Abstract
Using quantitative fluorescence in situ hybridization and flow cytometry, the telomere length of telomere repeat sequences after stem cell transplantation (SCT) were measured. The study included the telomeres of peripheral blood monocytes that should reflect the length of telomeres in stem cells and the telomeres of T lymphocytes that could shorten as a result of peripheral expansion. The loss of telomeres in monocytes and in memory T cells, although accelerated initially, became comparable to the loss of telomeres in healthy controls from the second year after transplantation. In addition, the telomere length in the naive T cells that were produced by the thymus was comparable to the telomere length in the naive T cells of the donor. Compared to the total length of telomeres available, the loss of telomere repeats in leukocytes after SCT resembles the accelerated shortening seen in early childhood and remains, therefore, relatively insignificant.
Introduction
Telomeres are specialized structures at the end of eukaryotic chromosomes that, in vertebrates, consist of hundreds to thousands of tandem repeats of the sequence TTAGGG.1 Because telomere length decreases with the number of cell divisions in vitro and with aging in vivo,2 it reflects the mitotic history of the cells. Furthermore, short telomeres might limit the cells' remaining replicative capacity.3 This may be of special significance after stem cell transplantation (SCT) because the telomere length of leukocytes in the patient may be up to 2000 base pair (bp) shorter than in the donor.4-9
Because the telomerase activity in stem cells does not prevent telomere shortening,10 the shorter telomeres may be the consequence of the expansion of the transplanted stem cells needed to repopulate the host. In addition, the telomere loss previously observed in peripheral blood mononuclear cells4,6 9 might be explained by the extensive proliferation of the T lymphocytes cotransfused with the marrow. In this report we show that both phenomena contribute significantly to the accelerated telomere shortening after SCT and that after an initial period of approximately one year, the rate of telomere shortening in patients becomes comparable to that in their donors.
Study design
The average length of telomere repeats in individual cells was measured by flow fluorescence in situ hybridization (FISH), as previously described,11 and expressed as molecules of equivalent soluble fluorochrome (MESF) units12 after calibration with fluorescein isothiocyanate (FITC)–labeled fluorescent calibration beads (Quantum-24 Premixed; Flow Cytometry Standards Corporation, San Juan, Puerto Rico). Interexperimental variation was monitored by analyzing aliquots of a batch of frozen cells13 and did not exceed 15%. Fluorescence-activated cell sorter (FACS) analysis of telomere length in monocytes and lymphocytes was performed with gates on single diploid cells with the characteristic light scatter properties.13 To analyze naive T cells and cells with a memory phenotype, 2-5 × 104 cells were fractionated into CD4+CD45RA+CD45RO− and CD4+CD45RA−CD45RO+ by FACS analysis (FACStar Plus; Becton Dickinson, Basel, Switzerland) after staining with anti–CD4-APC (allophycocyanin) (Leu4; Becton Dickinson), CD45RO-FITC (Becton Dickinson), and CD45RA-PE (phycoerythrin).
To obtain sufficient numbers of cells (106) for the analysis of telomere fluorescence, the cells were cultured for 10-15 days in Roswell Park Memorial Institute (RPMI 1640) medium (Life Technologies, Basel, Switzerland) containing 10% (vol/vol) human serum supplemented with 1 μg/mL phytohemagglutinin (Murex Biotech, Dartford, England), 100 U/mL recombinant interleukin–2 (rIL-2) (Roche, Nutley, NJ), and 106/mL irradiated allogeneic cells. This approach for telomere length analysis was validated by experiments showing no significant difference between telomere fluorescence in freshly isolated T lymphocytes and their 10- to 15-day-old blasts.
Results and discussion
We studied the telomere-length dynamics in peripheral blood mononuclear subpopulations in 4 patients (ages, 23-25 years) who received transplantations for acute myeloid or lymphoblastic leukemia (AML or ALL, respectively) during first remission. We selected the patients who received a T-cell–depleted14 bone marrow from a human leukocyte antigen (HLA)–identical sibling after a standard conditioning (120 mg/kg cyclophospamide and 12 Gy total body irradiation). The patients selected had received only a mild immune suppression regimen (1-2 months of cyclosporin A with a trough at 200 ng/mL [approximately 3mg/kg]), were free of major complications (acute or chronic graft-versus-host disease or relapse), and did not have a significant hematological chimerism (≤ 10%).
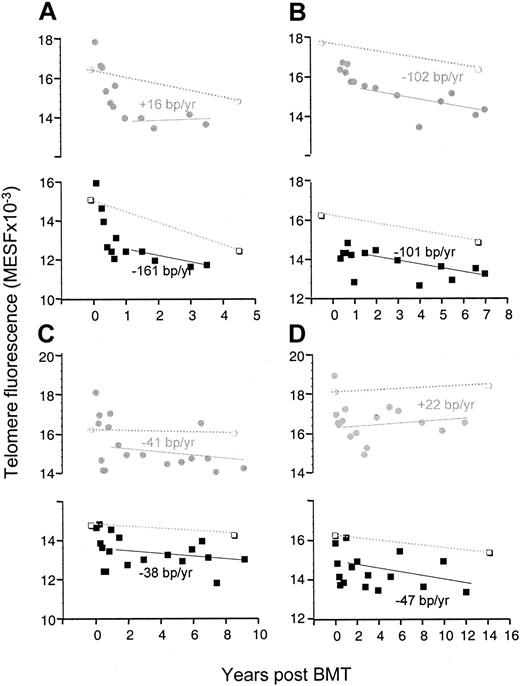
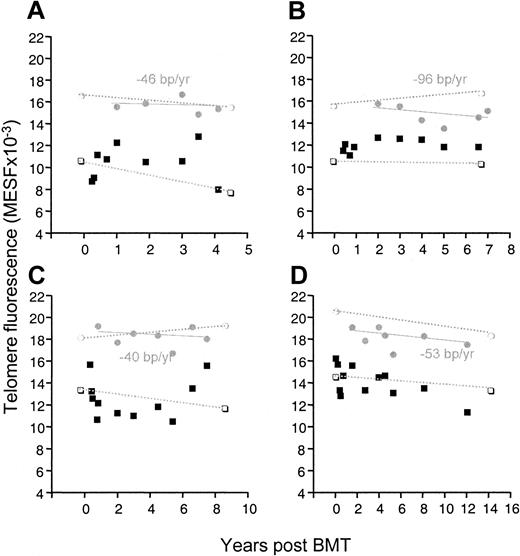
Figure 1 shows the telomere loss in monocytes and lymphocytes in the patients as well as in their donors. Already starting from the second year after transplantation, the telomeres in the patients shortened at a rate comparable to that of their donor, which was similar to the gradual loss found in healthy controls (varying between 27-59 bp/y from one report to another).4-6,13,15 Therefore, it is likely that the high rate of telomere loss during the first year is entirely responsible for the difference of 1-2 kb that was apparent through the entire transplantation period. It should be noted that although the initial accelerated telomere loss in monocytes may look quite similar to the loss in lymphocytes, it is unlikely that the mechanism of loss is the same for both cells. Whereas the telomere loss in monocytes is probably owed directly to the loss of telomeres in stem cells, the accelerated loss in lymphocytes may be caused primarily by the numerous divisions that mature T cells undergo when they repopulate the T-cell compartment.16,17 It is evident that during this phase, the effect of telomere shortening in stem cells contributes little, while in these patients the thymus does not produce T cells before the sixth month following transplantation.18 Figure 2shows that the telomere loss induced by this initial peripheral expansion of memory T cells could be as high as several kb/y (patient C, 3.4 kb/y; patient D, 2.1 kb/y).
Accelerated telomere loss after SCT is limited in time.
Analysis of telomere fluorescence of monocytes (shaded circles) and lymphocytes (black squares) isolated from blood samples from patients A-D at different time points after transplantation. Telomere loss (bp/y) was calculated by linear regression analysis (solid line) of the data after the first year following SCT only. Telomere fluorescence of monocytes (open circles) and lymphocytes (open squares) of the respective donors is shown both at the time of transplantation and at the time indicated (dotted line).
Accelerated telomere loss after SCT is limited in time.
Analysis of telomere fluorescence of monocytes (shaded circles) and lymphocytes (black squares) isolated from blood samples from patients A-D at different time points after transplantation. Telomere loss (bp/y) was calculated by linear regression analysis (solid line) of the data after the first year following SCT only. Telomere fluorescence of monocytes (open circles) and lymphocytes (open squares) of the respective donors is shown both at the time of transplantation and at the time indicated (dotted line).
Telomere fluorescence in CD4+ naive T cells and memory T cells from patients A-D.
CD4+ naive T cells are indicated by shaded circles, and memory T cells are indicated by solid squares. Telomere loss (bp/y) was calculated by linear regression analysis only for the naive cells of the patient. Telomere fluorescence of naive (open circles) and memory (open squares) cells from their respective donors is shown at the time of transplantation and at the time indicated (dotted line).
Telomere fluorescence in CD4+ naive T cells and memory T cells from patients A-D.
CD4+ naive T cells are indicated by shaded circles, and memory T cells are indicated by solid squares. Telomere loss (bp/y) was calculated by linear regression analysis only for the naive cells of the patient. Telomere fluorescence of naive (open circles) and memory (open squares) cells from their respective donors is shown at the time of transplantation and at the time indicated (dotted line).
With the emergence (6-12 months after transplantation) of CD4+CD45RA+RO− naive T cells with significantly longer telomeres, the interpretation of the changes in telomere length in the memory pool became more complex. During some periods the average telomere length even increased; this was probably due to the recruitment of naive cells into the memory pool. Regarding the telomere length in the naive cells, several other points are noteworthy. First, telomere shortening was not different from that of naive cells in their donors or in the much larger control group.13 Second, the telomeres of the naive cells were longer than in the monocytes in the same patient. This was in concordance with our previous finding that telomeres in naive T cells are significantly longer than in granulocytes and that this difference remains constant over an age range of 0-90 years.13 Then we reasoned that the number of cell divisions between granulocytes and stem cells must be higher than the number between naive T cells and stem cells. Alternatively, one could argue that during the maturation of T cells in the thymus, telomeres are elongated by the telomerase that is expressed in thymocytes.19
A completely different hypothesis would be that after SCT, the genuine pluripotent stem cell divides only at a low rate without a significant telomere shortening. In the mean time, the recipient is repopulated by more mature hematopoietic precursors that produce predominantly myeloid cells and erythrocytes. This would fit into the finding that during the first 6 months after SCT, only very few B and T cells are produced. Then, lymphocyte production manifests only when the number of stem cells is adequate, but most of the myeloid cells might still be produced by the precursors having repopulated the recipient during the first months. Although this is pure speculation, our data comprise some indications that this might be true. First, in patients C and D, the average length of telomeres in monocytes increased slightly after the first year following transplantation (Figure 1), which could be the result of a greater contribution by stem cells, in which telomere shortening was much less significant. Moreover, in all patients, the telomeres in naive T cells were not shorter than those in their donors, which is not easy to explain if the telomeres of the stem cells from which they were derived did have shorter telomeres than those in the donor.
In conclusion, we have shown that the accelerated telomere loss in leukocytes observed after SCT is limited in time. Because telomere shortening proceeds thereafter at a normal rate, the transplantation-induced loss of telomeres represents only 20% of the “telomere reserve” of the stem cell compartment and should therefore have little consequence for the function of the different hematological lineages.
Acknowledgments
We thank Solange Vischer for excellent technical assistance and Giovanna Cameron, Rick Zapfer, Gayle Thornbury, and Dominique Wohlwend for help with cell sorting.
Supported by grant 31-53774.98 from the Swiss National Science Foundation, Bern, Switzerland; a grant of the foundation Dr Henri Dubois-Ferrière-Dinu Lipatti, Geneva, Switzerland; grants AI29524 and GM56162 from the National Institutes of Health, Bethesda, MD; and a grant from the National Cancer Institute of Canada, with funds from the Terry Fox Run, Vancouver, BC, Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nathalie Rufer, Unité d'Immunologie de Transplantation, Hôpital Universitaire de Genève, 24 rue Micheli-Du-Crest, CH-1211 Genève 14, Switzerland; e-mail:rufer@cmu.unige.ch; or Peter Lansdorp, Terry Fox Laboratory, British Columbia Cancer Agency, 601 West 10th Ave, Vancouver, BC, V5Z 1L3 Canada; e-mail: plansdor@bccancer.bc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal