amplification system to spread the alarm by generating additional inflammatory mediators. In murine and human macrophages, extracellular ATP triggers release of TNF-α and interleukin (IL)-1β. P2 receptors involved in TNF-α release have not been identified and there is accordingly little insight into the molecular mechanism involved. An early report in Raw 264.7 murine macrophages99 suggests that ATP might act by enhancing the level of TNF-α mRNA, and this would not require priming by other proinflammatory factors, but a much more detailed investigation is clearly needed.
Participation of P2 receptors in IL-1β secretion is more firmly established and dissected mechanistically. It has been known for a while that LPS-dependent release of the proinflammatory cytokine IL-1β from macrophages and microglial cells, in contrast to peripheral blood monocytes, is a slow and inefficient process that leads to extracellular accumulation of minute amounts of this cytokine and mainly of the high-molecular-weight (31-34 kd), uncleaved, biologically inactive, procytokine form.100,101 This finding has led many investigators to postulate that a second stimulus is needed to trigger processing and secretion of the cytokine in its low-molecular-weight (17 kd) biologically active form, but the identity of this second stimulus has remained elusive. In 1991 Hogquist and colleagues observed that extracellular ATP triggered IL-1β processing and release,102 and in 1992 Gabel and coworkers reported that mature IL-1β formation could be induced by the K+ionophore nigericin.101 What is in common between nigericin and ATP? Perregaux and coworkers101 reasoned that both nigericin and ATP decrease intracellular K+levels60 and that perhaps this ionic perturbation was needed to activate the enzyme that cleaves pro–IL-1β into mature IL-1β, ie, IL-1β–converting enzyme (ICE), also known as caspase-1.103 Later studies fulfilled this prediction because ATP was shown to trigger IL-1β release via a nonlytic mechanism in many different mononuclear phagocytic cells, and release was inhibited by procedures that prevented K+ efflux85,104-106 (Figure 4). In support of a key role for K+ in ICE activation, Cheneval et al107 have shown that a reduction in the K+concentration leads to proteolytic cleavage of isolated recombinant ICE. Interestingly, although proteolytic activation of the isolated enzyme could be induced by a reduction in the concentration of other cations besides K+, autoprocessing of cytoplasmic ICE showed an absolute requirement for K+depletion.107 That ATP acts via ICE is also demonstrated by the ability of a specific ICE inhibitor, the tetrapeptide YVAD (Tyr-Val-Ala-Asp), to block ATP-dependent IL-1β maturation.106,108 Furthermore, macrophages isolated from mice deficient in ICE were unable to process pro–IL-1β upon challenge with LPS plus ATP.109 Finally, involvement of P2X7 in ATP-mediated ICE activation is supported by (a) agonist and antagonist profile of cytokine release,85 (b) blockade by a specific anti-P2X7 monoclonal antibody,55 and (c) detection of ICE proteolytic fragments (p20 and p10) in ATP-stimulated microglial cells.106,110 There are no clues as to how a decrease in K+ concentration may activate ICE autoprocessing; nevertheless, K+ provides a straightforward link between P2X7 and ICE because opening of the P2X7 channel/pore provides a very fast pathway for K+ efflux.60,104 It would be interesting to test whether the same K+-based mechanism of activation also applies to other caspases and how this may be involved in apoptosis.111
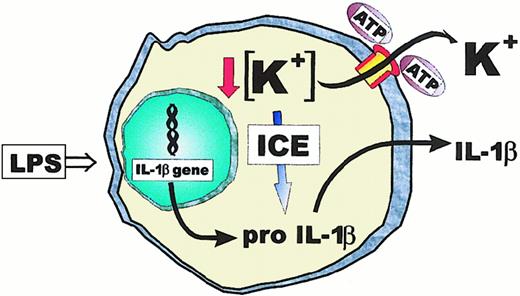
Model for P2X7-mediated ICE/caspase-1 activation and IL-1β maturation.
Stimulation with LPS triggers IL-1β gene transcription and pro–IL-1β accumulation. Opening of the P2X7 pore by extracellular ATP causes a large K+ efflux that triggers proteolytic activation of ICE and cleavage of pro–IL-1β. Mature IL-1β is then secreted through an unknown pathway.
Model for P2X7-mediated ICE/caspase-1 activation and IL-1β maturation.
Stimulation with LPS triggers IL-1β gene transcription and pro–IL-1β accumulation. Opening of the P2X7 pore by extracellular ATP causes a large K+ efflux that triggers proteolytic activation of ICE and cleavage of pro–IL-1β. Mature IL-1β is then secreted through an unknown pathway.
In human monocytes, ATP is a powerful stimulus not only for caspase-1 activation but also for the externalization of mature caspase-1 subunits.112 The meaning of this novel observation is elusive, but it may point to a possible function of activated caspase-1 either in the extracellular space or on the outer leaflet of the plasma membrane. In addition, ATP might trigger IL-1β release by alternative mechanisms, eg, by inducing exocytosis of IL-1β–containing specialized vesicles (late endosomes or lysosomes), as recently suggested by Rubartelli and coworkers.113 It is possible that the LPS signal for IL-1β release consists, at least in part, in an autocrine/paracrine stimulation mediated by ATP secretion, as suggested by studies in human and mouse macrophages and mouse microglia 9 114
Participation of P2X7 in LPS-dependent activation of immune cells might have very interesting and far-reaching practical applications in the treatment of sepsis caused by gram-negative bacteria. In 1994 Proctor and colleagues115 showed that the ATP analogue, 2-methylthio-ATP (2-MeS-ATP), inhibited endotoxin-stimulated release of toxic mediators such as TNF-α and IL-1β and protected mice from endotoxin-induced death. Interpretation of this early experiment is not straightforward, because 2-MeS-ATP is an agonist at P2Y as well as P2X purinoceptors2,3; however, at P2X7 2-MeS-ATP acts as a partial agonist and thus it is conceivable that it might antagonize P2X7stimulation by locally secreted ATP and reduce LPS-dependent TNF-α and IL-1β release. Altogether, these observations suggest that P2X7 (and maybe other P2 receptors) take part in some crucial but as yet unknown steps in the complex chain of events leading to septic shock, either as a component of a paracrine/autocrine loop9 or as a binding site for LPS.115 116
Stimulation with extracellular nucleotides also switches on the inducible nitric oxide synthase (iNOS),116-118 a key enzyme for the bactericidal activity of macrophages. Nucleotides per se are ineffective, but coexposure to low doses of ATP (or UTP) and LPS produces a much higher stimulation of iNOS activity compared with LPS alone. In murine Raw 264.7 macrophages a prolonged (18 hours) incubation was needed to elicit nitrite release, suggesting that P2 stimulation acted by increasing iNOS gene expression rather than by increasing enzyme activity. Other data suggest that P2 receptors are involved in NO generation in a rather more complex fashion. Denlinger and coworkers showed that pretreatment with 2-MeS-ATP prevented iNOS expression and NO generation due to the subsequent addition of LPS,117 raising the issue of the possible participation of P2 receptors in LPS-dependent signaling.116,117 In addition, it has been recently shown that NO production due toMycobacterium tuberculosis infection also occurs in P2X7 knockout mice and it is inhibited by P2 blockers,119 thus pointing to the participation of other P2X and P2Y receptors. There are an increasing number of papers suggesting that P2 receptors (namely P2X7) might have a role in endotoxin- or parasite-mediated macrophage stimulation. Besides the studies carried out in our laboratory showing that incubation of macrophages or microglia with oATP or apyrase inhibited LPS-dependent IL-1β release,9 other groups have reported that LPS-dependent NO release and nuclear factor (NF)-κB and mitogen-associated protein kinase (MAPK) activation are profoundly inhibited by oATP or by PPADS.116 MAPK in Raw 264.7 macrophages can also be stimulated via P2Y, but the putative purinergic receptor involved in LPS-dependent activation does not seem to be a member of the P2Y family because oATP or PPADS, which block LPS-dependent stimulation, do not affect MAPK stimulation by UTP.116 In the light of the report that ATP triggers NF-κB activation via P2X7 and that this activation is blocked by oATP,110 it is likely that the P2 receptor that participates in LPS-dependent macrophage activation is P2X7.
A common event observed in many reactions involving mononuclear phagocytes is multinucleation: often during chronic inflammatory reactions macrophages differentiate into epithelioid cells that eventually fuse into large polykarions named multinucleated giant cells (MGCs).120 Furthermore, in the bone, osteoclast precursors normally fuse to generate large elements with increasing bone resorption activity. MGCs are a common finding of widespread infectious diseases such as tuberculosis, but little is known about the molecular mechanism underlying fusion. In 1995, Falzoni et al66suggested that the P2X7 receptor could be involved in MGC formation. Monocyte-derived human macrophages can be induced to fuse in vitro by incubation with concanavalin A or phytohemagglutinin, provided that contaminating lymphocytes are also present.121Pretreatment with oATP fully inhibits this process, although other responses such as concanavalin A–dependent [Ca++]i changes, chemotaxis, or expression of plasma membrane molecules thought to take part in cell fusion (eg, CD11a, CD18, and CD54) are unaffected.66 We have extended these studies to J774 macrophages and selected several clones that either express P2X7 to a very high level (P2X7plus) or lack it altogether (P2X7less). P2X7plus cells spontaneously fuse in culture to form MGCs of different size and shape, containing from a few to 20 or more nuclei.77 A monoclonal antibody raised against the P2X7 outer domain prevents fusion of human macrophages in culture.122 123
The participation in ICE activation and IL-1β release, and in MGC formation establishes an intriguing link between the P2X7 receptor and chronic inflammation. Experiments from Molloy et al124 and Lammas et al125 further strengthen this link. Both groups investigated the effect of extracellular ATP on macrophage cultures infected withMycobacterium bovis (bacille Calmette-Guérin) and reported that P2X7 activation caused killing of the phagocyte as well as of the intracellular pathogen. The mechanism involved is unknown, but a recent paper suggests that it might require activation of phospholipase D.126 Another possibility is that the known vesiculating activity of ATP91,127,128affects viability of the intracellular pathogen by increasing phagosome-lysosome fusion, as suggested by some of the electron microscopy images reported by Molloy et al.124 In macrophages, stimulation of a phosphatidylcholine-selective phospholipase D by P2X7 agonists was reported as early as 1992 83 and shown to be independent of pore formation and of the ensuing Ca++ influx.129 The mechanism by which an activated phospholipase D might partake in parasite killing is unknown but might be related to the enhanced rate of vesicle fusion observed in ATP-stimulated phagocytes. Ability of macrophages to eliminate intracellular parasites is enhanced upon activation with interferon-γ125; thus it might not be a coincidence that this cytokine and other proinflammatory factors also up-regulate P2X7 expression.66 130 It is also intriguing that to kill the intraphagosomal parasite, ATP concentrations cytotoxic for the macrophage need to be used or, in other words, parasite killing appears to be a consequence of macrophage death, as if intracellular parasite elimination via P2X7 was not a primary function of the receptor but rather an accessory consequence of its primary cytotoxic activity.
That extracellular ATP is a potent cytotoxic factor for macrophages was immediately apparent as soon as a thorough investigation of ATP receptors was started in these cells, and P2X7 was quickly identified as the culprit. Initially in Silverstein's and later in our laboratory, murine macrophage clones were selected that showed an almost absolute refractoriness to ATP-mediated cytotoxicity.60,75,76,95 These cells showed a normal mobilization of Ca++ from intracellular stores in response to ATP, but no permeabilization of the plasma membrane, and accordingly lacked reactivity to anti-P2X7 antibodies.77Blockade of the P2X7 receptor by oATP or KN-62 abrogated ATP-dependent cytotoxicity. The role of P2 receptors in cytotoxicity is usually tested in the presence of exogenous ATP, but it cannot be excluded that ATP spontaneously released by cell monolayers may provide a chronic cytolytic stimulus by acting as an autocrine/paracrine factor. We have tested this hypothesis in P2X7plus J774 macrophage cultures grown to confluence. These macrophage clones show an unusually high rate of spontaneous cell death that can be significantly reduced by pretreatment with oATP or coincubation with apyrase or hexokinase.131 In contrast to the P2X7plus clones, the P2X7less cells have a low rate of spontaneous death that is not affected by the presence of P2X7 blockers or ATP-hydrolyzing enzymes. The mechanism of ATP-dependent death can be either necrosis or apoptosis, depending on the length of incubation in the presence of the nucleotide and the dose. In our hands, ATP-pulsed J774 macrophages appear to die mostly by colloido-osmotic lysis; on the contrary, monocyte-derived human macrophages, which incidentally are more resistant to ATP-mediated cytotoxicity, are prone to die by apoptosis.124,125 It is possible that the propensity of these cells to die by apoptosis is related to their lower susceptibility to ATP because we have previously observed132 that, to set in motion the complex machinery involved in apoptosis, a certain amount of time is needed that is clearly unavailable in those cells that are so sensitive to ATP as to decease quickly. An in-depth investigation of the apoptotic pathways triggered by ATP in macrophages has not been yet carried out, but we know from work in microglial cells that caspase-1, -3, and -8 are activated with the subsequent cleavage of the caspase substrates PARP (poly [ADP-ribose] polymerase) and lamin B.106,133In addition, the crucial transcription factors, NF-κB and NFAT (nuclear factor of activated T cells), are also stimulated.110 134
P2 receptors in dendritic cells
Dendritic cells are a newcomer in the purinergic field. It has been known for a while that epidermal Langerhans' cells posses a powerful plasma membrane formalin-resistant ecto-ATPase that has been used as a histochemical marker,135 but their physiologic function was never understood. In 1993 Girolomoni and coworkers136 demonstrated that human epidermal Langerhans' cells can be permeabilized by ATP, albeit to a lesser degree than human keratinocytes or J774 macrophages. However, inhibition of ecto-ATPase greatly enhanced sensitivity to ATP, and this led these authors to suggest that one of the possible physiologic functions of this ectoenzyme was protection of Langerhans' cells against the noxious effects of extracellular ATP. Scattered reports have then followed suggesting that phagocytic cells of the thymus reticulum express a P2X7-like ATP-permeabilizing receptor,137 but only during the last few years has a systematic study of these receptors been carried out in human and mouse dendritic cells.138-143
Human dendritic cells were found to express mRNA for P2X1, P2X4, P2X5, and P2X7 as well as for P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors.140 Immunohistochemistry with an anti-P2X7 monoclonal antibody performed in human tonsils shows that a cell population of the marginal zone identified as dendritic cells heavily expresses P2X7.55Scanty pharmacologic data suggest that at least P2Y1, P2Y2, and P2Y4 are functional and mediate a Ca++ signal in these cells.139P2X7 functions have been investigated in detail in human and mouse dendritic cells, and available evidence suggests that this receptor mediates cytokine release and might also particpate in antigen presentation.141,142 During their investigation of P2Y receptors in human dendritic cells, Liu et al139 observed that dendritic cells redirect their dendrites toward a nearby patch pipette leaking ATP, an incidental finding that might suggest that dendritic cells, like other mononuclear phagocytes, exhibit a P2Y-mediated chemotactic response to ATP. In addition, it has been shown that stimulation with UTP or uridine diphosphate (but surprisingly not with ATP) provided a potent stimulus for the cytokine gene transcription and secretion.144 Given the high expression of P2X7, it is not surprising that dendritic cells are exceedingly sensitive to the cytotoxic activity of ATP and readily die by apoptosis.141 145 Whether this may have relevance in the overall process of modulation of the immune response is presently unknown.
P2 receptors in lymphocytes
Lymphocyte responsivity to nucleotides has been known for many years: In 1978 Gregory and Kern reported that extracellular ATP stimulated proliferation of mouse thymocytes146; Fishman et al in 1980 observed that in human peripheral lymphocytes ATP suppressed proliferation,147 presumably via generation of adenosine. In 1981 Ikehara et al148 in some way reconcilied these contrasting observations by showing that ATP stimulation of DNA synthesis was observed in lymphoid cells from the thymus and inhibition in cells from spleen, lymph nodes, and peripheral blood. These early observations were followed by a few other studies that overall were of little help in building a coherent picture of the responses of lymphoid cells to extracellular nucleotides, and they remained basically anecdotal.149,150 It was not until the end of the 1980s that a systematic approach to the study of purinergic receptor expression and function in lymphocytes was started.151-157
Human B lymphocytes express P2Y receptors, as indicated by the ability of ATP and many other nucleotides (UTP, GTP, CTP, ITP, ADP, adenosine 5′-O-(3′-thiotriphosphate), ATPγS) to trigger Ca++ release from intracellular stores.158Human B lymphocytes also express P2X receptors, although of which particular subtype is still uncertain. Pharmacologic, electrophysiologic, and reverse transcriptase–polymerase chain reaction (RT-PCR) data suggest that the P2X7 receptor is present in these cells and might be up-regulated in chronic leukocytic leukemia cells (quite intriguingly it appears to be also up-regulated in lymphoblastoid cells from patients with Duchenne dystrophy).154,159-161 Rather surprisingly, however, B lymphocytes do not undergo the typical increase in permeability to aqueous solutes up to 900 d, suggesting that a pore of a smaller size, permeable to molecules up to 320 d, is generated by ATP: although ethidium bromide (314 d) is admitted, propidium bromide (414 d) is excluded.159-162 Furthermore, B lymphocytes are also poorly susceptible to ATP-mediated cytotoxicity. Human peripheral T lymphocytes lack P2Y receptors according to functional and pharmacologic studies but express a P2X-like ATP-activated channel.163 Unpublished data from our laboratory show that these cells express at the mRNA level P2X1, P2X4, and P2X7, although a significant variability is observed among samples from different subjects. ATP and BzATP cause in T lymphocytes a large influx of Na+ and Ca++ from the extracellular medium that is fully prevented by pretreatment with oATP. Like in B lymphocytes, ATP treatment of T lymphocytes generates a pore smaller than that seen in macrophages or in HEK293 cells transfected with the recombinant P2X7.48 62 This might be due to the assembly of P2X1 or P2X4 subunits together with P2X7 into the receptor complex, but this is purely speculative.
Expression of P2 receptors in mouse lymphocytes has been more thoroughly investigated. RT-PCR data show that murine thymocytes express the message for P2X1, P2Y1, and P2Y2 receptors and accordingly undergo Ca++release from intracellular stores and an increase in plasma membrane permeability to external cations when challenged with ATP.164-166 Steroid hormones or cross-linking of T-cell receptor (TCR) by anti-TCR monoclonal antibody causes a transient enhancement of P2Y2 mRNA, suggesting that this could be an early event in response to a variety of activatory stimuli.167 Sensitivity to ATP in thymocytes changes depending on the stage of maturation: CD4+CD8−TCRhigh thymocytes were found to be very sensitive to ATP-mediated lysis166; large double-positive proliferating thymocytes were much less responsive compared with those terminally differentiated.168 As in the case of human lymphocytes, the cut-off of the ATP-gated plasma membrane channel for mouse lymphocytes appears to be slightly over 300 d (ethidium is admitted, propidium is excluded).166 Functional and pharmacologic data support expression of P2X7 by mouse lymphocytes,155,156,166 but there is little molecular evidence to corroborate this claim. At variance with human lymphocytes, mouse thymocytes are readily killed by ATP.155,156 In double-positive (CD4+CD8+) immature thymocyte populations, expression of P2X1 correlated with susceptibility to undergo apoptosis upon dexamethasone treatment,164 and incubation in the presence of apyrase blocked the process, as if dexamethasone induced P2X1activation via autocrine/paracrine release of ATP. Ability of extracellular ATP to cause apoptosis of mouse cell lines was initially documented in P-815 mastocytoma and YAC lymphoid cells and subsequentely extended to thymocytes.132,169 Among mouse lymphocytes, cytotoxic T-lymphocyte clones, cytotoxic T lymphocytes from peritoneal exudates, and lymphokine-activated killer cells turned out to be fully insensitive to ATP.132,155 Whether this is due to lack of P2 purinergic receptors or to high expression of ecto-ATPase/ectonucleotidases it is not known. The potent cytotoxic activity of extracellular ATP and the remarkable resistance of cytotoxic T lymphocytes and lymphokine-activated killer cells instigated speculations on the possible participation of ATP receptors in target cell killing, as a pathway alternative or parallel to perforin or lymphotoxin.156,157,170However, it has been very difficult to provide sound experimental support to this hypothesis.171
The role of P2X receptors in the control of lymphocyte proliferation could be more complex than just being effectors of cell death. We have recently examined the effect of transduction of P2X7less human B-lymphoid cells with the P2X7 receptor complementary DNA and have surprisingly found that its expression confers a proliferation advantage in the absence of serum.172 We have not yet dissected the biochemical mechanism underlying the enhanced growth rate of the P2X7 transfectants, but we believe that it involves autocrine stimulation by ATP, because incubation with apyrase or hexokinase or pretreatment with oATP abrogated proliferation of P2X7 transfectants in the absence of serum.172 Whether these observations are relevant for tumors arising from hemopoietic cells is under investigation.
Stimulation with extracellular ATP causes shedding of the cell adhesion molecule L-selectin (CD62L) and the low-affinity receptor for IgE (CD23) from B chronic lymphocytic leukemic cells.173,174These cells express P2X7, and agonist/antagonist studies suggest that the receptor involved is P2X7.162CD23 and L-selectin are normally found in high amounts in sera from patients with B chronic lymphocytic leukemia, and this could be due to ATP-dependent shedding via P2X7 stimulation.
P2 receptors in polymorphonuclear granulocytes
Scattered evidence for a role of extracellular nucleotides in granulocyte responses has been present for a while,175,176but a systematic investigation was only started at the end of the 1980s.177-181 Most studies concentrated on neutrophils, showing that ATP was able to trigger an increase in [Ca++]i, stimulation of phosphoinositide breakdown, superoxide anion generation, and granule exocytosis (both specific and azurophilic).182,183 In human neutrophils, ATP and UTP were reported to be equipotent for both the [Ca++]i increase and superoxide anion formation,178,179 and ATP was also shown to stimulate phospholipase C and diacylglycerol generation as well as protein kinase C activity.183,184 It is of great interest in the light of the proposed proinflammatory role of extracellular ATP that this nucleotide also increases membrane expression of CD11b/CD18 and adhesion to albumin-coated latex beads.185 Because ATP is released by the endothelium and its local concentration is likely to increase during inflammation as a consequence of inactivation of ecto-ATPases by oxygen radicals,186 up-regulation of adhesion molecules by this nucleotide could be of relevance for leukocyte migration across the vessel wall. ATP also enhances the adhesion between neutrophils and pulmonary endothelial cells, a mechanism that might be relevant in syndromes such as adult respiratory distress syndrome and septic shock.187,188 Of course, it cannot be excluded that the ATP effect could be at least in part mediated by its hydrolysis to adenosine,189 but recent data suggest that ecto-ATPase activity has an inhibitory rather than stimulatory effect on granulocyte-endothelium interaction.190 Dubyak and coworkers reported that chronic stimulation with ATPγS or UTP could drive differentiation of myelomonocytic progenitor cells (HL-60 and U937).191 Later studies showed that myeloid progenitors express P2Y1 at earlier and P2Y2 at later stages of differentiation.192 The myeloid progenitors HL-60 cells also express P2 Y11.39
P2 subtype expression has not been thoroughly investigated in neutrophils, mainly because of the lack of selective antibodies. RT-PCR data show that human polymorphonuclear granulocytes express P2Y4 and P2Y6 but not P2Y1 or P2Y2 receptors.5 Among P2X receptors, the presence of P2X7 was shown by Northern blotting and immunocytochemistry.49 It has been suggested that human neutrophils might express receptors for diadenosine polyphosphates,193 but evidence for this is preliminary. Besides neutrophils, eosinophils also express P2 receptors coupled to [Ca++]i increases, actin reorganization, and stimulation of the NADPH oxidase.194,195 Interestingly, eosinophils show locomotive activity in response to ATP, ADP, and GTP.194 No data are available as to the P2 subtypes expressed.
P2 receptors in platelets
ADP is one of the best-known activators of platelet aggregation,25,196-198 but the receptors involved have been, at least partially, identified only during the last 5 years. Stimulation with ADP causes release of Ca++from intracellular stores, Ca++ influx, phospholipase C activation, inhibition of stimulated adenylate cyclase, shape change, activation of fibrinogen receptors, and aggregation.199-201 ATP and ATP analogues are potent inhibitors of these responses. It has also been shown that ADP causes granule release and thromboxane A2production.202,203 It was initially thought that these effects were mediated by only one receptor named P2T; however, later studies led to the molecular and pharmacologic characterization in platelets of at least 2 of the known members of the P2 family: P2X1 204-207 and P2Y1.208,209 With the availability of more selective platelet P2Y1 and P2X1 agonists and antagonist, it is becoming evident that the view that these are the P2 receptors solely responsible of ADP-mediated platelet activation is an oversimplification. It is clear that it is possible to block ADP-mediated inhibition of stimulated adenylate cyclase activity without decreasing the ADP-dependent [Ca++]irise. Thus it is postulated that ADP-triggered platelet activation is mediated by 3 receptors: One not yet cloned receptor (P2TAC) is coupled to inhibition of stimulated adenylate cyclase activity; a second (P2Y1) to phospholipase C activation, InsP3 formation, and Ca++ release from intracellular stores; and a third one (P2X1) to fast Ca++ influx across the plasma membrane.210,211 According to this proposal, the P2TAC receptor would coincide with the plateletP2YADP (written in italics to signify that it is not yet cloned) receptor of the nomenclature established by IUPHAR.2,27 Development of selective platelet P2 receptor antagonists has progressed further than in other cell types, and some have already reached clinical applications. Two thienopyridine compounds, ticlopidine and clopidogrel, inhibit ADP-triggered platelet aggregation presumably by selectively blockingP2YADP. The limited structure-relationship analysis so far carried out suggests that 2-alkylthio–substituted analogues of ATP and AMP (eg, 2-MeS-ATP; 2-methylthioadenylyl 5′-(β,γ-methylene)-diphosphonate [2-MeS-β,γ-Me-ATP]; 2-propylthioadenylyl 5′-(β,γ-difluoromethylene)-diphosphonate [ARL 66096]; and 2-propylthioadenylyl 5′-(β,γ-dichloromethylene)-diphosphonate [ARL 67085]) are selective competitive antagonists at P2YADP, and 3′-substituted AMP analogues (eg, adenosine 3′-phosphate 5′-phosphosulphate [A3P5PS]) are selective P2Y1antagonists.212 The pharmacology of the platelet P2Y1 receptor was clarified when only high-performance liquid chromatography–purified nucleotides were used and care was taken to avoid degradation of triphosphate analogues to the corresponding diphosphates. These precautions are seldom taken in the analysis of nucleotide effects in other cells, and this may lead to a re-evaluation of the agonist activity of ATP in other cell models. It is likely that full platelet activation requires stimulation and cooperative signaling of all 3 receptors, but the initial data from knockout mice suggest a central role for P2Y1, because P2Y1-deficient animals showed increased bleeding time and reduced collagen- and ADP-induced thromboembolism.213Interestingly, ADP-mediated adenylate cyclase inhibition was not reduced in platelets from the p2y1−/− mice. A P2X1-deficient (P2X1−/−) mouse is also available,214 but no data on platelet function in this animal have been published. A patient was described who was affected by what appears to be a selective deficit inP2YADP receptor expression215 and, on the other hand, expression of P2Y1 (and P2X1) was found to be normal in a patient affected by a severe deficiency of ADP-triggered platelet activation.216Presence of a functional ATP-activated P2X1 receptor raises intriguing questions on the interplay among different P2 receptors in platelet physiology, because ATP or ATP analogues were never shown to cause platelet activation. It might be that the P2X1receptor is chronically desensitized in vivo due to continuous leakage of ATP/ADP from blood or endothelial cells, but this issue clearly needs further scrutiny.217 Injury to blood cells or to the vessel wall releases ATP that is quickly dephosphorylated to ADP by ecto-ATPases expressed on the endothelium. Furthermore, platelets themselves are a major source of ATP and ADP that are stored within dense granules to a concentration of about 1 M. Thus, ADP-triggered secretion activates an autocatalytic cycle of autocrine/paracrine stimulation by released nucleotides.218 Release of ATP from platelets can also feed back on the endothelial cells, inducing secretion of other factors involved in hemostasis and inflammation, such as von Willebrand factor.219 The key role of extracellular ATP and P2 receptors in hemostasis has been underscored by the surprising phenotype of cd39/ATP diphosphohydrolase knockout (cd39−/−) mice.220 It was expected that these mice showed a thrombotic diathesis due to enhanced platelet aggregation, because CD39 has been considered an inhibitor of platelet activation. On the contrary,cd39−/−mice displayed prolonged bleeding times and failure to aggregate. These deficits were shown to be due to P2Y1 receptor desensitization dependent on an increased accumulation of extracellular ATP and were largely corrected by apyrase.
P2 receptors in erythrocytes
Effects of extracellular ATP on erythrocytes were initially reported in 1972 by Parker and Snow,221 who showed that this nucleotide caused Na+ influx and K+ efflux paralleled by an increase in water content. As later demonstrated in other cell types, ion fluxes were prevented by Mg2+ or hexokinase plus glucose and potentiated by ethylenediaminetetraacetic acid. All other nucleotides tested were ineffective. An increase in plasma membrane permeability of erythrocytes was also reported by Trams,222 who showed a dramatic accumulation of extracellular adenylates in the presence of extracellular ATP. These authors concluded that ATP caused a permeability change in erythrocyte plasma membrane that allowed for leakage of cytoplasmic ATP (“ATP-induced ATP release”). These data would suggest the expression by erythrocytes of a P2X7-like receptor, but no further characterization of this phenomenon was carried out. Release of ATP under hypoxic conditions has also been reported,223but the pathway involved was not elucidated. At variance with P2X, erythrocyte P2Y receptors are more thoroughly characterized. Avian red blood cells express a typical P2Y1 receptor coupled to phospholipase β activation via a G protein of the Gqfamily.224,225 Erythrocytes are an ideal “integrator unit” in the blood because they express P2 receptors and at the same time readily release ATP. These properties, on the one hand, make these cells sensitive to ATP released by other blood elements (eg, platelets) and, on the other hand, endow them with the ability to modulate the function of circulating or endothelial cells by secreting large amounts of this nucleotide. It has been proposed that ATP release from erythrocytes could contribute to regulation of local blood flow by acting at P2Y receptors on vascular endothelium.226 227ATP has a well-known NO releasing activity; thus, under ischemic conditions, when release from erythrocytes is maximal, ATP could be one of the local factors that counteract the decreased blood flow by inducing vasodilatation.
P2 receptors on bone marrow hemopoietic precursors
According to the few available studies, all hemopoietic precursors isolated from mouse bone marrow, as opposed to stromal cells, are highly sensitive to the cytotoxic effect of ATP.228,229This phenotypic property has made available a very efficient procedure for the isolation of highly purified marrow stromal cells or the deletion of hemopoietic cell precursors. The cytotoxic mechanisms appear to be dependent on the known pore-forming ability of ATP mediated by P2X7 activation and can be significantly enhanced by including in the reaction medium a low-molecular-weight nonpermeant poisonous agent such as potassium thiocyanate.228 229 This procedure might turn out helpful for the local treatment of tumors of hemopoietic origin.
Conclusions
For many years it was thought that receptors for extracellular nucleotides had a physiologic role only in excitable tissues; however, it is now increasingly clear that they are widespread and involved in signal transduction in several other tissues, including blood cells (Table 2). Drugs based onP2YADP antagonism are already in use as antithrombotic agents, and P2Y1 blockers are being developed for this same purpose. Besides thrombosis, another promising field of application of P2 agonist/antagonist is inflammation. Ability of P2 receptors to mediate chemotaxis (via P2Y), or cytotoxic responses and cytokine secretion (via P2X7), opens an entirely new perspective for the development of anti-inflammatory drugs. Chronic inflammatory diseases might be one of the first targets for the clinical application of selective P2X7 antagonists. These compounds might prove beneficial to reduce IL-1β release and granuloma formation. Finally, high expression of P2X7 by lymphocytic leukemia cells, and its participation in the control of cell death and proliferation, suggests a novel and as yet fully unexplored approach to the treatment of lymphoproliferative disorders.
P2 receptors expressed by blood cells
| Cell type . | P2Y . | P2X . | Reference no. . |
|---|---|---|---|
| Rat/mouse peritonel macrophages | P2Y | P2X7 | 49,77, 79-82, 94 |
| BAC1.2F5 macrophages | P2Y | P2X7-like | 83 |
| RAW 264.7 macrophages | P2Y | P2X7-like | 81, 116 |
| J774 macrophages | P2Y | P2X7 | 60,75-77 |
| THP-1 macrophages | P2Y2 | P2X7 | 5,88 |
| KG-1 myeloblastic cells | P2Y1 | 192 | |
| HL-60 myeloid cells | P2Y2, P2Y11 | P2X1 | 39, 192, 232 |
| U937 monocytes | P2Y2, P2Y6 | 5 | |
| Human monocytes | P2Y1, P2Y2, P2Y4, P2Y6 | 5, 89 | |
| Human macrophages | P2Y | P2X7 | 77, 79, 89 |
| FSDC | P2Y | P2X7 | 141 |
| Mouse dendritic cells* | P2X7 | 141 | |
| Human dendritic cells† | P2Y1, P2Y2, P2Y4, P2Y6, P2Y11 | P2X1, P2X4, P2X5, P2X7 | 139, 140, 142 |
| Human Langerhans' cells‡ | P2X7-like | 136 | |
| Human dendritic cells2-153 | P2X7 | 55, 137 | |
| P-815 mastocytoma | P2X7-like | 132 | |
| YAC lymphoma cells | P2X7-like | 132 | |
| Mouse lymphocytes | P2X7 | 49, 155, 166 | |
| Murine thymocytes | P2Y1, P2Y2 | P2X1 | 164, 165, 167,168 |
| Human B lymphocytes | P2Y | P2X7 | 154, 158, 160, 161,162 |
| Human T lymphocytes | P2X1, P2X4, P2X7 | 163, O.R.B. et al, unpublished data, 1999 | |
| Human PMN | P2Y4, P2Y6 | P2X7 | 5, 49 |
| Human platelets | P2Y1 | P2X1 | 204-209 |
| Erythrocytes | P2Y1 | P2X7-like | 224,225 |
| RBL and rat mast cells | P2Y | P2X7-like | 58, 59, 230,231 |
| Mouse hemopoietic precursors | P2X7-like | 228,229 |
| Cell type . | P2Y . | P2X . | Reference no. . |
|---|---|---|---|
| Rat/mouse peritonel macrophages | P2Y | P2X7 | 49,77, 79-82, 94 |
| BAC1.2F5 macrophages | P2Y | P2X7-like | 83 |
| RAW 264.7 macrophages | P2Y | P2X7-like | 81, 116 |
| J774 macrophages | P2Y | P2X7 | 60,75-77 |
| THP-1 macrophages | P2Y2 | P2X7 | 5,88 |
| KG-1 myeloblastic cells | P2Y1 | 192 | |
| HL-60 myeloid cells | P2Y2, P2Y11 | P2X1 | 39, 192, 232 |
| U937 monocytes | P2Y2, P2Y6 | 5 | |
| Human monocytes | P2Y1, P2Y2, P2Y4, P2Y6 | 5, 89 | |
| Human macrophages | P2Y | P2X7 | 77, 79, 89 |
| FSDC | P2Y | P2X7 | 141 |
| Mouse dendritic cells* | P2X7 | 141 | |
| Human dendritic cells† | P2Y1, P2Y2, P2Y4, P2Y6, P2Y11 | P2X1, P2X4, P2X5, P2X7 | 139, 140, 142 |
| Human Langerhans' cells‡ | P2X7-like | 136 | |
| Human dendritic cells2-153 | P2X7 | 55, 137 | |
| P-815 mastocytoma | P2X7-like | 132 | |
| YAC lymphoma cells | P2X7-like | 132 | |
| Mouse lymphocytes | P2X7 | 49, 155, 166 | |
| Murine thymocytes | P2Y1, P2Y2 | P2X1 | 164, 165, 167,168 |
| Human B lymphocytes | P2Y | P2X7 | 154, 158, 160, 161,162 |
| Human T lymphocytes | P2X1, P2X4, P2X7 | 163, O.R.B. et al, unpublished data, 1999 | |
| Human PMN | P2Y4, P2Y6 | P2X7 | 5, 49 |
| Human platelets | P2Y1 | P2X1 | 204-209 |
| Erythrocytes | P2Y1 | P2X7-like | 224,225 |
| RBL and rat mast cells | P2Y | P2X7-like | 58, 59, 230,231 |
| Mouse hemopoietic precursors | P2X7-like | 228,229 |
P2 receptor expression is based on functional and pharmacologic evidence, mRNA detection by reverse transcriptase–polymerase chain reaction, or reactivity with specific antibodies. For P2Y receptors, lack of a subscript indicates that, although functional and pharmacologic data show expression of P2Y receptors, the individual P2Y subtypes have not been yet identified. For P2X receptors, “P2X7-like” means that functional and pharmacologic evidence strongly suggest expression of P2X7, but molecular data are missing. Failure to list a P2Y or P2X receptor for a given cell type means that there is lack of evidence for its expression, whether at the functional, pharmacologic, or molecular level.
FSCD indicates fetal skin–derived dendritic cell; PMN, polimorphonuclear; RBL, rat basophilic leukemia.
Derived from bone marrow.
Derived from blood precursors.
Derived from epidermis.
Derived from tonsils and lymph nodes.
Supported by grants by the Italian Ministry of Scientific Research (Cofin and 60%), the National Research Council of Italy (Target Project on Biotechnology), the Italian Association for Cancer Research, and Telethon of Italy. J.M.S. supported by a fellowship from the European Community (training grant BMH4-98-5146).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
Author notes
Francesco Di Virgilio, Department of Experimental and Diagnostic Medicine, Section of General Pathology, University of Ferrara, Via Borsari, 46, I-44100 Ferrara, Italy; e-mail:fdv@dns.unife.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal