Polycomb-group (PcG) proteins, such as BMI-1 and EZH2, form multimeric gene-repressing complexes involved in axial patterning, hematopoiesis, and cell cycle regulation. In addition, BMI-1 is involved in experimental lymphomagenesis. Little is known about its role in human lymphomagenesis. Here, BMI-1 and EZH2 expression patterns are analyzed in a variety of B-cell non-Hodgkin lymphomas (B-NHLs), including small lymphocytic lymphoma, follicular lymphoma, large B-cell lymphoma, mantle-cell lymphoma, and Burkitt lymphoma. In contrast to the mutually exclusive pattern of BMI-1 and EZH2 in reactive follicles, the neoplastic cells in B-NHLs of intermediate- and high-grade malignancy showed strong coexpression of BMI-1 and EZH2. This pattern overlapped with the expression of Mib-1/Ki-67, a marker for proliferation. Neoplastic cells in B-NHL of low-grade malignancy were either BMI-1low/EZH2+ (neoplastic centroblasts) or BMI-1lowEZH2−(neoplastic centrocytes). These observations show that low-, intermediate-, and high grade B-NHLs are associated with increased coexpression of the BMI-1 and EZH2 PcG proteins, whose normal expression pattern is mutually exclusive. This expression pattern is probably caused by a failure to down-regulate BMI-1 in dividing neoplastic cells, because BMI-1 expression is absent from normal dividing B cells. These observations are in agreement with findings in studies of Bmi-1 transgenic mice. The extent of BMI-1/EZH2 coexpression correlated with clinical grade and the presence of Mib-1/Ki-67 expression, suggesting that the irregular expression of BMI-1 and EZH2 is an early event in the formation of B-NHL. This points to a role for abnormal PcG expression in human lymphomagenesis.
Introduction
B-cell non-Hodgkin lymphomas (B-NHLs) are clonal disorders with a mature phenotype and rearranged immunoglobulin genes.1 These tumors show a wide spectrum of morphologic features that vary between a nearly intact preservation of nodal architecture in follicular lymphoma and a diffuse growth pattern in most large B-cell lymphomas and Burkitt lymphoma.2-4 The pathogenic mechanism leading to B-NHL is probably a multistep process related to the inherent genetic instability associated with immunoglobulin rearrangement,5 external factors such as impaired or suppressed immunity, and a variety of environmental factors.6
Polycomb-group (PcG) proteins play a role in body plan formation (axial patterning through the repression of Hox genes), hematopoiesis, and checkpoints affecting cell cycle entry.7-11 Recent experiments also identified PcG proteins as a group of gene-regulatory factors that may contribute to oncogenesis and lymphomagenesis. PcG proteins form large, multimeric complexes that bind to chromatin and probably function by altering chromatin structure.12,13 Some PcG proteins may repress gene activity through histone deacetylation.14 So far, 2 PcG complexes have been identified: a complex containing the ENX/EZH2 and EED PcG proteins and another complex consisting of BMI-1, RING1, HPH1, HPH2, HPC1, HPC2, and HPC3.9,15,16 These complexes are hypothesized to have opposing roles: predominance of one complex may maintain cells in a proliferative state, whereas predominance of the other complex is seen in differentiated cells.17-19Previously, we demonstrated that the expression of PcG complexes during germinal center (GC) reaction is linked to the differentiation status of follicular B cells. We observed a mutually exclusive pattern of BMI-1/RING1 and EZH2/EED PcG proteins in reactive centroblasts and centrocytes. EZH2/EED expression was seen in dividing centroblasts of GC dark zones, and BMI-1/RING1 expression was dominant in resting B cells of the mantle zones and centrocytes in the light zones.20 21 These observations suggested that the expression of PcG complexes is highly regulated during GC reaction and that PcG proteins may contribute to antigen-specific B-cell maturation.
Deregulation of PcG gene expression in experimental model systems has clearly been linked to oncogenesis. For instance, the overexpression of Bmi-1 resulted in lymphomas in transgenic mice.10 In addition, the overexpression of RING1 caused anchorage-independent growth, cellular transformation, and metastatic activity in nude mice.12 Yet, little is known about a possible role for PcG genes in human lymphoma. We recently demonstrated that Mib-1/Ki-67+ Hodgkin–Reed-Sternberg (HRS) cells coexpress EZH2 and BMI-1.21 Because most HRS cells originate from B cells in reactive follicles, where the expression of BMI-1 and the expression of EZH2 are mutually exclusive, this pattern suggested that Hodgkin lymphoma is associated with deregulated expression of PcG complexes.
In the current study, we questioned whether B-NHL is also associated with BMI-1/EZH2 coexpression in Mib-1/Ki-67-expressing neoplastic B cells. Using unique antisera against BMI-1 and EZH2, we found BMI-1/EZH2 coexpression in Mib-1/Ki-67+ neoplastic large cells in intermediate- and high-grade B-NHL. Large Mib-1/Ki-67+ neoplastic cells in low-grade B-NHL showed weak coexpression of EZH2 and BMI-1. By contrast, small neoplastic cells in low-grade B-NHL showed reduced BMI-1 expression in the absence of EZH2 or Mib-1/Ki-67. We concluded that human B-NHL, such as Hodgkin lymphoma, is associated with irregular expression of BMI-1 and EZH2PcG genes. In addition, the level of BMI-1/EZH2 coexpression correlated with clinical grade and the presence of Mib-1/Ki-67 expression. These findings suggest that the irregular expression of BMI-1 and EZH2 is an early event in the formation of B-NHL, and they point to a role for abnormal PcG expression in human lymphomagenesis.
Materials and methods
Patient material
Fifty-two lymph nodes from patients with B-NHL were obtained after surgery, immediately frozen or fixed in 10% buffered formalin, and embedded in paraffin (Table1). Burkitt lymphoma (n = 5), mantle-cell lymphoma (MCL) (n = 6), follicular lymphoma (n = 30), diffuse large B-cell lymphoma (n = 6), and small lymphocytic lymphoma (n = 5) were retrieved from the files of the Department of Pathology, Academic Hospital of the Free University of Amsterdam. Both paraffin-embedded (n = 52) and frozen material (n = 38) were used. Biopsy specimens were taken from patients at the time of diagnosis. Lymphomas were classified according to REAL/WHO classification (WHO terminology is used in this text).22,23 In patients with follicular lymphoma, grading was done according to the method described by Mann and Berard.24 In addition, healthy control tissue, such as lymph nodes or extranodal lymphoid tissue, were used from regular surgical pathology archival material. BMI-1 and EZH2 expression in these tissues was described in 2 earlier studies20 21 and are not shown in detail in the current study.
Characteristics of 52 mature (peripheral) B-cell neoplasms tested
| . | Number . | Mean age, y (range) . | Nodal/ extranodal . |
|---|---|---|---|
| Small lymphocytic lymphoma | n = 5 | 55 (60-73) | 2/3 |
| Follicular lymphoma | n = 30 | 55 (12-91) | 13/12 |
| Mantle-cell lymphoma | n = 6 | 60 (31-88) | 5/1 |
| Diffuse large B-cell lymphoma | n = 6 | 52 (11-85) | 3/3 |
| Burkitt lymphoma | n = 5 | 5-21 (10) | −/5 |
| . | Number . | Mean age, y (range) . | Nodal/ extranodal . |
|---|---|---|---|
| Small lymphocytic lymphoma | n = 5 | 55 (60-73) | 2/3 |
| Follicular lymphoma | n = 30 | 55 (12-91) | 13/12 |
| Mantle-cell lymphoma | n = 6 | 60 (31-88) | 5/1 |
| Diffuse large B-cell lymphoma | n = 6 | 52 (11-85) | 3/3 |
| Burkitt lymphoma | n = 5 | 5-21 (10) | −/5 |
Antibodies used in this study
Monoclonal mouse antibodies raised against BMI-1 (IgG2b) and polyclonal rabbit antibodies against EZH2 have been described.13 The anti–Ki-67 monoclonal antibody (MIB1) was obtained from Immunotech (Marseilles, France).
Immunohistochemical detection of human PcGgene expression
Expression of the BMI-1 and EZH2 PcG proteins was detected using the 6C9 mouse monoclonal antibody (anti–BMI-1) and the polyclonal K358 rabbit antiserum (anti-EZH2), respectively.13 After deparaffinization, endogenous peroxidase was inhibited by incubation of the tissue sections for 30 minutes at room temperature in 0.3% H2O2, diluted in methanol. Antigens were retrieved by boiling for 10 minutes in citrate buffer (pH, 6), followed by successive rinses in phosphate-buffered saline (PBS) containing 0.5% Triton (1 × 5 minutes) and then in PBS only (3 × 5 minutes). Slides were incubated for 10 minutes in 0.1 M glycine (diluted in PBS) and rinsed in PBS only (3 × 5 minutes). Before application of the primary antiserum or antibody, sections were incubated for 10 minutes in normal swine serum (diluted 1:10 in PBS + 1% BSA) or normal rabbit serum (diluted 1:50 in PBS + 1% BSA). Secondary antisera were biotinylated goat-antimouse or biotinylated swine-antirabbit (Dako, Glostrup, Denmark). Immunostaining was performed with 3-amino-9-ethylcarbazole using the streptavidin-biotin complex–horseradish peroxidase method (Dako) and tyramine intensification. Sections were counterstained with hematoxylin. Photographs were taken with a Zeiss Axiophot microscope and digitized using an Agfa duoscan scanner.
Double immunofluorescence
Tissue sections were fixed in 2% formaldehyde, and endogenous peroxidase was inhibited as above. After preincubation with 5% BSA, a combination of 2 primary antibodies was applied overnight at 4°C—either anti–BMI-1 (6C9; mouse IgG2b monoclonal antibody) and anti-EZH2 (K358; rabbit polyclonal antiserum) or EZH2 with anti–Ki-67 (MIB1; mouse IgG1 monoclonal antibody; Immunotech). BMI-1 or Ki-67 was detected by biotinylated goat antimouse antiserum followed by streptavidin-Cy3 (Immunoresearch, Jackson, PA), whereas EZH2 was detected by swine-antirabbit Ig-fluorescein isothiocyanate (FITC; Dako). Alternatively, green fluorescence was performed using Alexa-linked goat-antirabbit immunoglobulin (Molecular Probes, Eugene, OR). For each double-immunofluorescence experiment, single-color controls were included.
Results
Coexpression of BMI-1, EZH2 in Mib-1/Ki-67+ neoplastic cells of large B-cell lymphoma
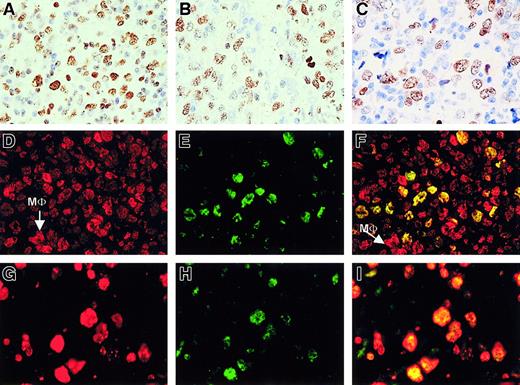
We started our study of PcG expression in human B-NHL with an analysis of large B-cell lymphoma (including follicular lymphoma grade III either with or without a residual follicular growth pattern) and diffuse large B-cell lymphoma. Neoplastic centroblasts in these lymphomas showed clear nuclear staining for BMI-1 (Figure1A), to an extent almost similar to that of EZH2 (Figure 1B). Staining in these large B-cell lymphomas appeared comparable to the pattern obtained for Mib-1/Ki-67 (Figure 1C). Using double immunofluorescence, we confirmed that neoplastic centroblasts expressed BMI-1 (red signal in Figure 1D) and EZH2 (green staining in Figure 1E) in the same nucleus (producing a yellow signal in Figure1F). Note that normal cells in the surrounding infiltrate are BMI-1+/EZH2− (see Figure 10). In addition, neoplastic cells expressed Mib-1/Ki-67 (red signal; Figure 1G) in combination with EZH2 (green signal; Figure 1H), resulting in yellow nuclear staining after combining the 2 signals (Figure 1I). From these patterns, we concluded that coexpression of BMI-1 and EZH2 coincided with cycling Mib-1/Ki-67+ neoplastic cells. We observed the same pattern in nodal and extranodal large B-cell lymphoma (not shown).
Large B-cell lymphoma centroblasts are BMI-1+/EZH2+ that coexpress MIB1+.
(A-C, first row) Immunohistochemistry (IHC) of large B-cell lymphoma with numerous centroblasts shows clear expression of BMI-1 (A), EZH2 (B), and Mib-1/Ki67 (C). Large brown-staining cells are positive. Small lymphocytes also stain for BMI-1 (A), whereas these cells are negative for EZH2 and Mib-1/Ki-67. Photographs are taken from different parts of the tumor. One representative example is shown. Original magnification, 63× objective. (D-F, second row) Immunofluorescence (IF) of large B-cell lymphoma shows red staining for BMI-1 (D), green staining for EZH2 (E), and overlay photographic exposure with yellow cells indicative of centroblasts expressing both BMI-1 and EZH2 (F). Note that bright BMI-1+ (red) cells are probably macrophages (MΦ), as indicated in panels D and F. Note that small (infiltrating) lymphocytes in panels D to F show expression of BMI-1 but not of EZH2, as expected. (G-I, third row) IF of large B-cell lymphoma shows expression of Mib/Ki67 in red (G) and EZH2 in green (H). Double fluorescence confirms the coexpression of these proteins in the nuclei of tumor cells (J, overlay photographic exposure with yellow nuclei). Same example as in panels D to F is shown in panels G to I. All IF pictures were taken with 63× objective.
Large B-cell lymphoma centroblasts are BMI-1+/EZH2+ that coexpress MIB1+.
(A-C, first row) Immunohistochemistry (IHC) of large B-cell lymphoma with numerous centroblasts shows clear expression of BMI-1 (A), EZH2 (B), and Mib-1/Ki67 (C). Large brown-staining cells are positive. Small lymphocytes also stain for BMI-1 (A), whereas these cells are negative for EZH2 and Mib-1/Ki-67. Photographs are taken from different parts of the tumor. One representative example is shown. Original magnification, 63× objective. (D-F, second row) Immunofluorescence (IF) of large B-cell lymphoma shows red staining for BMI-1 (D), green staining for EZH2 (E), and overlay photographic exposure with yellow cells indicative of centroblasts expressing both BMI-1 and EZH2 (F). Note that bright BMI-1+ (red) cells are probably macrophages (MΦ), as indicated in panels D and F. Note that small (infiltrating) lymphocytes in panels D to F show expression of BMI-1 but not of EZH2, as expected. (G-I, third row) IF of large B-cell lymphoma shows expression of Mib/Ki67 in red (G) and EZH2 in green (H). Double fluorescence confirms the coexpression of these proteins in the nuclei of tumor cells (J, overlay photographic exposure with yellow nuclei). Same example as in panels D to F is shown in panels G to I. All IF pictures were taken with 63× objective.
Neoplastic Mib-1/Ki-67+ cells in mantle-cell and Burkitt lymphoma coexpress BMI-1 and EZH2
Mantle-cell lymphoma and Burkitt lymphoma showed an expression pattern of BMI-1, EZH2, and Mib-1/Ki-67 that closely resembled the expression profile of large B-cell lymphoma. In mantle-cell lymphoma, neoplastic cells showed both BMI-1 (correlating with reactive mantle cells) and EZH2 expression (shown as double immunofluorescence in Figure 2A). Almost all EZH2-expressing cells expressed Mib-1/Ki-67 (shown as double immunofluorescence in Figure 2B). Therefore, expression patterns of EZH2 and BMI-1 showed considerable overlap in mantle lymphoma cells (single colors not shown), comparable to the pattern in large B-cell lymphoma. Note that large green EZH2+ and Mib-1/Ki-67− cells are also present: these are probably pre-existing blasts.
Neoplastic cells in mantle-cell lymphoma and Burkitt lymphoma coexpress BMI-1, EZH2, and Mib-1/Ki-67.
(A-B, first row) Double immunofluorescence of mantle cell lymphoma (MCL) shows BMI-1 and EZH2 with a majority of yellow (double-positive) tumor cells. The technique used is similar to that used in Figure 1with single red and single green colors (for clarity, only double-exposure overlay photographs are shown). Yellow cells are indicative of both green and red fluorescence. Note that bright red cells are BMI-1+ macrophages (MΦ). Three green cells (A, top) show distinct speckled yellow staining, whereas most other nuclei are evenly yellow (double-positive for BMI-1 and EZH2). (B) Double IF for EZH2 and Mib-1/Ki-67 (green and red, respectively) with most cells double-positive (yellow). Single green staining cells are referred to in the text. (C-D, second row) Double IF of Burkitt lymphoma (C) shows BMI-1 and EZH2 (red and green, respectively) with a majority of yellow (double-positive) tumor cells. (D) Double IF for Mib-1/Ki-67 and EZH2 (red and green, respectively) with most cells double-positive. Single-color controls in panels A to D are omitted. Photographs were taken from one representative experiment for each lymphoma (63× objective).
Neoplastic cells in mantle-cell lymphoma and Burkitt lymphoma coexpress BMI-1, EZH2, and Mib-1/Ki-67.
(A-B, first row) Double immunofluorescence of mantle cell lymphoma (MCL) shows BMI-1 and EZH2 with a majority of yellow (double-positive) tumor cells. The technique used is similar to that used in Figure 1with single red and single green colors (for clarity, only double-exposure overlay photographs are shown). Yellow cells are indicative of both green and red fluorescence. Note that bright red cells are BMI-1+ macrophages (MΦ). Three green cells (A, top) show distinct speckled yellow staining, whereas most other nuclei are evenly yellow (double-positive for BMI-1 and EZH2). (B) Double IF for EZH2 and Mib-1/Ki-67 (green and red, respectively) with most cells double-positive (yellow). Single green staining cells are referred to in the text. (C-D, second row) Double IF of Burkitt lymphoma (C) shows BMI-1 and EZH2 (red and green, respectively) with a majority of yellow (double-positive) tumor cells. (D) Double IF for Mib-1/Ki-67 and EZH2 (red and green, respectively) with most cells double-positive. Single-color controls in panels A to D are omitted. Photographs were taken from one representative experiment for each lymphoma (63× objective).
The pattern of BMI-1 and EZH2 in virtually all Burkitt lymphoma cells showed overlap similar to that of large B-cell blasts in B-NHL. Burkitt blasts coexpressed BMI-1 (red signal) and EZH2 (green signal), producing a yellow signal in Figure 2C. Overlap between EZH2+ neoplastic cells and Mib-1/Ki-67+ cells was observed for almost all Burkitt blasts (Figure 2D).
Decreased BMI-1 expression in neoplastic centrocytes of low-grade follicular lymphoma
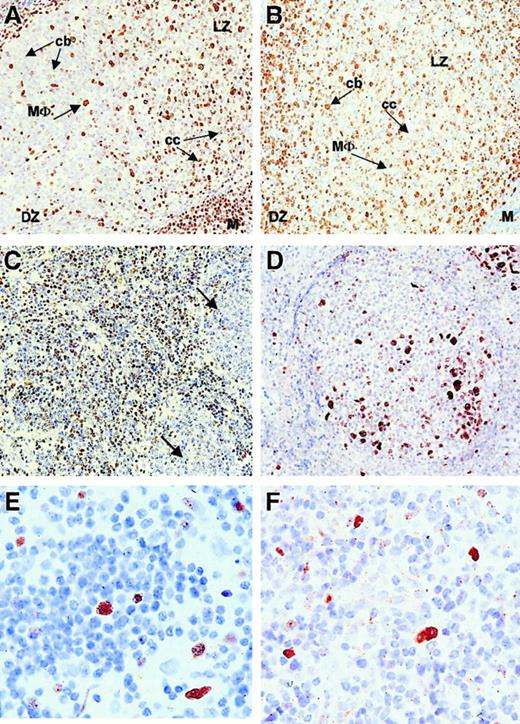
Because the coexpression of BMI-1 and EZH2 correlated with cycling Mib-1/Ki-67+ cells in intermediate- and high-grade lymphomas, we subsequently analyzed the expression profile of these PcG proteins in low-grade B-NHL. Figure 3shows representative immunohistochemical staining patterns for BMI-1 and EZH2 in follicular lymphoma. In control tissue, expression of BMI-1 (Figure 3A) and EZH2 (Figure 3B) was as determined previously.20 21 In general, mantle cells, intrafollicular macrophages, and light zone centrocytes in reactive follicles were positive for BMI-1. These cells did not stain for EZH2, which was mainly detectable in dark-zone centroblasts. In low-grade follicular lymphoma with preserved follicular architecture, we observed reduced BMI-1 staining in neoplastic centrocytes (Figure 3C, see legend) compared to the more intense staining pattern in surrounding infiltrating cells and centrocytes of reactive follicles (Figure 3A). EZH2 expression in low-grade follicle center lymphoma was confined to reactive and neoplastic centroblasts (Figure 3D; overview). In diffuse areas, small numbers of neoplastic centroblasts were observed (Figure 3E; detail) comparable to the number of Mib-1/Ki-67–expressing cells (Figure 3F). The pattern in small lymphocytic lymphoma was similar to that in low-grade follicular lymphoma (not shown). We concluded that BMI-1 expression is decreased in neoplastic centrocytes in follicular lymphoma and neoplastic small cells in small lymphocytic lymphoma. EZH2 expression in neoplastic centroblasts appeared unchanged compared to that in reactive centroblasts.
Immunohistochemistry (IHC) and immunofluorescence (IF) of low-grade follicular lymphoma showing BMI-1low/EZH2− neoplastic centrocytes and BMI-1low/EZH2+ centroblasts.
(A-B, first row) IHC on reactive follicle with BMI-1 (A) and EHZ2 (B). Note that macrophages are BMI-1+ (MΦ) and EZH2−, centrocytes (cc) are BMI-1+ and EHZ2−, whereas centroblasts (cb) are BMI-1−but EZH2+. M, mantle cells (BMI-1+EZH2−); DZ, dark zone; LZ, light zone. Original magnification, 20× objective. (C-D, second row) IHC on low-grade follicular lymphoma. (C) Reactive centrocytes mainly on the left and neoplastic centrocytes mainly on the right side of the image (arrows). Reactive centrocytes are clearly BMI-1+, whereas neoplastic (larger) centrocytes are weakly positive and negative. (D) Residual germinal center overrun by neoplastic centrocytes. Residual centroblasts are EZH2+. Original magnification, 20× objective. (E-F, third row) IHC on low-grade follicular lymphoma (detail) showing Mib-1/Ki-67+ neoplastic centroblasts (E) and, with similar magnification, EZH2+ centroblasts (F). Original magnification, 63× objective. All photographs are from a representative experiment with one low-grade follicular lymphoma (Berard grade I/II).
Immunohistochemistry (IHC) and immunofluorescence (IF) of low-grade follicular lymphoma showing BMI-1low/EZH2− neoplastic centrocytes and BMI-1low/EZH2+ centroblasts.
(A-B, first row) IHC on reactive follicle with BMI-1 (A) and EHZ2 (B). Note that macrophages are BMI-1+ (MΦ) and EZH2−, centrocytes (cc) are BMI-1+ and EHZ2−, whereas centroblasts (cb) are BMI-1−but EZH2+. M, mantle cells (BMI-1+EZH2−); DZ, dark zone; LZ, light zone. Original magnification, 20× objective. (C-D, second row) IHC on low-grade follicular lymphoma. (C) Reactive centrocytes mainly on the left and neoplastic centrocytes mainly on the right side of the image (arrows). Reactive centrocytes are clearly BMI-1+, whereas neoplastic (larger) centrocytes are weakly positive and negative. (D) Residual germinal center overrun by neoplastic centrocytes. Residual centroblasts are EZH2+. Original magnification, 20× objective. (E-F, third row) IHC on low-grade follicular lymphoma (detail) showing Mib-1/Ki-67+ neoplastic centroblasts (E) and, with similar magnification, EZH2+ centroblasts (F). Original magnification, 63× objective. All photographs are from a representative experiment with one low-grade follicular lymphoma (Berard grade I/II).
Weak coexpression of BMI-1 and EZH2 in neoplastic centroblasts of low-grade follicular lymphoma
To determine whether EZH2+ neoplastic centroblasts in low-grade follicular lymphoma coexpress BMI-1, we analyzed the expression of these proteins with double immunofluorescence. BMI-1 was detected in neoplastic centrocytes of low-grade follicular lymphoma and neoplastic small lymphocytes in small lymphocytic lymphoma. As can be seen in Figure 4A, BMI-1 expression was seen throughout, both in neoplastic centrocytes and in neoplastic centroblasts. EZH2 expression was detected in a limited number of centroblasts (Figure 4B), and double immunofluorescence showed that large cells/blasts weakly expressed BMI-1 in the presence of strong EZH2 expression. This produced a yellowish hue in these centroblasts (Figure 4C). A similar pattern was observed in lymphoplasmacytoid lymphoma—weak but detectable BMI-1 expression (Figure 4D) in small numbers of EZH2-expressing neoplastic cells (Figure 4E), resulting in weak BMI-1/EZH2 coexpression (Figure 4F). Note that coexpression of BMI-1 and EZH2 rarely occurs in normal reactive follicles, whereas BMI-1 and EZH2 expression in centroblasts and centrocytes is mutually exclusive (not shown, but discussed in detail elsewhere).20 21
Low-grade follicular lymphoma and small lymphocytic lymphoma show BMI-1low/EZH2+ centroblasts associated with cycling cells.
(A-C, first row) IF on follicular lymphoma (Berard grade I/II) with BMI-1 (red fluorescence) and EZH2 (green fluorescence). BMI-1 expression is detectable in most centrocytes (A). Large, bright red cells are macrophages (MΦ). Weak but distinct BMI-1 expression is detectable in neoplastic centroblasts (dotted arrow in A), which are also EZH2 (B; arrow). This produces a yellow signal in C. (D-F, second row) IF for BMI-1 (red fluorescent signal) and EZH2 (green fluorescent signal) on lymphocytic lymphoma. BMI-1 expression is detectable in almost all tumor cells (D), and a limited number of cells are EZH2+ (E). These cells coexpress BMI-1, producing a weak yellow signal in panel F (overlay photographic exposure) similar to that in panel C. (G-I, third row) IF for Mib-1/Ki-67 (red fluorescence) and EZH2 (green fluorescence) on follicular lymphoma. (G) Mib1/Ki67+ cells. (H) EZH2+ cells (using a stronger Alexa fluorescent probe than the FITC probe in panel B). (I) Overlay photographic exposure showing overlap between EZH2 and Mib/Ki67. One representative example is shown. Lymphocytic lymphoma gave a comparable result (not shown). All photographs were taken with a 63× objective.
Low-grade follicular lymphoma and small lymphocytic lymphoma show BMI-1low/EZH2+ centroblasts associated with cycling cells.
(A-C, first row) IF on follicular lymphoma (Berard grade I/II) with BMI-1 (red fluorescence) and EZH2 (green fluorescence). BMI-1 expression is detectable in most centrocytes (A). Large, bright red cells are macrophages (MΦ). Weak but distinct BMI-1 expression is detectable in neoplastic centroblasts (dotted arrow in A), which are also EZH2 (B; arrow). This produces a yellow signal in C. (D-F, second row) IF for BMI-1 (red fluorescent signal) and EZH2 (green fluorescent signal) on lymphocytic lymphoma. BMI-1 expression is detectable in almost all tumor cells (D), and a limited number of cells are EZH2+ (E). These cells coexpress BMI-1, producing a weak yellow signal in panel F (overlay photographic exposure) similar to that in panel C. (G-I, third row) IF for Mib-1/Ki-67 (red fluorescence) and EZH2 (green fluorescence) on follicular lymphoma. (G) Mib1/Ki67+ cells. (H) EZH2+ cells (using a stronger Alexa fluorescent probe than the FITC probe in panel B). (I) Overlay photographic exposure showing overlap between EZH2 and Mib/Ki67. One representative example is shown. Lymphocytic lymphoma gave a comparable result (not shown). All photographs were taken with a 63× objective.
In follicular lymphoma Mib-1/Ki-67 and EZH2 expression overlapped (Figure 4G-I), similar in pattern to that in small lymphocytic lymphoma (not shown). We concluded from this pattern that Mib-1/Ki-67+ cells in low-grade B-NHL are cells that weakly coexpress BMI-1 and EZH2.
In summary, all B-NHL tested showed aberrant PcG expression compared to reactive follicular cells. In general, small centrocytes and lymphocytes in low-grade follicular lymphoma and small lymphocytic lymphoma expressed BMI-1 at reduced levels compared to their reactive counterparts. In these lymphomas, EZH2 expression was limited to large Mib-1/Ki-67+ cells that weakly coexpress BMI-1. In contrast, neoplastic mantle cells, Burkitt cells, and blast cells in large-cell B-NHL showed strong double expression of BMI-1/EZH2 that always overlapped with Mib-1/Ki-67.
Discussion
PcG genes encode a new class of gene regulatory factors that contribute to normal lymphoid development and lymphomagenesis. They were originally discovered in Drosophila, where they regulate embryonic development as inhibitors of homeobox gene expression. Polycomb proteins function by forming multimeric protein complexes that bind chromatin. Two fundamental complexes have been identified, but their composition can differ in various cell types. This variation is most likely related to target gene specificity9 and the role of PcG complexes in the maintenance of cellular identity during cell division.
The 2 human PcG complexes are expressed at various stages of GC B-cell development.20 21 However, their expression depends on differentiation stage and stage in the cell cycle: dividing centroblasts express the complex identified by the EZH2 PcG protein, whereas resting mantle cells and centrocytes use the complex identified by BMI-1. BMI-1 and EZH2 are rarely detected in the same nucleus of follicular B cells, suggesting that expression of the 2 complexes is mutually exclusive and highly regulated.
There is increasing evidence that the deregulation of PcG expression is related to the formation of lymphomas. A well-studied example is the Bmi-1 transgenic mouse, which exhibits increased lymphoproliferation and induction of lymphomas.10,25,26 We recently demonstrated that one malignant counterpart of follicular B cells, the HRS cell in Hodgkin lymphoma, coexpresses BMI-1 and EZH2.21 This suggested that deregulated PcG expression may be related to human lymphomagenesis as well. In the current study, we analyzed BMI-1 and EZH2 expression in various classes of B-NHL and questioned whether neoplastic B cells coexpress BMI-1 and EZH2. We observed 2 aberrant expression patterns of these PcG proteins. Tumor cells in intermediate- and high-grade B-NHL (large B-cell NHL, Burkitt lymphoma, and mantle-cell lymphoma) expressed BMI-1 at high levels (BMIhigh), virtually always in the presence of EZH2. By contrast, tumor cells in low-grade B-NHL (follicular lymphoma, small lymphocytic lymphoma) expressed low levels of BMI-1 (BMI-1low), either in the presence (neoplastic centroblasts) or absence (neoplastic centrocytes) of EZH2. Furthermore, the detection of EZH2 in B-NHL neoplastic cells overlapped with expression of the Mib-1/Ki-67+ proliferation marker.
The irregular expression profile of BMI-1 and EZH2 in B-NHL suggests that the distinct balance between the BMI-1 and EZH2-containing PcG complex is disturbed in these lymphomas. Furthermore, the extent of irregular PcG expression correlated with the type of lymphoma (and, therefore, clinical behavior). Small neoplastic centrocytes in low-grade B-NHL exhibited decreased BMI-1 expression in the absence of EZH2, and larger neoplastic blasts in these low-grade B-NHL showed weak coexpression of BMI-1 and EZH2. By contrast, tumor cells in intermediate- and high-grade B-NHL were strongly positive for BMI-1 and EZH2. These results suggest that the balance between BMI-1 and EZH2 expression is progressively disturbed in dividing cells of intermediate- and high-grade B-NHL lymphomas. Because PcG complexes are involved in the maintenance of the cellular differentiation program, altered PcG expression patterns could at least partially explain the different behavior of neoplastic cells.
Although our study does not resolve the mechanism that accounts for BMI-1/EZH2 coexpression in neoplastic cells, the expression profile in the normal counterparts of these cells allows us to speculate about a possible mechanism. Expression of EZH2 in Mib-1/Ki-67+neoplastic B cells appears to be natural, because normal Mib-1/Ki-67+ follicular B cells express EZH2 as well.20,21 In addition, in vitro up-regulation of EZH2 transcription has been reported during the entry of lymphocytes into the cell cycle.27 We conclude that the aberrant PcG expression pattern in B-NHL is related to the presence of BMI-1 in dividing neoplastic cells, suggesting overexpression of thisPcG gene in neoplastic cells. Normal follicular B cells do not express BMI-1 when they are dividing, and BMI-1 is only detected in EZH2− resting centrocytes and mantle cells.20,21 Furthermore, the absence of BMI-1 expression in dividing healthy B cells is most likely related to the fact that expression of this PcG gene is cell cycle dependent; it dissociates from chromosomes during the late S-/G2-M phase of the cell cycle.28 Consequently, the presence of BMI-1 in dividing neoplastic cells may reflect a failure to down-regulate BMI-1 expression. Theoretically, this occurs early during lymphomagenesis because the intensity of BMI-1/EZH2 coexpression, and the number of neoplastic cells in which this occurs, increases in B-NHL of higher grade. A recent study29 noted that a subset of mantle-cell lymphoma with blastoid transformation contained amplification of theBmi-1 gene. Although the overexpression of BMI-1 in human neoplastic cells is in line with the induction of lymphomas in Bmi-1 transgenic mice, it is unclear whether BMI-1/EZH2 coexpression precedes cellular transformation or whether it is a consequence of this process.
One important aspect of PcG complex expression that should be addressed in future studies is the fine composition of the complexes expressed in normal and transformed cells. It is unknown whether PcG expression patterns, as determined in Hodgkin lymphoma and B-NHL, represent normally assembled PcG complexes. We recently found that BMI-1+/RING1+/EZH2−/EED−mantle cell lymphomas (MCL) up-regulate EZH2 when stimulated to proliferation.30 This resulted in the coexpression of BMI-1 and EZH2 in the presence of RING1. However, whereas the EED PcG protein is present in dividing normal EZH2+ centroblasts, EED remained unexpressed in the proliferating MCL cells. EED expression appears associated with the negative control of proliferation,19 and the imbalance between EZH2 and EED in MCL could be an alternative or additional contributing factor to MCL proliferation. In addition, we should mention at this point that the mutually exclusive expression of BMI-1/RING1 and EZH2/EED, as observed in follicular B cells, does not appear to be entirely universal for normal lymphoid cells. For instance, we recently found BMI-1/EZH2 coexpression in healthy thymocytes, whereas mature T cells expressed BMI-1 and EZH2 in a mutually exclusive pattern.3 This suggests that the fine composition of PcG complexes in normal and transformed cells could affect cellular behavior because a PcG complex may be composed of PcG proteins in different ratios. For instance, a BMI-1 PcG protein can interact with RING, HPC2, and HPC3, but also with other BMI-1 proteins.9,15 32 Therefore, the ratio in which PcG proteins are present in a complex may differ between cell types or between normal and diseased cells. Theoretically, a change in the relative ratio of PcG proteins could be an additional, or an alternative, explanation for altered cellular behavior. We are investigating whether the variation in PcG complex composition correlates with different clinical behaviors of lymphomas.
In conclusion, we demonstrated that low-, intermediate-, and high-grade B-NHL are associated with increased coexpression of the BMI-1 and EZH2 PcG proteins, whose normal expression pattern is mutually exclusive. The underlying mechanism of this expression pattern is most likely related to a failure to down-regulate BMI-1 in dividing neoplastic cells, which is in agreement with observations in Bmi-1 transgenic mice. The extent of BMI-1/EZH2 coexpression correlated with clinical grade and the presence of Mib-1/Ki-67 expression. This suggests that the irregular expression of BMI-1 and EZH2 is an early event in the formation of B-NHL and points to a role for abnormal PcG expression in human lymphomagenesis.
F.J.v.K. and F.M.R. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Folkert J. van Kemenade, Department of Pathology, VU University Hospital, Rm PA-001, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: f.vkemenade@azvu.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal