Abstract
Bone morphogenetic proteins (BMPs) can be isolated from organic bone matrix and are able to initiate de novo cartilage and bone formation. Here it is shown that BMP-4 inhibited DNA synthesis in a dose-dependent manner in 3 IL-6–dependent multiple myeloma (MM) cell lines (OH-2, IH-1, and ANBL-6). In contrast, no effect on DNA synthesis was observed in 3 IL-6–independent MM cell lines (JJN-3, U266, and RPMI 8226). BMP-4 induced cell cycle growth arrest in the G0/G1 phase in OH-2 and ANBL-6 cells but not in IH-1 cells. BMP-4 induced apoptosis in OH-2 and IH-1 cells, but not significantly in ANBL-6 cells. Furthermore, BMP-4 induced apoptosis in freshly isolated MM cells from 4 of 13 patients. In the OH-2 and ANBL-6 cell lines and in a patient sample, immunoblotting showed that BMP-4 down-regulated IL-6–induced tyrosine phosphorylation of Stat3, suggesting a mechanism for the apparent antagonism between IL-6 and BMP-4. BMP-4 or analogues may be attractive therapeutic agents in MM because of possible beneficial effects on both tumor burden and bone disease.
Introduction
Multiple myeloma (MM) is a B-cell malignancy characterized by a low-grade proliferation of plasma cells in the bone marrow. Tumor progression is frequently accompanied by osteolysis caused by increased bone resorption and decreased bone formation. In vitro, myeloma cells can be responsive to several cytokines with proliferative and anti-apoptotic effects, of which IL-6 is the most important.1 2
Few cytokines with the ability to induce apoptosis or to inhibit proliferation of myeloma cells have been identified. Interferon (IFN)-γ inhibits IL-6–dependent proliferation of myeloma cell lines and patient samples.3,4 In vitro effects of IFN-α are more complex because induction of proliferation, increased survival, and inhibition of proliferation have been reported.2IFN-α treatment of patients with MM may prolong the plateau phase, but it does not significantly prolong survival.5,6 IFN-γ was evaluated in one small clinical trial and showed no beneficial effect.7 Activin A, a member of the transforming growth factor (TGF)–β superfamily, induces apoptosis and inhibits proliferation in murine plasmacytoma.8-11 In addition, Fas ligand (FasL) and TRAIL (Apo-2 ligand) may induce apoptosis in some myeloma cells.12 13
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily and were originally identified by their unique ability to induce ectopic cartilage and bone formation in vivo.14,15To date, at least 15 BMPs have been characterized.16 In bone, osteoblasts and chondrocytes synthesize BMPs, and these proteins play important roles in bone formation and bone cell differentiation by stimulating alkaline phosphatase activity and synthesis of proteoglycan, collagen, and osteocalcin.17,18 BMPs also induce osteoblast differentiation of uncommitted bone marrow stromal precursor cells and are involved in postnatal bone remodeling.19,20 Furthermore, in animal models, BMPs promote the healing of large segmental fractures and bone defects.16 21
BMPs and their receptors are expressed in tissues other than bone, and it has been demonstrated that BMPs play multiple roles in the regulation of growth, differentiation, and apoptosis of various cell types. This may be exemplified by the essential role of BMP-4 in ventralization of the embryo.22-24 In malignant disease, BMPs modulate cell proliferation, frequently in an inhibitory manner, and have been implicated in the osteosclerotic pattern of prostate cancer bone metastasis.25-29 Recently, BMPs have emerged as proteins of significance in hematology. BMP-4 strictly controls the formation of the ventral hematopoietic islands in Xenopusembryos, and a complete hematopoietically active bone marrow cavity is formed in BMP-induced ectopic bone.30-32 Human hematopoietic cell lines and normal bone marrow cells express mRNA from multiple BMPs.33 Finally, BMPs modulate growth and differentiation of hematopoietic stem cells in adult mice.34 35 In this report, we show that BMP-4 is able to inhibit DNA synthesis and to induce apoptosis in myeloma cells.
Materials and methods
Cell lines and cell culture conditions
We used the human myeloma cell lines ANBL-6 (kind gift from Dr Diane Jelinek, Mayo Clinic, Rochester, MN), JJN-3 (kind gift from Dr J. Ball, University of Birmingham, United Kingdom), OH-2,36U-266, and RPMI 8226 (both from ATCC, Rockville, MD). We recently established an IL-6–dependent human myeloma cell line, IH-1, isolated from pleural fluid from patient 11 in Table1. Cells were grown in a medium of RPMI 1640 (Life Technologies, Paisley, United Kingdom) supplemented with 2 mM L-glutamine and 40 μg/mL gentamicin. The stocks of these cell lines were maintained in this medium supplemented with 10% fetal calf serum (FCS), except for RPMI 8226 (15% FCS), and OH-2 and IH-1 cells (10% human A Rh+ serum from the Blood Bank, Trondheim University Hospital, Trondheim, Norway). IL-6–dependent cell lines IH-1, ANBL-6, and OH-2 were maintained in media containing 2 ng/mL IL-6. Media were replenished twice weekly. The IL-6–dependent cell lines were washed 4 times in Hanks balanced salt solution to deplete cells of IL-6 before assays. For experiments, all cells were grown in medium containing 10% FCS supplemented with cytokines as indicated.
Clinical characteristics of patients and cytokine responses
| Patient no. . | Stage* . | Ig class . | Age . | Debut† . | Treatment response‡ . | IL-6 response1-153 . | BMP-4 response1-153 . | IL-6/BMP-4 interaction1-153 . |
|---|---|---|---|---|---|---|---|---|
| 1 | IIIA | IgG | 75 | No | No | + | + | + |
| 2 | IIA | IgA | 74 | Yes | Yes | − | − | − |
| 3 | IIA | IgA | 79 | No | ND | − | − | − |
| 4 | IIA | IgD | 48 | Yes | Yes | − | − | − |
| 5 | IIIA | IgG | 76 | No | No | − | + | + |
| 6 | IIIA | IgG | 60 | No | No | − | − | − |
| 7 | IIIA | IgA | 73 | Yes | Yes | − | − | − |
| 8 | IA | IgG | 84 | No | Yes | − | − | − |
| 9 | IIA | IgA | 76 | Yes | Yes | + | + | + |
| 10 | IIA | IgA | 70 | Yes | Yes | − | − | − |
| 11 | IIIA | IgA | 76 | No | No | + | + | + |
| 12 | IIIA | 51 | No | No | − | − | − | |
| 13 | IIIA | IgG | 76 | Yes | Yes | − | − | − |
| Patient no. . | Stage* . | Ig class . | Age . | Debut† . | Treatment response‡ . | IL-6 response1-153 . | BMP-4 response1-153 . | IL-6/BMP-4 interaction1-153 . |
|---|---|---|---|---|---|---|---|---|
| 1 | IIIA | IgG | 75 | No | No | + | + | + |
| 2 | IIA | IgA | 74 | Yes | Yes | − | − | − |
| 3 | IIA | IgA | 79 | No | ND | − | − | − |
| 4 | IIA | IgD | 48 | Yes | Yes | − | − | − |
| 5 | IIIA | IgG | 76 | No | No | − | + | + |
| 6 | IIIA | IgG | 60 | No | No | − | − | − |
| 7 | IIIA | IgA | 73 | Yes | Yes | − | − | − |
| 8 | IA | IgG | 84 | No | Yes | − | − | − |
| 9 | IIA | IgA | 76 | Yes | Yes | + | + | + |
| 10 | IIA | IgA | 70 | Yes | Yes | − | − | − |
| 11 | IIIA | IgA | 76 | No | No | + | + | + |
| 12 | IIIA | 51 | No | No | − | − | − | |
| 13 | IIIA | IgG | 76 | Yes | Yes | − | − | − |
According to Durie and Salmon.60
Denotes whether primary cells were harvested at the time of diagnosis or not.
Response to treatment with melphalan–prednisone or vincristine, doxorubicin, and dexamethasone regimens was defined as reduction of the M-component by 50% or more.
Purified myeloma cells were incubated for 3 days with or without IL-6 (1 ng/mL) or BMP-4 (50 ng/mL), and cellular viability was determined. A positive response was defined as a 10% or greater absolute change in viability after BMP-4 or IL-6 stimulation compared with unstimulated cells. Interaction was defined to be present if the response of either cytokine was counteracted by ≥ 10% by the other. Patient 12 had nonsecretory myeloma.
ND, not determined.
Isolation of myeloma cells from patients
Myeloma cells from 13 patients admitted to the Section of Hematology, University Hospital of Trondheim were used in this study. Clinical characteristics of the patients are shown in Table 1.
Plasma cells from bone marrow aspirates or pleural fluid were purified by positive immunomagnetic separation using the monoclonal antibody B-B4 (Serotec, Oxford, United Kingdom), which recognizes syndecan-1.37 To release cells from beads, cells were treated with 0.25% (wt/vol) trypsin (Life Technologies) at 37°C for 5 minutes and vigorously pipetted. Thereafter, beads were collected on the magnet, and cells were retrieved after centrifugation. This method yields a population of more than 98% myeloma cells. Because the separation method may interfere with responses to cytokines, we have compared responses to IL-6 and BMP-4 in immunomagnetically selected and unselected OH-2 cells. Responses to both cytokines were similar under both experimental conditions.
Cytokines
BMP-4 was a kind gift from Genetics Institute (Cambridge, MA). We also used BMP-4 from R&D Systems (Abingdon, United Kingdom). IL-6 was a gift from Sandoz (Basel, Switzerland). All cytokines used were of recombinant human type; 1 ng/mL is equivalent to 40 pM IL-6 and approximately 30 pM BMP-4.
DNA synthesis
Cells were seeded in 96-well plastic culture plates (Corning Costar, Corning, NY) at a density of 1 to 4 × 104 cells per well in 200 μL medium supplemented with 10% FCS and cytokines as indicated. After 54 hours, cells were pulsed with 1 μCi methyl-[3H]-thymidine (NEN Life Science Products, Boston, MA) per well and were harvested 18 hours later with a Micromate 196 cell harvester (Packard, Meriden, CT), and β radiation was measured with a Matrix 96 beta counter (Packard).
Apoptosis assay
Viability and apoptosis were evaluated by flow cytometry using cellular annexin V binding (APOPTEST-FITC kit; Nexins Research, Hoeven, Netherlands). 105 cells were incubated in 24-well culture plates with cytokines as indicated. Cells were washed once in phosphate-buffered saline, resuspended in 250 μL binding buffer, and incubated in the dark with 0.25 μL annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) to 2 μg/mL for 10 minutes, according to instructions by the manufacturer. Cells were classified as PI- or annexin V–positive or -negative using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). All PI-positive cells were considered dead (upper 2 quadrants of dot plots), PI-negative and annexin V–positive cells were considered apoptotic (lower right quadrant), and remaining cells (lower left quadrant) were considered viable.
Cell cycle analysis
In concentrations as indicated, 2 × 105 cells were washed and incubated with IL-6, with or without BMP-4. Cells were washed and resuspended for 30 minutes in ice-cold permeabilization buffer containing 1 mM Tris HCl, pH 8.0, 0.1% Triton-X, 3.4 mM sodium citrate, 0.1 mM ethylenediaminetetraacetic acid, and 20 μg/mL PI (all reagents from Sigma, St Louis, MO). Thereafter, cellular DNA content was analyzed by flow cytometry, and histograms were analyzed with ModFit LT software (Verity Software House, Topsham, ME).
Surface IL-6 receptor
The expression of the IL-6 receptor α chain (gp80) in OH-2 cells was determined by flow cytometry. Cells were incubated with 0.5 μg mouse monoclonal antibody (clone BR-6; Biosource, Camarillo, CA) or irrelevant isotype-matched IgG1 monoclonal antibody (DAKO, Glostrup, Denmark) as a control. As a secondary antibody, FITC-conjugated goat anti-mouse immunoglobulin (Becton Dickinson) was used. The cells were simultaneously incubated with PI to exclude dead cells.
Stat3 Western blot analysis
1.5 × 106 cells were incubated with or without cytokines. Cells were washed once in cold phosphate-buffered saline and centrifuged, and the resultant cell pellets were frozen at −70°C. The cell pellets were solubilized in 100 μL sodium dodecyl sulfate (SDS) sample buffer (100 mM Tris/HCl, pH 6.8, 10% SDS, 40% glycerol, and 0.005% bromophenol blue). DNA was sheared by repeated pipetting, samples were boiled, and aliquots of 25 μL were loaded on 10% SDS polyacrylamide gels. Proteins in the gels were transferred to nitrocellulose filters (Bio-Rad, Hercules, CA). Filters were blocked in 5% skimmed milk in 0.05% Tween 20–Tris-buffered saline, pH 7.4, before probing with antibodies against Stat3 or against Stat3 containing phosphotyrosine at amino acid residue 705 (catalog numbers 9132 and 9131, respectively; New England Biolabs, Beverly, MA). Bound antibodies were visualized by enhanced chemiluminescence using horseradish peroxidase-conjugated goat antirabbit immunoglobulin, as described by the manufacturer (Amersham Pharmacia Biotech, Uppsala, Sweden).
Results
BMP-4 inhibits DNA synthesis and induces apoptosis in IL-6–dependent myeloma cell lines
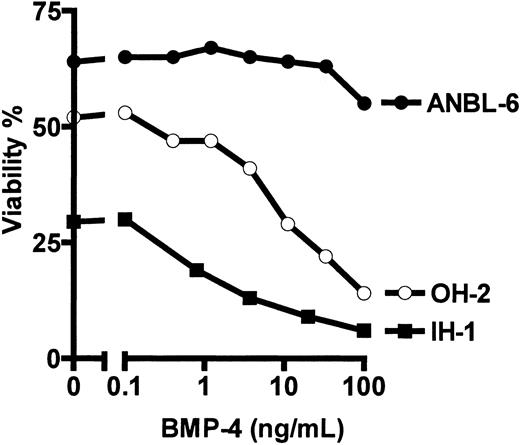
Measured by [3H] thymidine incorporation, BMP-4 dose dependently reduced DNA synthesis in 3 available IL-6–dependent cell lines, IH-1, OH-2, and ANBL-6 (Figure 1). In the IL-6–independent cell lines JJN-3, RPMI 8226, and U266, there was little or no effect of BMP-4 (not shown).
BMP-4 inhibits DNA synthesis in IL-6–dependent myeloma cell lines.
Cell lines were stimulated with 0.1 ng/mL IL-6 (IH-1 and OH-2) or 1 ng/mL IL-6 (ANBL-6), with or without BMP-4, in concentrations as indicated for 72 hours. DNA synthesis was measured by [3H]-thymidine incorporation. Counts were normalized; 100% represents the count obtained with IL-6 alone (absolute counts were 1500 counts per minute (cpm) for IH-1, 2400 cpm for ANBL-6, and 900 cpm for OH-2).
BMP-4 inhibits DNA synthesis in IL-6–dependent myeloma cell lines.
Cell lines were stimulated with 0.1 ng/mL IL-6 (IH-1 and OH-2) or 1 ng/mL IL-6 (ANBL-6), with or without BMP-4, in concentrations as indicated for 72 hours. DNA synthesis was measured by [3H]-thymidine incorporation. Counts were normalized; 100% represents the count obtained with IL-6 alone (absolute counts were 1500 counts per minute (cpm) for IH-1, 2400 cpm for ANBL-6, and 900 cpm for OH-2).
To determine whether the effect of BMP-4 on DNA synthesis was caused by apoptosis, cells were labeled with annexin V–FITC and PI and analyzed by flow cytometry. In Figure 2 we show that BMP-4 dose dependently induced apoptosis in OH-2 and IH-1 cells. In ANBL-6 cells, we observed a decrease in viability of 8% at the highest BMP-4 concentration (100 ng/mL). A high fraction of dead cells was regularly observed in IH-1 cells after cell washing and pipetting, and only 30% of cells were viable in cultures with IL-6 but without BMP-4. The kinetics of BMP-4–induced loss of viability in OH-2 cells are shown in Figure 3. Within the timeframe of observation, the BMP-4 effect on viability did not wane. The relation between IL-6 and BMP-4 effects on viability (OH-2) and DNA synthesis (ANBL-6) is shown in Figure 4. In these cell lines, the effects of BMP-4 could only partially be overridden by increasing concentrations of IL-6. Furthermore, BMP-4 decreased cellular viability to lower levels than IL-6 starvation alone in OH-2 cells.
BMP-4 reduces viability of OH-2 and IH-1 cells but not in ANBL-6 cells.
Annexin V–FITC and PI binding were measured by flow cytometry in myeloma cell lines incubated for 72 hours with BMP-4 in concentrations as indicated and 1 ng/mL IL-6 (ANBL-6) or 0.1 ng/mL IL-6 (IH-1 and OH-2). Percentage of viable cells, located in the lower left quadrant of dot plots, as exemplified in Figure 7, was recorded.
BMP-4 reduces viability of OH-2 and IH-1 cells but not in ANBL-6 cells.
Annexin V–FITC and PI binding were measured by flow cytometry in myeloma cell lines incubated for 72 hours with BMP-4 in concentrations as indicated and 1 ng/mL IL-6 (ANBL-6) or 0.1 ng/mL IL-6 (IH-1 and OH-2). Percentage of viable cells, located in the lower left quadrant of dot plots, as exemplified in Figure 7, was recorded.
The effect of BMP-4 in OH-2 cells does not wane with time.
Cellular viability in OH-2 cells was measured daily by annexin V–FITC and PI flow cytometry after incubation without cytokines or with 10 ng/mL BMP-4 or 1 ng/mL IL-6, as indicated. Data are expressed as the percentage of viable cells—that is, the percentage of cells in the lower left quadrant of dot plots, as exemplified in Figure7.
The effect of BMP-4 in OH-2 cells does not wane with time.
Cellular viability in OH-2 cells was measured daily by annexin V–FITC and PI flow cytometry after incubation without cytokines or with 10 ng/mL BMP-4 or 1 ng/mL IL-6, as indicated. Data are expressed as the percentage of viable cells—that is, the percentage of cells in the lower left quadrant of dot plots, as exemplified in Figure7.
Combined effects of BMP-4 and IL-6 in OH-2 and ANBL-6 cells.
Cells were incubated with cytokines as indicated for 72 hours. Effects on cellular viability measured by annexin V–FITC and PI flow cytometry in OH-2 cells. Effects on DNA synthesis measured by [3H]-thymidine incorporation in ANBL-6 cells.
Combined effects of BMP-4 and IL-6 in OH-2 and ANBL-6 cells.
Cells were incubated with cytokines as indicated for 72 hours. Effects on cellular viability measured by annexin V–FITC and PI flow cytometry in OH-2 cells. Effects on DNA synthesis measured by [3H]-thymidine incorporation in ANBL-6 cells.
Effect of BMP-4 on cell cycle distribution in ANBL-6, IH-1, and OH-2 cells
To characterize further the effect of BMP-4 on DNA synthesis, analysis of cell cycle distribution was performed. ANBL-6, IH-1, and OH-2 cells were cultured with IL-6, with or without BMP-4. As shown in Figure 5, BMP-4 induced growth arrest in the G0/G1 phase in both OH-2 and ANBL-6 cells. In contrast, no effect on cell cycle distribution was observed in IH-1 cells (data not shown).
BMP-4 induces cell cycle arrest in OH-2 and ANBL-6 cells.
OH-2 cells were incubated with 0.1 ng/mL IL-6, with or without 20 ng/mL BMP-4 (upper histograms). ANBL-6 cells were incubated with 1 ng/mL IL-6, with or without 100 ng/mL of BMP-4 (lower histograms). After cytokine stimulation for 18 hours, cells were washed, permeabilized, and stained with PI. Cellular DNA content was measured by flow cytometry. The percentage of cells in the G0/G1phase of the cell cycle is indicated in each histogram.
BMP-4 induces cell cycle arrest in OH-2 and ANBL-6 cells.
OH-2 cells were incubated with 0.1 ng/mL IL-6, with or without 20 ng/mL BMP-4 (upper histograms). ANBL-6 cells were incubated with 1 ng/mL IL-6, with or without 100 ng/mL of BMP-4 (lower histograms). After cytokine stimulation for 18 hours, cells were washed, permeabilized, and stained with PI. Cellular DNA content was measured by flow cytometry. The percentage of cells in the G0/G1phase of the cell cycle is indicated in each histogram.
BMP-4 reduces Stat3 tyrosine phosphorylation in myeloma cell lines
The anti-apoptotic effect of IL-6 in myeloma cells is possibly mediated by tyrosine phosphorylation of the Stat3 protein; therefore, we investigated whether this was affected by BMP treatment.38 A Western blot showing tyrosine 705 phosphorylation of Stat3 in OH-2 and ANBL-6 after 4 hours of incubation with cytokines is displayed in Figure 6. IL-6 dose dependently up-regulated Stat3 phosphorylation in both cell lines (lanes 1-3). The constitutive phosphorylation of tyrosine 705 in unstimulated OH-2 cells (lane 1) was abrogated by BMP-4 (lanes 4 and 5). BMP-4 counteracted IL-6–dependent Stat3 phosphorylation in a dose-dependent manner (compare lanes 2, 6, and 7 for 0.1 ng/mL IL-6 and lanes 3, 8, and 9 for 1 ng/mL IL-6) in both cell lines. The decrease in Stat3 phosphorylation was present as early as 30 minutes after the addition of BMP-4 in OH-2 cells (data not shown).
BMP-4 inhibits IL-6–induced Stat3 phosphorylation in IL-6–dependent myeloma cell lines.
Cells were incubated for 4 hours with cytokines in concentrations (ng/mL) as indicated. Cellular lysates were analyzed by Western blot. The lower panels show the total Stat3 protein staining, and the upper panels show specific tyrosine phosphorylation of Stat3 amino acid residue 705.
BMP-4 inhibits IL-6–induced Stat3 phosphorylation in IL-6–dependent myeloma cell lines.
Cells were incubated for 4 hours with cytokines in concentrations (ng/mL) as indicated. Cellular lysates were analyzed by Western blot. The lower panels show the total Stat3 protein staining, and the upper panels show specific tyrosine phosphorylation of Stat3 amino acid residue 705.
Surface IL-6R (gp80) on OH-2 cells is down-regulated late in the apoptotic process
The surface expression of gp80 was measured by flow cytometry. After 48 hours, we found that BMP-4 reduced the mean fluorescence intensity by 34% (range, 10%-52%, 5 experiments, data not shown). However, when samples were stimulated for 20 hours only, no reduction of gp80 expression was found. Consequently, it appears that the apoptotic process began before the expression of gp80 was affected (Figure 3).
Effects of BMP-4 in primary myeloma cells
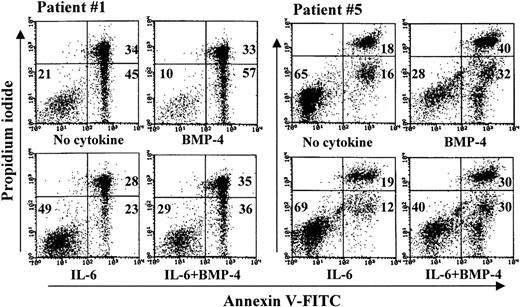
Purified MM cells from 13 patients were incubated without cytokines, with BMP-4 and IL-6 alone or in combination, and assayed by annexin and PI flow cytometry as shown in Table 1. Response to cytokine was defined as a more than 10% absolute change in viability compared with unstimulated cells. Using this definition, we observed effects of BMP-4 alone in 4 of 13 samples. In 3 of these patients (patients 1, 9, and 11), we also observed effects of IL-6. In addition, when cells were stimulated with BMP-4 and IL-6 in combination, BMP-4 modified the effect of IL-6 in accordance with our definition in these 3 patients. This interaction is exemplified in Figure7 (patient 1). Next, we show that primary cells from patient 5 responded to BMP-4 but it gave no clear response to IL-6 alone (Figure 7). However, the effect of BMP-4 was partly counteracted by IL-6, suggesting an interaction between BMP-4 and IL-6 signaling also in these cells. In patient 5 we determined cellular viability at 2 time points, at 3 and 5 days, and found that the effect of BMP-4 increased with time in comparison with our findings in cell lines (data not shown).
BMP-4 induces apoptosis in samples of purified primary myeloma cells.
Primary myeloma cells from patients 1 and 5 were stimulated with 50 ng/mL BMP-4 1 ng/mL IL-6, or both, as indicated, and annexin V–FITC and PI binding were measured by flow cytometry. Numbers represent the percentage of cells in the respective quadrants. Cells in the lower left quadrant were considered viable, in the lower right quadrant apoptotic, and in the upper quadrants dead.
BMP-4 induces apoptosis in samples of purified primary myeloma cells.
Primary myeloma cells from patients 1 and 5 were stimulated with 50 ng/mL BMP-4 1 ng/mL IL-6, or both, as indicated, and annexin V–FITC and PI binding were measured by flow cytometry. Numbers represent the percentage of cells in the respective quadrants. Cells in the lower left quadrant were considered viable, in the lower right quadrant apoptotic, and in the upper quadrants dead.
In Figure 8 we show Stat3 phosphorylation of cells from patient 11, with a pattern similar to that shown previously for OH-2 and ANBL-6 cells. Of note, these primary purified myeloma cells derive from the same sample as cells used for the establishment of the IH-1 cell line. Apparently, there was no constitutive phosphorylation of Stat3, but IL-6 induced phosphorylation in a dose-dependent manner. Again, BMP-4 was able to counteract this effect.
BMP-4 inhibits IL-6–induced Stat3 phosphorylation in primary myeloma cells.
Cells from patient 11 were incubated for 4 hours with cytokines, as indicated. Cellular lysates were analyzed by Western blot. Lower panels show total Stat3 protein, and upper panels show specific tyrosine phosphorylation of Stat3 amino acid residue 705.
BMP-4 inhibits IL-6–induced Stat3 phosphorylation in primary myeloma cells.
Cells from patient 11 were incubated for 4 hours with cytokines, as indicated. Cellular lysates were analyzed by Western blot. Lower panels show total Stat3 protein, and upper panels show specific tyrosine phosphorylation of Stat3 amino acid residue 705.
Discussion
The results from this study provide evidence that a member of the BMP family can inhibit proliferation or induce apoptosis of human myeloma cells. This was found in 3 IL-6–dependent, but not in 3 IL-6–independent, cell lines. The relevance to MM is strengthened by the observation that the pro-apoptotic effect of BMP-4 was not merely a cell line phenomenon but was also found in 4 of 13 freshly isolated primary samples.
Given the role of IL-6 as a main myeloma cell growth factor in vivo and in vitro, it is important to identify factors that may counteract IL-6, particularly because few substances have been described that inhibit myeloma cell growth or survival. Effects of BMP-4 on DNA synthesis and apoptosis were found in IL-6–dependent myeloma cell lines only. Furthermore, in 4 primary samples with effects of BMP-4 on viability, 3 were responsive to IL-6 alone. Myeloma cells from the fourth patient (patient 5 in Table 1) that responded to BMP-4 were not clearly affected by the addition of IL-6 alone. However, the effect of BMP-4 on viability was partly counteracted by IL-6, also suggesting that in these cells there was an interaction between BMP-4 signaling and IL-6 signaling. IL-6 binds to its specific α-receptor subunit (gp80), and this complex in turn recruits gp130, the common receptor subunit for members of the IL-6 cytokine family. Receptor signaling is mediated by gp130 and involves 2 downstream pathways—the Ras-dependent mitogen-activated protein kinase pathway and the Janus kinases/signal transducer and activator of transcription (JAK/STAT) cascade.1,2,39,40 In the OH-2 and ANBL-6 cell lines and in the primary sample from which IH-1 cells were derived, the effects of BMP-4 correlated with dephosphorylation of Stat3. The exact mechanism for this modulation of Stat3 phosphorylation is unknown. BMPs bind to transmembrane receptors with serine–threonine kinase activity, which subsequently activate the Smad signaling pathway.41,42 In OH-2 cells, dephosphorylation of Stat3 was evident as early as 30 minutes after the addition of BMP-4. It is, therefore, possible that BMP-4 directly activates one or more proteins, such as a protein tyrosine phosphatase, to interact with Stat3 or proteins directly upstream of Stat3 in the IL-6–signaling pathway.43
Although BMP-4 clearly inhibited DNA synthesis in all 3 IL-6–dependent cell lines, a heterogeneous effect on apoptosis and cell cycle arrest was observed. In IH-1 cells, we were unable to detect effects on cell cycle distribution. Furthermore, the BMP-4–induced effects on DNA synthesis and cell cycle distribution in ANBL-6 cells did not correspond with any significant increase in apoptosis. This finding may reflect differences in the expression of factors involved in the apoptotic process. For instance, overexpression of anti-apoptotic members of the Bcl-2 family, such as Bcl-2 and Bcl-XL, may lead to a high resistance to apoptosis.44-46
Although the effect of BMP-4 on DNA synthesis and viability in myeloma cells correlated with effects on Stat3 phosphorylation, various other mechanisms mediating the effect of BMP-4 may also be present. In other cell types, the effects of BMPs are mediated through signaling pathways that induce growth arrest and apoptosis independently of IL-6.41,42,47-49 For instance, BMP-2 has been reported to induce apoptosis in an IL-6–independent hybridoma cell line.47 Furthermore, in B9 hybridoma cells, which are dependent on IL-6 for cell growth, we found no effect on Stat3 phosphorylation, in spite of readily detectable apoptosis and growth arrest induced by BMP-4 (our unpublished data). Therefore, BMP-4 may affect proliferation and apoptosis in myeloma cells by interference with the JAK/STAT pathway but possibly also through other pathways.
Various compounds induce apoptosis in myeloma cells, including FasL, retinoic acid, IFN-γ, IFN-β, and chemotherapeutic agents. However, the effects of these compounds on growth and apoptosis of myeloma cells are variable in different cell lines and between individual patients, similar to our findings with BMP-4. In many instances, however, IL-6 counteracts these effects, frequently by regulating proteins of the Bcl-2 family.3,9,38,44,45,50-57 Resistance to the induction of apoptosis is common in myeloma. For instance, JJN-3 cells are extremely sensitive whereas OH-2 cells are relatively resistant to the induction of apoptosis by FasL.12 This is the reverse of the responses to BMP-4 reported here, and it demonstrates that in both cell lines, the apoptotic machinery is present and inducible, but with different agents. This suggests that successful treatment with apoptosis-inducing agents should include a combination of approaches; in this context, BMP-4 represents a novel strategy.
The role of BMPs has been addressed in several malignancies in which BMPs frequently decrease the proliferation of tumor cells in comparison with our findings in myeloma.25-27,58 59 Induction of apoptosis by BMPs has to our knowledge not been demonstrated before in human tumor cells.
In conclusion, we have found that BMP-4 inhibits proliferation and induces apoptosis in myeloma cells. Although difficulties concerning the mode of administration and the safety of BMP-4 are unresolved, our data suggest that treatment with BMP-4 or its analogues should be tried in animal models and possibly in patients with MM. The activation of BMP receptors may reduce tumor burden and ameliorate bone disease, and it represents a new target for the treatment of this disease.
Acknowledgments
We thank Berit F. Stördal and Hanne Hella for excellent technical work.
Supported by grants from the Norwegian Cancer Society, Blix' legat, and the Cancer Fund, Trondheim University Hospital.
Ö.H. and H.H.-H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Öyvind Hjertner, Institute of Cancer Research and Molecular Biology, Norwegian University of Science and Technology, MTFS, N-7489 Trondheim, Norway; e-mail:oyvind.hjertner@medisin.ntnu.no.

![Fig. 1. BMP-4 inhibits DNA synthesis in IL-6–dependent myeloma cell lines. / Cell lines were stimulated with 0.1 ng/mL IL-6 (IH-1 and OH-2) or 1 ng/mL IL-6 (ANBL-6), with or without BMP-4, in concentrations as indicated for 72 hours. DNA synthesis was measured by [3H]-thymidine incorporation. Counts were normalized; 100% represents the count obtained with IL-6 alone (absolute counts were 1500 counts per minute (cpm) for IH-1, 2400 cpm for ANBL-6, and 900 cpm for OH-2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.516/5/m_h80210582001.jpeg?Expires=1767791417&Signature=DyWEHoKd~mx7ShuzW-3oRxzia3emTSGALir6v5boP5hU8l~pPMdfv65g2-aLvxh8yW69ExPnVGGCgsCVlLoJFpaN8vF3rjQ0-1KkQZXj66rN44ygZJRrHRnTcHCT9V0m58bSZWZnK69f2AvQR76SZLO5KRDcVmCJGvUSqJ4716crNeJlR9xllOau9t43CZb7QPDuSZ0KQpDPe63Ph82jqPB4mXeDySLdCGUw6veU4ClPVK0FDGi4bds9w3vGcC84ui~LtXTR-i73Y5EucwbD1uaSBeGvxz~X~0lWFx0~IFpXHVMNxyLo9shycIVoBlcLOFz~0mKuseMA7indPvOBzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Combined effects of BMP-4 and IL-6 in OH-2 and ANBL-6 cells. / Cells were incubated with cytokines as indicated for 72 hours. Effects on cellular viability measured by annexin V–FITC and PI flow cytometry in OH-2 cells. Effects on DNA synthesis measured by [3H]-thymidine incorporation in ANBL-6 cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.516/5/m_h80210582004.jpeg?Expires=1767791417&Signature=IBXmOux1VY42~Z5fdgTSPszhn4VK7vk8KO3KUSYAjPaL~OUVOUEorhLq0eZ5hg7Rzbaaz-cxLgVrh0AE~G~u8am6afXc5QnD5PKC0kDqdYhEci~yyu4cEc98OyXIF8YywBVxlnD1TbXbgfsiwtwte1GYkqsq-pmcBNVMS1v7R-hyc0NOXYOQfNMN52fay6vlkDY0U9by3NgoocQ3hvzDe1yqdY43MfoB4jgO3CAiLary-UBM3917WNJymk8Nz3QeLRIKGjPMHCHi8YJBcT34DCyV-VjKXeINBWUFSUvMUrfGvtOcOiWrTlUC~fHPLVSoDCaN1hQfdSRodgulg47Hkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal