Abstract
The induction of anergy in T cells, although widely accepted as critical for the maintenance of tolerance, is still poorly understood at the molecular level. Recent evidence demonstrates that in addition to blockade of costimulation using monoclonal antibodies (mAbs) directed against cell surface determinants, treatment of mixed lymphocyte reaction (MLR) cultures with interleukin 10 (IL-10) and transforming growth factor-β (TGF-β) results in induction of tolerance, rendering alloreactive murine CD4+ T cells incapable of inducing graft-versus-host disease (GVHD) after in vivo transfer to histoincompatible recipients. The present study, using these cells prior to adoptive transfer, determined that IL-10 + TGF-β–tolerant CD4+ T cells exhibit an altered pattern of T-cell receptor (TCR) + CD28-mediated signaling and are incapable of progressing out of the G1 phase of the cell cycle during stimulation with HLA class II disparate antigen-presenting cells. TGFβ + IL-10–tolerant cells were incapable of phosphorylating TCR-ζ, or activating ZAP-70, Ras, and MAPK, similarly to T-cell tolerized by blockade of B7/CD28 and CD40/CD40L pathways. Moreover, these cells were incapable of clonal expansion due to defective synthesis of cyclin D3 and cyclin A, and defective activation of cyclin-dependent kinase (cdk)4, cdk6, and cdk2. These cells also exhibited defective down-regulation of p27kip1 cdk inhibitor and lack of cyclin D2-cdk4 activation, Rb hyperphosphorylation, and progression to the S phase of the cell cycle. These data link anergy-specific proximal biochemical alterations and the downstream nuclear pathways that control T-cell expansion and provide a biochemical profile of IL-10 + TGF-β–tolerant alloreactive T cells that do not induce GVHD when transferred into MHC class II disparate recipients in vivo.
Introduction
Nonspecific immunosuppression has been increasingly successful in controlling T-cell alloreactivity during allogeneic bone marrow and solid organ transplantation. However, the requirement for the prolonged administration of immunosuppressive drugs with its unwanted side effects and limited availability of suitable donors remain significant obstacles to improving the outcome of allogeneic transplantation. As a consequence of using broadly immunosuppressive drugs, beneficial functions of T cells such as those needed for mediating antiviral responses are also suppressed. In a bone marrow transplantation (BMT) setting, it is known that donor T cells, which recognize major histocompatibility complex (MHC) or minor antigen disparities in the recipient, expand and mediate multiorgan system distraction known as graft-versus-host disease (GVHD). Therefore, approaches that selectively inactivate alloreactive donor T cells would be desirable.
Anergy in vitro and its in vivo counterpart, tolerance, are defined as the inability of antigen-specific T cells to proliferate and produce interleukin (IL)-2 on rechallenge with fully competent antigen-presenting cells (APCs) delivering T-cell receptor (TCR) and costimulatory signals. Anergy can be induced when T cells are stimulated via TCR in the absence of costimulation. To date, ex vivo or in vivo blockade of B7/CD28 and CD40/CD40L costimulatory pathways has shown great promise for the prevention of GVHD in vivo without nonspecific immunosuppression in murine and subhuman primate models.1-4 More recent studies in humans have shown that blockade of the B7/CD28 pathway may reduce the incidence of severe GVHD reactions when donor T cells are tolerized to host alloantigens ex vivo.5 However, blockade of these costimulatory pathways may not be uniformly effective in all individuals. Therefore, additional approaches that may improve the maintenance of tolerance are required.
Recent evidence indicates that in addition to blockade of costimulation, other mechanisms are operative in inducing peripheral tolerance. Soluble T-cell factors, most notably IL-10, can suppress clonal expansion of human and murine helper T cells in response to antigen.6,7 In vitro studies have shown that IL-10 has a direct inhibitory effect on CD4+ T cells by suppressing IL-2 secretion.8 In addition, IL-10 inhibits APC-dependent T-cell activation by preventing expression of MHC class II, CD54, and the members of the B7 family of costimulatory molecules B7-1 (CD80) and B7-2 (CD86) on APC.9-13 In vivo, high levels of IL-10 correlate with prevention of skin, cardiac, and islet graft rejection.14,15 Moreover, the isolation of IL-10–producing T cells from the peripheral blood of patients with severe combined immunodeficiency (SCID) in which tolerance had been induced after HLA-mismatched hematopoietic stem cell transplantation suggests that IL-10 may play a key role in the maintenance of tolerance in vivo.16 The transforming growth factor (TGF)-β family members are also known to be key negative regulators of immune homeostasis, because absence of TGF-β1 signaling in mice with targeted mutation of TGF-β117 or mice with transgenic expression of a dominant negative TGF-β receptor in T cells leads to the development of autoimmune disease with inflammatory infiltration in multiple organs, immunopathology, and secretion of autoimmune antibodies.18 In contrast, in vivo injection of T cells engineered to express latent TGF-β1suppresses experimental allergic encephalomylitis.19
Using a murine model of histoincompatible BMT, we have shown that culture of CD4+ cells from C57BL/6(Hb) (termed B6) mice with T-cell–depleted spleen cells from MHC class II disparate B6.C-Hbm12/KhEg (termed bm12) mice, results in the growth of primed B6 CD4+ T cells, which have high alloresponsiveness in vitro and induce GVHD lethality when transferred into irradiated bm12 recipients in vivo.20 Addition of IL-10 and TGF-β during the culture of B6 T cells with bm12 stimulators inhibited primary and secondary mixed lymphocyte reaction (MLR) but more importantly significantly diminished GVHD lethality.21 Interestingly, although each individual cytokine significantly reduced primary and to a lesser extent secondary MLR responses, only the combination of these cytokines in MLR cultures was able to protect the majority of recipients from GVHD-induced lethality when cultured cells were adaptively transferred in vivo.21
Because these findings have potential clinical significance and applicability in therapeutic approaches, we sought to determine the molecular basis of unresponsiveness induced by IL-10 and TGF-β, because this information will give insights not only into anergy but to determining clinical applications. Here, using this in vivo murine model of histoincompatible transplantation we show that CD4+ T cells rendered tolerant by treatment with IL-10 and TGF-β are incapable of phosphorylating TCR-ζ or activating ZAP-70, Ras, and MAPK, similarly to T cells tolerized by blockade of costimulation. Moreover, these cells are incapable of clonal expansion due to defective activation of cdk4 and cdk2 and defective synthesis of cyclin D3 and cyclin A. These cells also exhibited defective down-regulation of p27kip1 cdk inhibitor and lack of cyclin D2–cdk4 activation, Rb hyperphosphorylation, and entry to the S phase of the cell cycle. None of these biochemical alterations were observed when cells were cultured under the same conditions with either IL-10 or TGF-β alone, providing evidence for the synergistic effect of these cytokines in the induction of T-cell hyporesponsiveness and blockade of clonal expansion. These data link anergy-specific proximal biochemical alterations and the downstream nuclear pathways that control T-cell expansion and provide a mechanism by which IL-10 + TGF-β–tolerant cells are prevented from expansion and, therefore, they do not induce GVHD. Moreover, these data provide evidence for a more generalized significance of the biochemical events that characterize T-cell anergy because this pattern has been observed in T cells rendered anergic by either blockade of costimulation or, as shown here, by the use of immunosuppressive cytokines IL-10 and TGF-β.
Materials and methods
Animals
The C57BL/6 (H2b) (termed B6) mice were purchased from the National Institutes of Health (Bethesda, MD). B6.C-H2bm12/KhEg (termed bm12) mice, which differ from B6 mice only by a mutation in MHC class II region, were purchased from the Jackson Laboratory (Bar Harbor, ME). Donors and recipients were 8 to 10 weeks of age at the time of BMT. All mice were housed in a specific pathogen-free facility in microisolator cages.
Ex vivo priming and tolerization cultures
To purify CD4+ T cells, single cell suspensions of axillary, mesenteric, and inguinal lymph node cells were mashed, passed through a wire mesh, and collected into phosphate-buffered saline supplemented with 2% fetal calf serum. Cell preparations were depleted of natural killer (NK) cells (hybridoma PK136, rat IgG2a, provided by Dr Gloria Koo, Rahway, NJ) and CD8+ cells (hybridoma 2.43, rat IgG2b, provided by Dr David Sachs, Cambridge, MA) by coating with the relevant monoclonal antibodies (mAbs) followed by passage through a goat antimouse and goat antirat Ig-coated column (Biotex, Edmonton, Alberta, Canada). The goat antimouse-coated column also removes B cells and dendritic cells. The final composition of T cells in the donor graft was determined by flow cytometry and was always 94% or more CD4+ T cells. Responders were mixed with irradiated (30 Gray) anti-NK and anti-Thy1.2 mAb (30-H12, rat IgG2b provided by David Sachs and hybridoma PK136, mouse IgG2a, provided by G. Koo) plus complement-treated splenocyte stimulators. B6 CD4+ T cells were mixed with bm12 splenic stimulators. Responders and stimulator cells were suspended at a final concentration of 0.5 × 106 cells/mL in 24-well plates (Costar, Acton, MA) containing Dulbecco modified Eagle medium (Biowhittaker, Walkersville, MD) with 10% fetal calf serum (Hyclone, Logan, UT), 2-mercaptoethanol (5 × 10−5 M; Sigma Chemical, St Louis, MO), 10 mM Hepes buffer, 1 mM sodium pyruvate (Gibco Brl, Grand Island, NY), amino acid supplements (1.5 mM l-glutamine,l-asparagine, and l-arginine; Sigma), and antibiotics (penicillin 100 U/mL, streptomycin 100 mg/mL; Sigma). For ex vivo tolerization, IL-10 (Schering-Plough Research Institute, Kenilworth, NJ; specific activity, 3.1 × 107 U/mg) was added at a final concentration of 1000 U/mL and TGF-β2(Genzyme, Framingham, MA; (specific activity, 5 × 107U/mg) was added at a final concentration of 1 ng/mL and plates were incubated at 37°C and 10% CO2. After 8 days of culture, primed and IL-10 + TGF-β2–tolerant CD4+cells were isolated and were either injected intravenously to bm12 recipients to monitor the induction of GVHD,20 or used for the in vitro signaling experiments. As control, unstimulated cells were isolated and purified as above and used directly.
In vitro activation of T cells
Primed and tolerant T cells from B6 mice (2 × 107cells/sample) were resuspended at 107 cells/mL and incubated with anti-CD3ε (hybridoma 145-2C11, hamster IgG1, provided by Dr Jeffrey Bluestone, Chicago, IL; 1 μg/mL) and anti-CD28 (hybridoma 37.51, hamster IgG1, provided by Dr James Allison, Berkeley, CA; 5 μg/mL) at 4°C for 20 minutes. Cells were washed and incubated with goat antihamster IgG (10 μg/mL) at 37°C for various time intervals and cell lysates were prepared as described.22
Immunobloting, immunoprecipitation, and in vitro kinase reactions
Cell lysates from unstimulated, primed, and tolerant cells were prepared and equal amounts of protein (25 μg/sample) were analyzed by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on microgels, transferred onto nitrocellulose membranes, and immunoblotted with the indicated mAbs or antiserum: cbl, ZAP-70, SLP-69, fyn, pERK1/2, cyclin D2, cyclin D3, cyclin A, cdk4, cdk6, and cdk2 (Santa Cruz Biotechnology, Santa Cruz, CA); antiphosphotyrosineG10,4p21cip1 and ERK1/2 (Upstate Biotechnology, Lake Placid, NY); p27kip1 (Transduction Laboratories, Lexington, KY). Anti–TCR-ζ antiserum was kindly provided by Dr Paul Anderson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA). To examine the phosphorylation status of Rb, proteins were analyzed by 6% SDS-PAGE, transferred onto nitrocellulose membrane, and blotted with Rb-specific mAb (Pharmingen, San Diego, CA). After immunoblotting with mAbs or antiserum, immunodetection was performed by incubation with horseradise peroxidase–conjugated antimouse IgG (1:5000) or antirabbit IgG (1:10 000; Promega, Madison, WI) as indicated by the host origin of the primary antibody. Stripping and reprobing of the immunoblots were done as described.
For in vitro kinase reactions, immunoprecipitations were done using equal amounts of protein (150 μg/sample) with anti–cdk2 specific antiserum agarose conjugate (Santa Cruz Biotechnology) and in vitro kinase reactions were performed using histone H1 (Sigma) as exogenous substrate, according to described protocol.23 When immunoprecipitation was performed with anti-cdk4–specific antiserum, in vitro kinase reactions were performed using Rb-GST (Santa Cruz Biotechnology) as exogenous substrate.23 Reactions were analyzed by 10% SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membrane and exposed to x-ray film.
Results
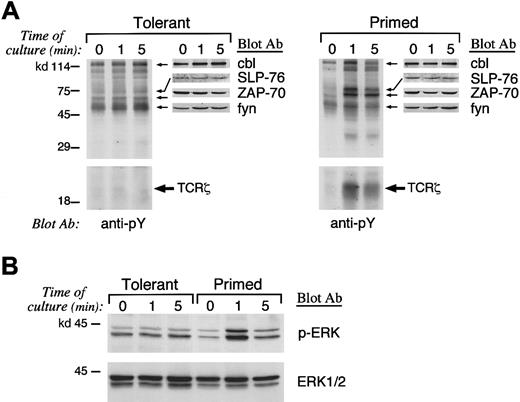
Anergy-specific pattern of tyrosine phosphorylation is detected in IL-10 + TGF-β–tolerant T cells
Previous studies have shown that an altered pattern of proximal biochemical events is induced when anergic T cells are activated via their TCR even in the presence of signals delivered via the CD28 costimulatory pathway, which mediates a highly potent stimulatory combination to T cells.22,24,25 After 8 days of primary culture, primed and IL-10 + TGF-β–tolerant CD4+ T cells were isolated and prior to injection into histoincompatible recipients were used for in vitro signaling experiments. Because activation of protein tyrosine phosphorylation is the earliest biochemical event occurring after T-cell stimulation, we examined the activation of protein tyrosine phosphorylation after stimulation of primed control and IL-10 + TGF-β–tolerant cells. Stimulation of tolerant T cells induced an altered pattern of protein tyrosine phosphorylation compared with that induced in primed control T cells (Figure 1A). Stimulation of primed control but not of IL-10 + TGF-β–tolerant T cells resulted in significant phosphorylation of TCR-ζ (Figure 1A, bottom panel). To confirm the identity of the differentially tyrosine phosphorylated complex of 18- to 23-kd size as the phosphorylated form of TCR-ζ, immunoprecipitation was performed with anti–TCR-ζ antiserum and immune complexes were analyzed by SDS-PAGE and immunobloted by antiphosphotyrosine mAb (Figure 1A, bottom panel). Stimulation of primed control cells also resulted in activation of ZAP-70 and transient phosphorylation of fyn and cbl (Figure 1A, top panel). In contrast, IL-10 + TGF-β–tolerant T cells exhibited constitutively increased and sustained phosphorylation of fyn, and cbl and defective activation of ZAP-70 (Figure 1A, top panel). The identity of these substrates was predicted by their electrophoretic mobility and was confirmed by peptide-specific immunoblot using the relevant mAbs or antiserum. Immunoblot with these specific mAbs or antiserum also demonstrated that the total amount of these differentially phosphorylated substrates was equivalent in primed control and IL-10 + TGF-β–tolerant T cells (Figure 1A, top panel). The identity of these substrates that underwent differential tyrosine phosphorylation in IL-10 + TGF-β–tolerant cells was consistent with observations in in vitro–anergized T-cell clones.24-32
Altered pattern of tyrosine phosphorylation and defective Ras activation in IL-10
+ TGF-β–tolerant T cells. (A) Primed and tolerant T cells from B6 mice were stimulated in vitro as described in “Materials and methods” for the indicated time intervals. Cell lysates were prepared and were analyzed by SDS-PAGE, transferred to nitrocellulose membrane, and blotted with antiphosphotyrosine mAb. Blots were stripped and reblotted with the indicated peptide-specific antiserum to determine the identity of the differentially phosphorylated substrates. (B) As an indicator of Ras activation, the serine phosphorylation of ERK1 and ERK2 MAPK was examined by blotting with phospho-ERK–specific antibody that recognizes only the activated phosphorylated form of ERK1 and ERK2 (top panel). The blot was stripped and reprobed with ERK-specific antibody, which recognizes total ERK (bottom panel).
Altered pattern of tyrosine phosphorylation and defective Ras activation in IL-10
+ TGF-β–tolerant T cells. (A) Primed and tolerant T cells from B6 mice were stimulated in vitro as described in “Materials and methods” for the indicated time intervals. Cell lysates were prepared and were analyzed by SDS-PAGE, transferred to nitrocellulose membrane, and blotted with antiphosphotyrosine mAb. Blots were stripped and reblotted with the indicated peptide-specific antiserum to determine the identity of the differentially phosphorylated substrates. (B) As an indicator of Ras activation, the serine phosphorylation of ERK1 and ERK2 MAPK was examined by blotting with phospho-ERK–specific antibody that recognizes only the activated phosphorylated form of ERK1 and ERK2 (top panel). The blot was stripped and reprobed with ERK-specific antibody, which recognizes total ERK (bottom panel).
Tyrosine phosphorylation of SLP-76 and activation of ERK1/2 is defective in IL-10 + TGF-β–tolerant T cells
SLP-76 is an adaptor protein that is tyrosine phosphorylated after TCR stimulation and has a critical role on IL-2 transcription. SLP-76 is required for activation of Ras and therefore links activation of proximal tyrosine kinases to downstream signaling pathways.33 34 Induction of SLP-76 tyrosine phosphorylation was defective in IL-10 + TGF-β–tolerant in comparison to primed control CD4+ T cells, although the total amount of SLP-76 was equivalent as determined by SLP-76–specific immunoblot (Figure 1A, top panel). Because tyrosine phosphorylation of SLP-76 is mandatory for activation of Ras after TCR stimulation, these results suggest that IL-10 + TGF-β–tolerant cells may be incapable of activating the Ras pathway after TCR- and CD28-mediated stimulation.
Raf-1 serine/threonine kinase, a downstream effector of Ras,35-37 is recruited to the plasma membrane by activated Ras,38,39 where it becomes activated in a Ras-independent manner. Activated Raf-1 phosphorylates and activates MEK-140,41 (a member of the MEK [MAP/ERK] kinases), leading to activation of the mitogen-activated protein (MAP/ERK) kinase cascade, which regulates IL-2 gene transcription.42,43 To determine whether IL-10 + TGF-β–tolerant cells could activate the Ras pathway after stimulation, we examined whether activation of the ERK1 and ERK2 kinases of the MAP/ERK kinase family, which are downstream of Ras, was induced. Immunoblot with an antibody specific for activated, phosphorylated ERK1 and ERK2 demonstrated that activation of these MAP kinases was induced after stimulation in control-primed but not in IL-10 + TGF-β–tolerant cells (Figure 1B, top panel). Reblotting of the same blot with an antibody specific for total ERK showed that equivalent amounts of total ERK1 and ERK2 were expressed in control and IL-10 + TGF-β–tolerant cells (Figure 1B, bottom panel). Taken together, the above described results demonstrate that a combination of biochemical findings previously established as the hallmark of the anergic cells generated by blockade of costimulation or ligation of the TCR by altered peptide ligands24-32 is also detected in IL-10 + TGF-β–tolerant T cells, which are anergic in vitro and in vivo.
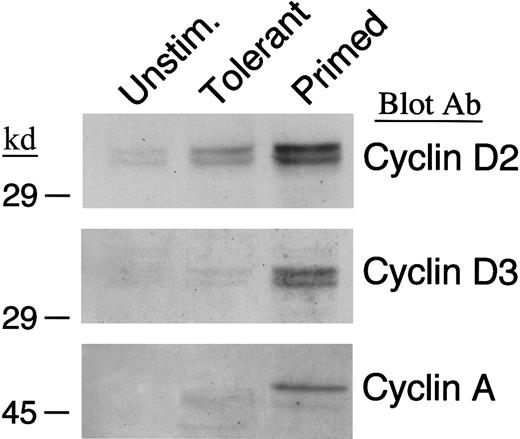
IL-10 + TGF-β–tolerant cells enter G1 but fail to progress into the late G1 and enter the S phase of the cell cycle
Previous studies have shown that alloantigen-specific human T-cell clones rendered anergic by lack of B7/CD28 costimulation are arrested at the G1 phase of the cell cycle. Similarly, alloreactive murine T cells that have been rendered tolerant by blockade of CD40/CD40L interactions and do not induce GVHD in vivo, are arrested at the G1 phase of the cell cycle.20 Therefore, we examined whether IL-10 + TGF-β–tolerant cells could enter the cell cycle, progress into the late G1, and enter the S phase. In T cells, cyclin D2 is expressed at very low levels in unstimulated cells and it is rapidly up-regulated after entry into the G1; cyclin D3 is synthesized at the late G1 and cyclin A at the S phase.44 45 To examine whether primed and IL-10 + TGF-β–tolerant cells were capable of entering G1, we examined whether cyclin D2 was up-regulated after in vitro culture of T cells in the presence or absence of IL-10 + TGF-β. As shown in Figure 2(top panel), cyclin D2 was induced in both primed and tolerant cells above background. In contrast, immunoblot with antibodies specific for cyclin D3 and cyclin A demonstrated that although both these cyclins were synthesized in primed cells, they were not detected in IL-10 + TGF-β–treated T cells (Figure 2, middle and bottom panels). These results show that tolerant cells enter G1phase and synthesize cyclin D2 although in lower levels than primed cells, but only primed cells progress to the late G1 phase and synthesize cyclin D3 and subsequently enter the S phase and synthesize cyclin A.
IL-10 + TGF-β–tolerant cells synthesize cyclin D2 but not cyclin D3 or cyclin A.
Lysates from unstimulated, tolerant, and primed T cells were analyzed by SDS-PAGE and immunoblotted with an mAb specific for cyclin D2. Blots were stripped and reprobed with an mAb specific for cyclin D3 or a rabbit polyclonal antibody specific for cyclin A.
IL-10 + TGF-β–tolerant cells synthesize cyclin D2 but not cyclin D3 or cyclin A.
Lysates from unstimulated, tolerant, and primed T cells were analyzed by SDS-PAGE and immunoblotted with an mAb specific for cyclin D2. Blots were stripped and reprobed with an mAb specific for cyclin D3 or a rabbit polyclonal antibody specific for cyclin A.
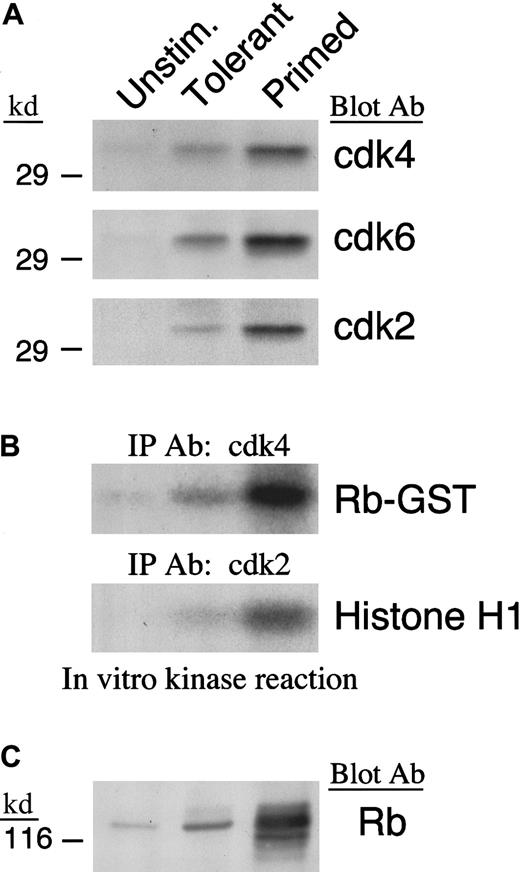
IL-10 + TGF-β treatment prevents down-regulation of p27kip1 and results in defective activation of cyclin-dependent kinases
To drive cell cycle progression, cyclins associate with specific cdks to form active holoenzymes. D-type cyclins associate with cdk4 and its homologue cdk6, whereas cyclin A associates with cdk2. The cdks are constitutively expressed at low levels in unstimulated T cells and are up-regulated after activation.46 Because up-regulation of cyclin D2 was defective and up-regulation of cyclin D3 and cyclin A were not detected in IL-10 + TGF-β–treated cells, we sought to determine whether cdk4, cdk6, and cdk2 also failed to be synthesized after culture in these cells. Immunoblot with cdk4-, cdk6-, and cdk2-specific antibodies showed that up-regulation of these cdks was defective in IL-10 + TGF-β–treated T cells in comparison to what is observed in primed control cells (Figure3A). In addition, enzymatic activation of these cdks was defective in IL-10 + TGF-β–treated cells as determined by in vitro kinase reaction (Figure 3B). However, the defective activation of these cdks was not simply secondary to their reduced quantitative expression in IL-10 + TGF-β–tolerant cells compared with their expression in primed cells, because it was observed even when increased numbers of IL-10 + TGF-β–tolerant cells were used so that the total amount of isolated cdk4, cdk6, and cdk2 protein was equivalent to that isolated from primed cells (data not shown).
Defective expression and enzymatic activation of cdk4 and cdk2 in IL-10 + TGF-β–tolerant cells.
(A) Lysates from unstimulated, tolerant, and primed T cells were analyzed by SDS-PAGE and immunoblotted with antiserum specific for cdk4, cdk6, or cdk2. (B) Immunoprecipitations were performed with anti-cdk4 or anti-cdk2 specific antiserum agarose conjugate and in vitro kinase reactions were done using Rb-GST or histone H1 as exogenous substrate, respectively. Reactions were analyzed by 10% SDS-PAGE, transferred to PVDF membrane, and exposed to x-ray film. (C) Lysates from unstimulated, tolerant, and primed T cells were analyzed by 6% SDS-PAGE, transferred onto nitrocellulose membrane, and immunoblotted with mAb specific for Rb.
Defective expression and enzymatic activation of cdk4 and cdk2 in IL-10 + TGF-β–tolerant cells.
(A) Lysates from unstimulated, tolerant, and primed T cells were analyzed by SDS-PAGE and immunoblotted with antiserum specific for cdk4, cdk6, or cdk2. (B) Immunoprecipitations were performed with anti-cdk4 or anti-cdk2 specific antiserum agarose conjugate and in vitro kinase reactions were done using Rb-GST or histone H1 as exogenous substrate, respectively. Reactions were analyzed by 10% SDS-PAGE, transferred to PVDF membrane, and exposed to x-ray film. (C) Lysates from unstimulated, tolerant, and primed T cells were analyzed by 6% SDS-PAGE, transferred onto nitrocellulose membrane, and immunoblotted with mAb specific for Rb.
To determine whether our findings of in vitro activation of cdk4, cdk6, and cdk2 kinase activity corresponded to the in vivo events, we examined the phosphorylation status of Rb protein, which is one of the most important intracellular substrates of activated cdks.47 Consistent with the in vitro findings on cdk activation, Rb was hyperphosphorylated in primed cells, whereas in IL-10 + TGF-β–tolerant cells the inactive hypophosphorylated form predominated (Figure 3C).
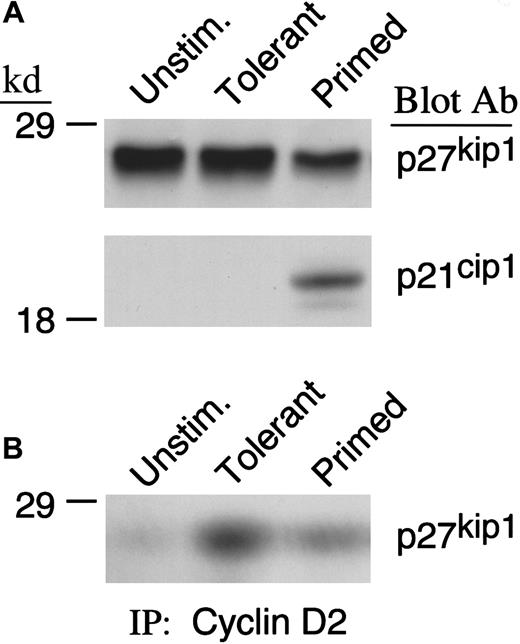
Because cdk4 and cdk2 kinase activity was observed to be defective in IL-10 + TGF-β–treated cells, we considered the possibility that cdk4 and cdk2 activity was reduced by forming complexes with inhibitory proteins. The major inhibitors of cdk4 and cdk2 activity are the members of the INK (p15INK4b, p16INK4a, p18INK4c, and p19INK4d) and the cip/kip (p21cip1, p27kip1, and p57kip2) families.48-52 INK family members specifically bind to and inhibit D-type cyclins complexed with cdk4 and cdk6, whereas p21cip1 and p27kip1 can inhibit many cyclin/cdk complexes.53 Previous studies from our group have shown that none of the INK family members was detected in murine primed T cells or T cells rendered tolerant by CD40L/CD40 blockade. Moreover, p21cip1 was up-regulated after activation in control primed cells. In contrast, p27kip1 was expressed at higher levels in tolerant cells compared with primed control and had an active role in maintaining the tolerant state.54 For these reasons, we examined the expression of p21cip1 and p27kip1in IL-10 + TGF-β–treated and primed T cells. As shown in Figure4A, IL-10 + TGF-β treatment prevented down-regulation of p27kip1 that was induced during culture in the primed control group. Moreover, higher amounts of p27kip1 were associated with cyclin D2 in IL-10 + TGF-β–tolerant cells compared with primed control cells (Figure 4B). In contrast, p21cip1 was detected in primed but not in IL-10 + TGF-β–treated cells. The paradoxical increase of p21cip1 during cell proliferation has also been observed in other systems55 and has been hypothesized to induce a brief arrest of the expanding cells to allow DNA repair.56-58
IL-10 + TGF-β treatment prevents down-regulation of p27kip1 in IL-10 + TGF-β–tolerant cells.
(A) Lysates from unstimulated, tolerant, and primed T cells were analyzed by SDS-PAGE and immunoblotted with mAb specific for p27kip1. Blots were subsequently stripped and reblotted with mAb specific for p21cip1. (B) Immunoprecipitations were performed with cyclin D2–specific antibody followed by immunoblot with a monoclonal antibody specific for p27kip1.
IL-10 + TGF-β treatment prevents down-regulation of p27kip1 in IL-10 + TGF-β–tolerant cells.
(A) Lysates from unstimulated, tolerant, and primed T cells were analyzed by SDS-PAGE and immunoblotted with mAb specific for p27kip1. Blots were subsequently stripped and reblotted with mAb specific for p21cip1. (B) Immunoprecipitations were performed with cyclin D2–specific antibody followed by immunoblot with a monoclonal antibody specific for p27kip1.
Functional studies on the same murine model showed that each one of these cytokines (IL-10 and TGF-β) independently was not sufficient to inhibit MLR and protect against GVHD lethality.21Therefore, we examined the effect of each individual cytokine on the molecular mechanisms that regulate T-cell expansion. As determined by immunoblot, there was no difference in the expression of cdk4, cdk6, cdk2, p27kip1, and p21cip1 in T cells treated with either IL-10 or TGF-β in comparison to control primed cells (data not shown). These results show that IL-10 + TGF-β was additive or synergistic in inducing anergy-specific biochemical effects on TCR-mediated activation and cell cycle progression, which results in the inability of IL-10 + TGF-β–treated cells to clonally expand and induce GVHD.
Discussion
Our present studies show that stimulation of TGF-β–tolerant T cells induced an altered pattern of protein tyrosine phosphorylation compared with that induced in primed T cells. This altered pattern was characterized by defective phosphorylation of TCR-ζ, defective activation of ZAP-70, and sustained phosphorylation of fyn and cbl. Moreover, the adaptor protein SLP-76, which is required for activation of Ras, was not tyrosine phosphorylated, resulting in defective activation of ERK1 and ERK2 MAP kinases, which are downstream of Ras. Altered tyrosine phosphorylation and defective Ras activation in anergic cells has been reported previously in systems in which anergy was induced by delivery of a TCR signal with altered peptide ligands or by blockade of B7/CD28 or CD40/CD40L costimulation.24,25,28-32,54 Our present data extend those observations and provide evidence for the more generalized significance of the biochemical events that underlie the induction and maintenance of the anergic state, because they are detected in T cells rendered tolerant by using a different approach, the addition of IL-10 + TGF-β. Importantly, our results provide evidence for the in vivo biologic relevance of these findings, because our ex vivo– anergized cells remain tolerant and prevent GVHD lethality after transfer in vivo.21
Although the mechanisms responsible IL-10 + TGF-β– induced tolerance might appear entirely distinct from tolerance induced by mAb directed against cell surface costimulatory determinants present on cells in MLR culture, IL-10 + TGF-β tolerance induction may be functioning by regulating cell surface events required for T-cell costimulation. For example, IL-10 is known to inhibit MHC class II expression and to negatively regulate T-cell costimulation.9-12 Although TGF-β has a direct antiproliferative effect on T cells, TGF-β also inhibits the APC capacity of granulocyte/macrophage colony-stimulating factor–stimulated bone marrow cells.59 Recent studies have shown that culture of T cells in the presence of IL-10 induces the differentiation of a unique subset of antigen-specific T cells termed regulatory cells 1 (Tr1).7 These cells have antigen-specific and antigen– nonspecific immunosuppressive properties, produce IL-10, interferon-γ, and TGF-β, but not IL-2 or IL-4, and proliferate poorly in response to antigenic stimulation. Whether in our system the effect of IL-10 + TGF-β is uniform on all T cells or only on a subset that differentiates into Tr1 cells and subsequently mediates anergy induction to the remaining T cells is to be determined. Regardless of the mechanism, our studies demonstrate that T cells cultured ex vivo with IL-10 + TGF-β acquire anergy-specific molecular and biochemical findings, remain tolerant in vivo, and do not induce GVHD. Because of these properties and our previous finding of common aspects between the biochemical events that characterize anergic T cells generated in MLR cultures by using mAbs against CD28/B7 and CD40L/CD40 interactions, such biochemical analyses may provide a powerful tool in quantifying the degree of tolerance induction in individual patients who receive T cells tolerized by different approaches.
Our results show that one of the most critical consequences of the altered TCR-proximal signaling in the anergic cells is the altered control of the expression and activation of the cell cycle regulatory molecules. Regulation of the cell cycle is mediated by synthesis of cyclins and their association with specific cdks to form active holoenzymes. The various stages of the cell cycle are characterized by the expression and activation of distinct cyclins and cdks.53,60 Our studies show that cyclin D2, which characterizes the early G1 phase as well as cdk4 and cdk6 which associate with D-type cyclins, are induced in IL-10 + TGF-β–tolerant cells albeit at significantly lower levels than in primed cells. Most strikingly, the enzymatic activity of these cdks is activated only in primed and not in IL-10 + TGF-β–tolerant cells. These findings correlate with lack of down-regulation of p27kip1 cdk inhibitor in tolerant cells, resulting in increased association of this inhibitor with cyclin D2/cdk4 complexes. Recently we have shown that induction of anergy by blockade of CD40/CD40L pathway or lack of B7 costimulation results in up-regulation of p27kip1 that has a central role in blockade of clonal expansion and IL-2 transcription in anergic cells.54Although in those systems anergic cells could synthesize cyclin D2, cdk4, and cdk6 at equivalent levels as the primed cells, the presence of increased p27kip1 resulted in an aberrant ratio of p27kip1 over cyclin D/cdk holoenzyme, leading to blockade of its enzymatic activity. Taken together with those results our present observations suggest that p27kip1 may be a critical downstream target of anergy induced by various approaches including the blockade of the costimulatory pathways B7/CD28 and CD40/CD40L and the use of the inhibitory cytokines IL-10 + TGF-β.
Our results provide a link between the altered TCR-proximal signaling events and the blockade of clonal expansion in tolerant T cells that prevent the induction of GVHD. Degradation of p27kip1requires its prior phosphorylation. Active cdk2 and formation of cdk2/cyclin E enzymatic complex is required for phosphorylation and subsequent ubiquitination and degradation of p27kip1.53 In addition, activation of Ras and MAP kinase is necessary for phosphorylation and degradation of p27kip1 during activation.61-64 Because activation of cdk2, Ras, and MAPK pathway is defective in alloreactive T cells cultured with IL-10 + TGF-β, these events are likely to be responsible for the inability of the IL-10 + TGF-β–anergic cells to down-regulate p27kip1 expression, clonally expand, and induce GVHD. Importantly, IL-2, which prevents the induction of anergy in all in vitro systems,65-67 the induction of tolerance in our murine in vivo system,20,21 and has a dominant role in the pathogenesis of GVHD,68directly mediates down-regulation of p27kip1.69
An apparent paradox exists for the finding that biochemical alterations are detected in bulk populations, which contain tolerized alloreactive T cells. This is even more striking when one considers the low frequency of such alloreactive T cells in the tolerized culture at the end of the primary culture, based on Vβ repertoire analysis (B.R.B., unpublished data, August 2000). The low frequency of alloreactive T cells in the tolerized culture would imply that the altered signaling pattern should be difficult to observe if these signaling alterations were restricted only to the tolerant T cells. Our recent data indicate that alloantigen-specific T cells tolerized by the use of anti-CD40L mAb, anti-B7 mAb, or with IL-10 + TGF-β acquire regulatory functions, which can under certain conditions down-regulate the response of both specific-alloreactive and nonspecific T cells (B.R.B., unpublished data, August 2000). Thus, the altered signaling pattern in response to CD3 + CD28 observed at the end of the primary tolerizing culture most likely involves antigen-specific alloreactive tolerized T cells and non–antigen-specific alloreactive T cells. Despite the presence of such regulatory cells at the end of the primary MLR culture, nominal antigen responses are retained as formally measured by the response of TCR transgenic cells in secondary culture.21 These secondary cultures did not contain alloantigen-bearing stimulators, which are required to maintain the regulatory properties of tolerized alloreactive T cells and they did not contain IL-10 that may prevent activation of DO11.10 cells by the specific antigen. Moreover, the APC of DO11.10 origin used to present antigen during the secondary cultures were never exposed to the effects of IL-10 and TGF-β, which as mentioned above, may also result in impaired function of APC.
In conclusion, the fundamental observation of our studies is that common features of biochemical signaling alterations are induced in T cells rendered anergic by blockade of B7/CD28 or CD40/CD40L costimulation as well as by IL-10 + TGF-β and that these biochemical findings correlate with GVHD prevention. Therefore, identification of such biochemical signaling alterations may be a highly useful indicator of sufficient magnitude of tolerance induction to predict that GVHD may be prevented in an individual patient undergoing allogeneic BMT.
Supported by National Institutes of Health grants AI 43552, AI 41584, HL 54785, AI 34495, HL 56067, and AI 35225, and a Research Grant from the National Marrow Donor Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vassiliki A. Boussiotis, Dana-Farber Cancer Institute, Mayer 547, 44 Binney St, Boston, MA 02115; e-mail:vassiliki_boussiotis@macmailgw.dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal