Successful autologous hematopoietic stem cell (HSC) transplantation in childhood acute lymphoblastic leukemia (ALL) requires the ability to either selectively kill the leukemia cells or separate normal from leukemic HSC. Based on previous studies showing that more than 95% of childhood B-lineage ALL express CD38, this study evaluated whether normal CD34+CD38− progenitors from children with B-lineage ALL could be isolated by flow cytometry. CD34+ cells from bone marrow samples from 10 children with B-lineage ALL were isolated at day 28 of treatment, when clinical remission had been attained. The CD34+ progenitor cells were flow cytometrically sorted into CD34+CD38+and CD34+CD38− populations. The absolute numbers of CD34+CD38− cells that could be isolated ranged from 401 to 6245. The cells were then analyzed for the presence of clonotypic rearrangements of the T-cell receptor (TCR) Vδ2-Dδ3 locus. Only patients whose diagnostic marrow had an informative TCR Vδ2-Dδ3 rearrangement were included in this study. Detection thresholds were typically 10−4 to 10−5 leukemic cells in normal marrow. In 6 of 10 samples analyzed, the sorted CD34+CD38− cells had no detectable Vδ2-Dδ3 rearrangements. In 4 cases, the clonotypic leukemic Vδ2-Dδ3 rearrangement was detected in the CD34+CD38− population, indicating that the putative normal HSC population also contained leukemic cells. The data indicate that although most childhood ALL cells express CD34 and CD38, leukemic cells are also frequently present in the CD34+CD38− population. Therefore, strategies to isolate and transplant normal HSC from children with ALL will require a more stringent definition of the normal HSC than the CD34+CD38− phenotype.
Introduction
Acute lymphoblastic leukemia (ALL) represents the clonal proliferation of malignantly transformed lymphoid progenitors in the bone marrow and is the most common malignancy in the pediatric population. Close to 80% of ALL cases are a result of clonal expansion of B-cell precursors and contain rearrangements in the heavy-chain immunoglobulin (IgH) gene.2 In addition, cross-lineage T-cell receptor (TCR) gene rearrangements frequently occur in B-cell precursor ALL: rearranged TCR β, TCR γ, and TCR δ are found in 35%, 55%, and 90% of cases, respectively.1 Thus polymerase chain reaction (PCR) analysis of immunoreceptor gene rearrangements can be used for clonality studies in lymphoid leukemias.1 CompleteTCR δ gene rearrangements can be analyzed easily with the PCR technique because the TCR δ genes contain a limited number of V and J gene segments; hence, only a limited number of oligonucleotide primers is needed.
The optimal treatment for recurrent ALL is allogeneic bone marrow transplant (BMT). Unfortunately, only 30% of patients who require a transplant will have an HLA-identical sibling donor.3Other potential marrow donors include HLA-phenotypically identical unrelated donors. However, there is a higher risk of serious or fatal graft-versus-host disease after unrelated donor BMT.
Autologous BMT is an alternative solution to the problems associated with unrelated BMT, because all patients would have a donor. However, difficulty in obtaining leukemia-free stem cells from patients with ALL has hindered the ability to perform autologous BMT. The major problem after autologous BMT is relapse of the disease. Autologous marrow may contain leukemic cells that are responsible for the relapse.4 To overcome this problem, autologous BMT could be performed either after depletion of leukemic cells or after positive selection of normal hematopoietic stem cells (HSCs). Methods to deplete autologous marrow of leukemic cells have generally included in vitro chemotherapy, immunologic depletion, or the use of immunotoxins, but these may be either toxic to normal HSCs or fail to completely remove leukemic cells.5-7
Therefore, we tested the feasibility of positive selection of normal HSCs based on immunophenotype. Our previous studies showed that more than 95% of childhood B-lineage ALL express CD38.8Terstappen and colleagues identified a subpopulation of progenitor cells with the CD34+CD38− immunophenotype, which may define the most primitive progenitors.9 Hao and coworkers have identified a similar subpopulation in umbilical cord blood.10 This rare, quiescent subpopulation has the ability to produce colony-forming units from extended long-term cultures. The CD34+CD38− cells lack expression of most lineage-specific antigens.
This study was designed to test whether normal CD34+CD38− progenitor cells, free of leukemia, could be isolated by flow cytometry from children with B-lineage ALL. Only patients with B-cell precursor ALL whose diagnostic marrow had an informative TCR Vδ2-Dδ3 rearrangement were included in this study. CD34+ cells were isolated at day 28 of treatment, when clinical remission had been attained. These cells were then flow cytometrically sorted into CD34+CD38+ and CD34+CD38− populations and then analyzed for the presence of clonotypic rearrangements of the TCR Vδ2-Dδ3 locus. The TCR Vδ2-Dδ3 rearrangements were detected by PCR amplification and Southern hybridization to an oligonucleotide probe derived from the sequence of the TCR Vδ2-Dδ3 N-region in the patients' diagnostic marrow sample.
Patients, materials, and methods
Patients
Bone marrow samples were obtained for the study from children diagnosed with ALL at Childrens Hospital Los Angeles (CHLA). B-lineage ALL was classified on the basis of FAB morphology. The patients included in the study were FAB L1 or L2 and had expression of B-lineage markers on their leukemia blasts, namely, HLA-DR, CD19+/−, and CD10. Most patients had CD34 expression on their leukemic blasts. The diagnostic leukemia samples were CD38+. The sample obtained prior to beginning therapy was designated the diagnostic marrow. A second bone marrow sample was obtained from patients who were informative for a TCR Vδ2-Dδ3 rearrangement, when clinical remission had been attained, usually on day 28 of treatment. The studies were performed in accordance with a protocol approved by the CHLA Committee on Clinical Investigations (institutional review board) and with the informed consent of the patients and parents.
Analysis of clonal rearrangements
DNA extraction.
Mononuclear cells were obtained from the diagnostic marrow by gradient centrifugation over Ficoll-Hypaque (Amersham Pharmacia, Piscataway, NJ), if adequate marrow was available. The cells were then lysed in a buffer containing 1 M Tris, 0.5 M EDTA, and 10% sodium dodecyl sulfate, and incubated at 37°C overnight in the presence of 0.5 mg/mL proteinase K (Life Technologies, Rockville, MD). If availability of diagnostic marrow was limited to less than 200 μL, the erythrocytes were lysed by resuspending the cells in 1 mL Orthomune lysis buffer (Ortho, Raritan, NJ) for 5 minutes at room temperature. After centrifugation, the erythrocyte lysis step was repeated. DNA was isolated by phenol-chloroform extraction, followed by ethanol precipitation.
PCR.
The PCR analysis was performed on DNA from diagnostic bone marrow allowing the detection of TCR Vδ2-Dδ3 rearrangements. Reaction mixtures contained 500 ng DNA, 20 pmoles of each 5′ and 3′ primer (primers 9 and 1011; Table1), 200 μM of each deoxynucleotide triphosphate, 1.5 mM MgCl2, 1 × GeneAmp buffer (10 mM Tris, pH 8.3, 50 mM KCl) (PerkinElmer, Branchburg, NJ) in a 50-μL volume. The reaction mixture was subjected to denaturation at 95°C for 5 minutes, followed by annealing at 52°C, for 2 minutes 43 seconds. Primer extension was started by the addition of 1.5 U Amplitaq Polymerase (PerkinElmer) and allowed to continue for 5 minutes at 72°C. Subsequent denaturation, annealing, and extension steps were carried out at 92°C for 50 seconds, 52°C for 1 minute 40 seconds, and 72°C for 1 minute 50 seconds for 35 cycles. Amplification products were analyzed on 1.5% agarose gels and discrete bands (∼100 bp) corresponding to clonal rearrangements were excised from the gel.
N-regions and flanking germline sequences in B-cell precursor acute lymphoblastic leukemia patients showing T-cell receptor Vδ2-Dδ3 rearrangements. The sequence used to generate patient-specific clonogenic probes is shown in bold and underlined
| Patient . | Vδ2 . | N . | Dδ3 . |
|---|---|---|---|
| Germline | GTGCCTGTGACACC | ACTGGGGGATAC | |
| 1 | GTGCCTGTGACACC | GGGGAGAGGGTCCCACAC | ACTGGGGGATAC |
| 2 | GTGCCTGTGAC | CTCGGGTCCTT | ACTGGGGGATAC |
| 3 | GTGCCTGTGACACC | CGGGGGTTCT | TGGGGGATAC |
| 4 | GTGCCTGTGAC | CCCCCCCCGAGG | GGGGGATAC |
| 5 | GTGCCTGTGAC | CCCCGGAGAACCCCAGGCCGGACAGACAGACTAGT | ACTGGGGGATACGC |
| 6 | GTGCCTGTGACACC | TCTGGTACCCTTCGCCCGTTCGCTCCCTAGGCG | TGGGGGATACGC |
| 7 | GTGCCTGTGAC | CC | ACTGGGGGATACGC |
| 8 | GTGCCTGTGACAC | GGGG | TGGGGGATACGC |
| 9 | GTGCCTGT | CTG | GGGGGATACGC |
| 10 | GTGCCTGTGA | GGGG | TGGGGGATACGC |
| Patient . | Vδ2 . | N . | Dδ3 . |
|---|---|---|---|
| Germline | GTGCCTGTGACACC | ACTGGGGGATAC | |
| 1 | GTGCCTGTGACACC | GGGGAGAGGGTCCCACAC | ACTGGGGGATAC |
| 2 | GTGCCTGTGAC | CTCGGGTCCTT | ACTGGGGGATAC |
| 3 | GTGCCTGTGACACC | CGGGGGTTCT | TGGGGGATAC |
| 4 | GTGCCTGTGAC | CCCCCCCCGAGG | GGGGGATAC |
| 5 | GTGCCTGTGAC | CCCCGGAGAACCCCAGGCCGGACAGACAGACTAGT | ACTGGGGGATACGC |
| 6 | GTGCCTGTGACACC | TCTGGTACCCTTCGCCCGTTCGCTCCCTAGGCG | TGGGGGATACGC |
| 7 | GTGCCTGTGAC | CC | ACTGGGGGATACGC |
| 8 | GTGCCTGTGACAC | GGGG | TGGGGGATACGC |
| 9 | GTGCCTGT | CTG | GGGGGATACGC |
| 10 | GTGCCTGTGA | GGGG | TGGGGGATACGC |
Primers used for detection of TCR Vδ2-Dδ3 rearrangements were as described by Campana and coworkers.11 Primer 8: 5′ GAGTCATGTCAGCCATTGAG 3". Primer 9: 5′ GCACCATCAGAGAGAGATGA 3′. Primer 10: 5′ TTGTAGCACTGTGCGTATCC 3′. Primer 11: 5′ AGGGAAATGGCACTTTTGCC 3′. Primers 9 and 10 are nested within the region amplified by primers 8 and 11.
Generation of clonospecific probes.
Gel slices were minced and DNA eluted in TE using a Spin-X column (Corning, Corning, NY). DNA was precipitated in ethanol and dissolved in 3 μL water. Purified amplification products were cloned into pGEM plasmids (Promega, Madison, WI) and used to transform DH5α competent cells. Minipreps of plasmid DNA from 6 to 12 individual clones were PCR amplified using the plasmid promoter sequences T7 and SP6, and sequenced. Oligonucleotide probes corresponding to the N-region of each patient's TCR Vδ2-Dδ3 rearrangements were synthesized, if there was consensus among the 6 or 12 clones. The probes included flanking germline sequences, minus exonuclease trimmed sequences as appropriate. Clonogenic probes were generated in this manner for each patient in whom a TCR Vδ2-Dδ3 rearrangement was found.
Evaluation probe specificity.
Genomic DNA (500 ng) from the patients' diagnostic marrow and DNA from peripheral blood of healthy normal controls was amplified in a 50-μL reaction containing 20 pmoles of each of the 5′ and 3′ primers (primers 8 and 11,11 Table 1), 200 μL of each deoxynucleotide triphosphate, 1.5 mM MgCl2, 1 × GeneAmp buffer (10 mM Tris, pH 8.3, 50 mM KCl). The PCR reaction mixture was incubated for 5 minutes at 95°C and for 2 minutes 37 seconds at 58°C. Primer extension was started by the addition of 1.5 U Amplitaq polymerase and allowed to continue for 5 minutes at 72°C. After this initial cycle, denaturing, annealing, and extension steps were carried out at 92°C for 50 seconds, 58°C for 1 minute 34 seconds, and 72°C for 1 minute 44 seconds for 35 cycles. The amplification product, which ranged from 350 to 380 bp, was electrophoresed on 1.5% agarose gels and transferred onto a charged nylon membrane (MSI, Westborough, MA). Southern hybridization was carried out using clonospecific probes end-labeled with 32P using T4 polynucleotide kinase, to be sure that the probe only hybridized to the leukemic cell DNA of the patient from whom it was derived. Probes that showed cross-hybridization to DNA from normal controls were not included in the study.
Processing of remission marrow
Fluorescent antibody labeling and cell sorting.
Mononuclear cells were isolated from the remission marrow by gradient centrifugation over Ficoll-Hypaque and washed with Hanks balanced saline solution (HBSS). After erythrocytes were lysed with Orthomune lysis buffer, the cells were washed with phosphate-buffered saline (PBS) and 200 000 cells were set apart to be used as a positive control for PCR amplification of the Vδ2-Dδ3 rearrangement. MAC MS+ separation columns (Miltenyi Biotech, Auburn, CA) were used for positive selection of CD34+cells. CD34+ cells were resuspended in PBS at 106 cells/100 μL for incubation with fluorescently labeled antibodies. Cells were incubated for 20 minutes at 4°C, with 20 μL of fluorescein isothiocyanate (FITC)-conjugated anti-CD34 (Becton Dickinson Immunocytometry Systems, San Jose, CA) and 20 μL of phycoerythrin (PE)-conjugated anti-CD38 (Becton Dickinson Immunocytometry Systems). CD34− cells used for isotype controls were incubated for 20 minutes in 50 μL FITC-murine IgG (diluted 1:100; Coulter, Hialeah, FL) and 50 μL PE-murine IgG (diluted 1:50, Coulter). Following incubation, cells were washed with PBS and sorted into CD34+CD38+ and CD34+CD38− subpopulations on a FACSVantage (Becton Dickinson Immunocytometry Systems). CD34+CD38− cells were defined as those that were CD34bright and having CD38 PE fluorescence less than one half of the maximum PE fluorescence of the isotype control.
DNA was extracted from each of the sorted cell fractions. Cells were spun down and lysed overnight in 200 μL of the proteinase K–containing buffer at 37°C, followed by phenol-chloroform extraction. DNA was ethanol precipitated using 0.1 M ammonium acetate and 120 μg glycogen (Boehringer Mannheim, Indianapolis, IN) as a carrier and resuspended in 10 μL water.
Detection of TCR Vδ2-Dδ3 rearrangements by PCR and Southern hybridization.
All of the 10 μL DNA obtained from the sorted cell populations was amplified by PCR in a 50-μL reaction as described earlier, using primers 8 and 1111 (Table 1). DNA (500 ng) from the diagnostic marrow and DNA from the unsorted remission marrow were included as positive controls. In addition, to determine the limit of sensitivity of detection of leukemic cell populations, genomic DNA from diagnostic marrow was diluted into DNA from peripheral blood of normal controls at 10−1 to 10−7 and amplified in the same manner. PCR amplification products were electrophoresed on 1.5% agarose gels and transferred onto charged nylon membranes. Clonospecific probes were end labeled and hybridization carried out as described earlier.
Results
Identification of Vδ2-Dδ3 rearrangements in the diagnostic bone marrow of patients with childhood ALL
Ten patients whose diagnostic marrow had an informative TCR Vδ2-Dδ3 rearrangement were included in this study. The Vδ2-Dδ3 sequences of the patients are given in Table 1. Rearrangement of the TCR δ locus sometimes results in the trimming of the germline sequences flanking the inserted N regions, with a resulting increase in diversity. The region used to generate patient-specific clonogenic probes (ranging from 17-35 bp) is given in bold and underlined in each case.
Variability in the detection of clonotypic Vδ2-Dδ3 rearrangements in the CD34+CD38− population from the remission marrow of patients with childhood ALL
The remission marrow was enriched for CD34+ cells, then stained with anti-CD34 FITC and anti-CD38 PE and flow cytometrically sorted into CD34+CD38+ and CD34+CD38− populations. To minimize the likelihood of contamination of the CD34+CD38−population with CD34+CD38+ cells during the sort, a very rigid criterion was used to define CD34+CD38− cells as only those that were CD34bright, and having CD38 PE fluorescence less than one half of the maximum PE fluorescence of the isotype control. The numbers of CD34+CD38+ cells isolated ranged from 20 006 to 472 271 cells and that of the CD34+CD38− cells ranged from 401 to 6245 cells (Table 2). Samples in which the number of CD34+CD38− cells fell below 250 cells were excluded from the study. Both populations were analyzed for the presence of clonotypic rearrangements of the TCR Vδ2-Dδ3 locus by PCR.
Yield of CD34+CD38+ and CD34+CD38− cells by FACS and detection of clonotypic T-cell receptor Vδ2-Dδ3 rearrangements in remission marrow
| Patient . | Remission marrow* . | Detection limit of leukemic cell DNA diluted into normal DNA . | Immunophenotype of diagnostic bone marrow . | Presence of clonotypic TCR Vδ2-Dδ3 rearrangement in CD34+CD38− cells . | ||
|---|---|---|---|---|---|---|
| No. CD34+CD38+ cells . | No. CD34+ CD38−cells . | % CD34+ . | % CD38+ . | |||
| 1 | 20 160 | 6245 | 1:105 | 87 | 90 | + |
| 2 | 96 736 | 1970 | 1:104 | 27 | 58 | − |
| 3 | 271 889 | 1022 | 1:104 | 83 | 90 | − |
| 4 | 261 749 | 3330 | 1:106 | 80 | 76 | + |
| 5 | 216 307 | 732 | 1:104 | 81 | 30 | − |
| 6 | 207 308 | 480 | 1:103 | 48 | 99 | − |
| 7 | 249 144 | 583 | 1:103 | 80 | 97 | − |
| 8 | 472 271 | 401 | 1:104 | 35 | 97 | + |
| 9 | 271 344 | 5070 | 1:104 | 97 | 62 | + |
| 10 | 20 006 | 485 | 1:103 | 3 | 99 | − |
| Patient . | Remission marrow* . | Detection limit of leukemic cell DNA diluted into normal DNA . | Immunophenotype of diagnostic bone marrow . | Presence of clonotypic TCR Vδ2-Dδ3 rearrangement in CD34+CD38− cells . | ||
|---|---|---|---|---|---|---|
| No. CD34+CD38+ cells . | No. CD34+ CD38−cells . | % CD34+ . | % CD38+ . | |||
| 1 | 20 160 | 6245 | 1:105 | 87 | 90 | + |
| 2 | 96 736 | 1970 | 1:104 | 27 | 58 | − |
| 3 | 271 889 | 1022 | 1:104 | 83 | 90 | − |
| 4 | 261 749 | 3330 | 1:106 | 80 | 76 | + |
| 5 | 216 307 | 732 | 1:104 | 81 | 30 | − |
| 6 | 207 308 | 480 | 1:103 | 48 | 99 | − |
| 7 | 249 144 | 583 | 1:103 | 80 | 97 | − |
| 8 | 472 271 | 401 | 1:104 | 35 | 97 | + |
| 9 | 271 344 | 5070 | 1:104 | 97 | 62 | + |
| 10 | 20 006 | 485 | 1:103 | 3 | 99 | − |
Shown are numbers of FACS isolated cells from which DNA was extracted. PCR analysis was performed on 100% of cells isolated.
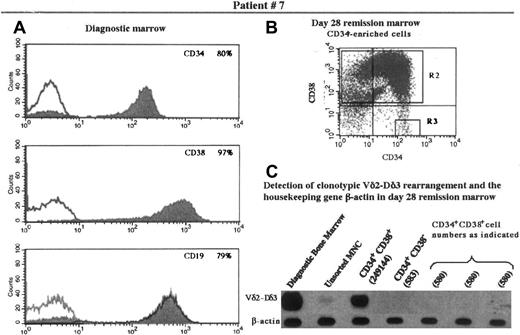
Six of the 10 patients in this study had no detectable clonotypic rearrangement in the CD34+CD38− population. Patient 7 (Figure 1) is representative of the 6 such patients. The Vδ2-Dδ3 rearrangement typical of this patient is easily detectable in the diagnostic bone marrow. When large numbers of CD34+CD38+ cells (approximately 250 000) from remission bone marrow were studied, the clonotypic rearrangement was detectable. However, no rearrangement was detected either in the CD34+CD38− cells or in equivalent numbers of CD34+CD38+ cells.
Immunophenotype of diagnostic bone marrow and flow cytometric analysis of and detection of TCR rearrangement in the progenitor population of the remission marrow in patient 7.
(A) Expression of CD34, CD38, and CD19 in the diagnostic bone marrow. (B) The day 28 remission marrow was enriched for CD34+cells and analyzed for expression of CD34 and CD38. Quadrants containing cells expressing CD34+CD38+ and CD34+CD38− were designated as the sorting regions R2 and R3, respectively. (C) Cells fractionated into CD34+CD38+ and CD34+CD38− underwent PCR amplification and Southern hybridization, along with other controls as appropriate, for detection of the clonotypic Vδ2-Dδ3 rearrangement originally identified in this patient. The B-actin gene was used as a control for the presence of DNA in the fractions tested. MNC indicates mononuclear cells.
Immunophenotype of diagnostic bone marrow and flow cytometric analysis of and detection of TCR rearrangement in the progenitor population of the remission marrow in patient 7.
(A) Expression of CD34, CD38, and CD19 in the diagnostic bone marrow. (B) The day 28 remission marrow was enriched for CD34+cells and analyzed for expression of CD34 and CD38. Quadrants containing cells expressing CD34+CD38+ and CD34+CD38− were designated as the sorting regions R2 and R3, respectively. (C) Cells fractionated into CD34+CD38+ and CD34+CD38− underwent PCR amplification and Southern hybridization, along with other controls as appropriate, for detection of the clonotypic Vδ2-Dδ3 rearrangement originally identified in this patient. The B-actin gene was used as a control for the presence of DNA in the fractions tested. MNC indicates mononuclear cells.
Four of the 10 patients in the study had detectable clonotypic rearrangements in the CD34+CD38−population
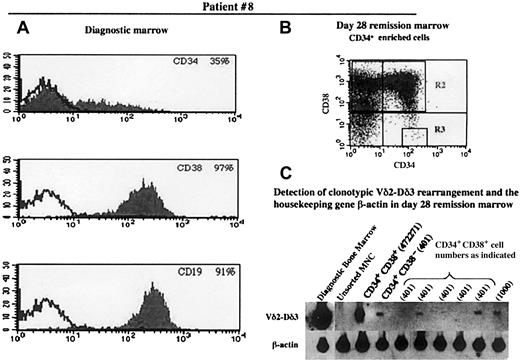
Figure 2 (patient 8) shows an example of a patient whose bone marrow was positive for the presence of the clonotypic rearrangement in the CD34+CD38−population. Note the strong signals indicating the presence of several copies of the rearrangement in the diagnostic marrow and the CD34+CD38+ cells (472 271 cells). The signal was several-fold lower in the CD34+CD38− lane (401 cells), but this was well within the limits of sensitivity, as indicated by the presence of signals of similar intensity in the lanes representing similar numbers of CD34+CD38+cells. It appears, therefore, that the number of CD34+CD38− cells with a clonotypic rearrangement is a small percentage of the total CD34+CD38− population. The low frequency of cells positive for the clonotypic rearrangement was also borne out by the fact that there is a detectable signal in only 2 of the 5 lanes of 401 CD34+CD38+ cells. Patient 1 showed a similar pattern of low frequency of cells positive for the clonotypic rearrangement in the CD34+CD38−population.
Immunophenotype of diagnostic bone marrow and flow cytometric analysis of and detection of TCR rearrangement in the progenitor population of the remission marrow in patient 8.
Panels A, B, and C are as designated in Figure 1.
Immunophenotype of diagnostic bone marrow and flow cytometric analysis of and detection of TCR rearrangement in the progenitor population of the remission marrow in patient 8.
Panels A, B, and C are as designated in Figure 1.
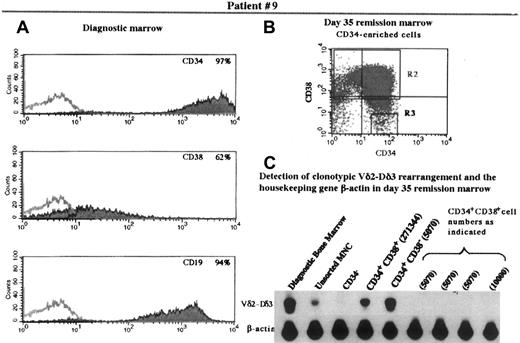
Bone marrow from patients 9 and 4 were also positive for the presence of the clonotypic rearrangement in the CD34+CD38− population. In these cases, however, the percentage of leukemic cells was greater in the CD34+CD38− population than in the CD34+CD38+ population. Figure3 shows the analysis of patient 9. Note that there was no detectable signal representing Vδ2-Dδ3 rearrangements in the lanes representing CD34+CD38+ cells equivalent in number to the CD34+CD38− cells (5070 cells), although B-actin signals could be visualized.
Immunophenotype of diagnostic bone marrow and flow cytometric analysis of and detection of TCR rearrangement in the progenitor population of the remission marrow in patient 9.
Panels A, B, and C are as designated in Figure 1.
Immunophenotype of diagnostic bone marrow and flow cytometric analysis of and detection of TCR rearrangement in the progenitor population of the remission marrow in patient 9.
Panels A, B, and C are as designated in Figure 1.
In summary, rearrangements were detected in the CD34+CD38− cells in 4 of 10 patients. In the 6 patients in whom rearrangements were not detected, rearrangements if present, occurred at a frequency below the limit of detection.
Discussion
Our previous studies,8 have shown that more than 95% of childhood B-lineage ALL, express CD38. CD34, an HSC-associated antigen, is expressed in 70% of B-cell precursor ALL. Thus one could postulate that CD34+CD38− cells in the leukemic samples could potentially be normal HSC and could be devoid of leukemic progenitors.
We analyzed the remission marrow from 10 patients being treated for B-cell precursor ALL, to determine if CD34+CD38− HSC free of leukemia could be isolated by flow cytometry. Our findings indicate that in 4 of the 10 patients, the clonotypic TCR Vδ2-Dδ3 rearrangement characteristic of each patient's leukemia could be detected unequivocally in the CD34+CD38− population. However, no rearrangement could be detected in the remaining 6 patients. These findings demonstrate that our original hypothesis cannot be universally applicable. Failure to detect a rearrangement could have one of 2 implications—either the CD34+CD38− HSC population in these patients does not contain leukemic cells, or the number of cells carrying the rearrangement was below the detection threshold. The detection threshold of the TCR δ rearrangement ranged from 10−3 to 10−6 dilution of leukemic cell DNA into normal DNA for the 10 patients in this study (Table 2). At a dilution of 10−3 the cell equivalents were calculated to range between 9 and 74 cells among the various patients (data not shown). It may not therefore be possible to identify samples with less than 10% of leukemic progenitors.
Several possible reasons could account for the presence of clonotypic rearrangements in the CD34+CD38− population. Although the CD34+CD38− population was selected on the basis that the cells were CD34bright and had CD 38 PE fluorescence less than one half of the maximum PE fluorescence of the isotype control, it is possible that there was some contamination of the CD34+CD38− population with CD34+CD38+ cells. However, in all patients showing the presence of a clonotypic rearrangement in the CD34+CD38− population, the intensity of the signal by Southern hybridization was far greater than would be expected by low level contamination with CD34+CD38+cells and suggests that a subset of the CD34+CD38− cells from these patients are leukemic.
A second possibility is that there is genomic instability among the leukemic cells. If there is no selective pressure to maintain the CD38 marker in the leukemic B-cell progenitor population, the CD34+CD38− cells sorted out by FACS may be a subpopulation of leukemic cells that have lost the CD38 marker, not true stem cells. Such a model would predict that leukemic progenitors would be present in both the CD34+CD38+ and CD34+CD38− populations.
A third hypothesis to explain the presence of leukemic cells in the CD34+CD38− population is that the leukemic progenitor has a different immunophenotype (CD34+CD38−) from the predominant phenotype (CD34+CD38+) seen among the leukemic cells at diagnosis. If this were true, then there may be limited differentiation of transformed ALL stem cells. Discrepancies between the phenotype of the leukemic stem cell and the predominant leukemic population have been observed in acute nonlymphocytic leukemia (ANLL) and chronic myeloid leukemia (CML). In studies with acute myeloid leukemia (AML), Bonnet and Dick have demonstrated that the cell capable of giving rise to human AML in nonobese diabetic-severe combined immunodeficiency (NOD-SCID) mice, termed the SCID leukemia-initiating cell or SL-IC, has both the differentiative and proliferative properties as well as the capacity for self-renewal expected of a leukemic stem cell.12 The SL-ICs were exclusively CD34+CD38−. Bonnet and Dick's study concluded that critical transformation events leading to leukemia occur in normal very primitive cells, rather than in more mature committed cells. More recently, Edwards and coworkers reported CD34 positivity in 41% of acute promyelocytic leukemia (APL) cases studied and using a FACS-fluorescence in situ hybridization (FISH) approach demonstrated the t(15;17) translocation in the CD34+CD38−fraction in 2 of the 17 cases studied, indicating that in certain cases at least, APL originates in very primitive hematopoietic progenitor cells.13 However, it should be noted that the findings of Edwards and colleagues are in contrast to those of Turhan and associates who demonstrated from a study of 3 APL patients, that primitive CD34+CD38− HSCs lacked PML-RARA rearrangements characteristic of APL.14
In a study of patients with Philadelphia chromosome (Ph1)-ALL it was found that the SL-ICs from all Ph1-ALL analyzed were exclusively CD34+CD38− .15 This cell surface phenotype is similar to that of normal SCID-repopulating cells and therefore supports the idea that a leukemia-initiating genetic event might occur at the stem cell level. The inability of this study to find any SL-IC activity in the CD34+CD38+ fraction suggests that leukemogenic events do not occur in committed progenitors. In our study, only 3 patients were found to have Ph1-ALL. Of these, only patient 9 showed a clonotypic Vδ2-Dδ3 rearrangement in the CD34+CD38−cells.
A previous study by Quijano and colleagues using FISH demonstrated that cytogenetically abnormal cells were present in the CD34+CD38−CD33−CD19−cells in 5 of 19 children with ALL.16 With the exception of one case, the analyses were performed on CD34+CD38−CD33−CD19−cells from diagnostic marrow samples. In the one case that was performed on remission marrow, no cytogenetically abnormal cells were found in the CD34+CD38−CD33−CD19−population. The current study differs from that of Quijano and coworkers in that all of the analyses were performed on remission marrow samples. This strategy was chosen to minimize the possibility that sorter errors in samples in which most of the cells were leukemic would lead to false-positive results. An alternative strategy might be to analyze peripheral blood populations, although this might not be feasible because of the small numbers of CD34+CD38− cells available from small children.
It has been proposed that a subset of leukemia stem cells with the capacity for self-renewal and proliferation must maintain the leukemia, because leukemic blasts themselves have little proliferative capacity.17 Two theories offer possible explanations for the manner in which leukemia develops and progresses.12The first theory suggests that cells at various stages of commitment in the stem cell or progenitor hierarchy are targets for malignant transformation.18 The phenotype of the resulting leukemia is dependent on the degree of commitment of the target cell. The second theory suggests that although oncogenic mutations occur in primitive cells, the differences in phenotype and the morphology characteristic of the leukemia are a result of variations in genetic and environmental factors.17 The detection of a TCR Vδ2-Dδ3 rearrangement in the CD34+CD38− fraction in 4 of the 10 patients indicates that at least in some cases of childhood ALL, the leukemogenic mutation occurs in very primitive progenitor cells or HSCs. It should also be noted that these cells appear to be resistant to induction chemotherapy, in contrast to the majority of the leukemic clone. Differences in the involvement of stem cell compartments between patients may be responsible for variations in response to treatment.
Our studies with normal umbilical cord blood cells (data not shown) show that TCR Vδ2-Dδ3 rearrangements occur very infrequently in the CD34+CD38− populations. Of a total of 1.1 × 104 FACS-sorted CD34+CD38 cells from 5 cord blood samples, 17 clones were analyzed and with the exception of a single clone, no Vδ2-Dδ3 rearrangements were found. Rearrangements were found with much greater frequency in the CD34+CD38dim and CD34+CD38− fractions, and were possibly concomitant with lymphoid commitment as demonstrated by the expression of CD7 and CD19. The question of why TCR δ rearrangements are observed in the very primitive progenitor population in some of the ALL cases remains speculative. It could be hypothesized that the etiology of events causing a leukemogenic mutation in the primitive progenitor population was simultaneously also the cause of the TCR Vδ2-Dδ3 rearrangement.
In vivo studies with immunodeficient mice may be necessary to establish if the CD34+CD38− populations from the 2 types of patients seen in this study contain leukemic progenitors or not. It may also be necessary to refine the immunophenotypic definition of the normal HSCs to allow resolution of the leukemic and normal stem cell populations. In early chronic phase CML, Delforge and coworkers have shown that CD34+ HLA-DR− progenitor cells that are Ph− and BCR/ABL messenger RNA− are polyclonal and therefore not leukemic progenitors.19 We are currently evaluating CD34+CD38−γc− as the phenotype of normal HSC20 and CD34+CD38+/−γc+ as a phenotype of the leukemic stem cell in ALL,21 using the same strategies described here to detect clonotypic rearrangements in B-lineage ALL.
We gratefully acknowledge Lora Barsky and Felix Burotto for their technical expertise in flow cytometry.
Supported by National Institutes of Health grants PO1 CA 59318 and IP50 HL 55850 (R.P., G.M.C., and K.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Weinberg, Div of Research Immunology/Bone Marrow Transplantation, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: kweinberg@chla.usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal