Viability-promoting genes such as BCL2 play an important role in human cancer but do not directly cause aggressive tumors. BCL2 transgenic mice develop lymphoma at low frequency, hindering studies of tumorigenesis and its inhibition in the presence of such gene products. MCL1 is a member of theBCL2 family that is highly regulated endogenously and that promotes cell viability and immortalization when introduced exogenously. Mice expressing an MCL1 transgene in hematolymphoid tissues have now been monitored for an extended period and were found to develop lymphoma with long latency and at high probability (more than 85% over 2 years). In most cases, the disease was widely disseminated and of clonal B-cell origin. A variety of histologic subtypes were seen, prominently follicular lymphoma and diffuse large-cell lymphoma. MCL1 thus sets the stage for the development of lymphoma as does BCL2, disease occurring with high probability and recapitulating a spectrum of subtypes as seen in human patients. These findings with the transgene underscore the importance of the normal, highly regulated pattern of MCL1expression, in addition to providing a model for studying tumorigenesis and its inhibition in the presence of a viability promotingBCL2 family member.
Introduction
The BCL2 gene family is involved in the control of cell viability, some family members inhibiting and others promoting apoptotic cell death.1 These genes also play a role in cancer,2 as exemplified by their involvement in several types of human leukemia and lymphoma.3-9Follicular B-cell lymphoma is a low-grade, slowly progressing disease, which typically exhibits deregulated BCL2 expression because of the t(14;18) translocation that juxtaposes BCL2 to the immunoglobulin heavy-chain locus. Diffuse large-cell lymphoma is a high-grade, rapidly progressing disease that also frequently exhibits elevated expression of BCL2.4 Follicular lymphoma eventually undergoes transformation to high-grade, aggressive disease.10 In addition to these 2 prevalent disease manifestations, a variety of other lymphoma subtypes occur,11 and the disease can be of B-cell, T-cell, or indeterminate phenotype.
The process of tumor development and evolution in the presence of antiapoptotic gene products such as BCL2 is not fully understood. Notwithstanding its involvement in cancer, BCL2rearrangements are seen in nontumorigenic cells at low frequency.12-14 In addition, elevated levels ofBCL2 occur in autoimmune diseases involving lymphocyte activation rather than tumorigenesis (eg, lupus erythematosus15). BCL2 transgenic mice provide parallels to these observations in human disease. These mice initially exhibit enhanced lymphocyte survival and splenic hyperplasia and can develop a lupuslike autoimmune disorder.16-23 With long-term observation, a fraction of the mice develop high-grade lymphoma (10% to 25% at 1 to 2 years), and this involves additional clonal genetic changes such as alterations in c-MYC.20,21 24 A key question, which arises from the observations in both human and murine systems, is that of what distinguishes cells that undergo transformation from those that do not. Of further importance is the question of how the events involved in the initiation of indolent disease differ from those mediating progression to high-grade disease, for example how the development of follicular lymphoma differs from subsequent conversion to diffuse large-cell lymphoma. A final critical issue is whether tumorigenesis can be inhibited, either at initial stages or during disease evolution.
The low incidence of tumor formation in BCL2 transgenic mice presents a limitation to studies aimed at further addressing the above questions and at determining whether targeting of viability-promoting gene products can inhibit tumor development. The low incidence of tumor formation is not simply a property of a particular BCL2transgenic line, as it has been consistently seen in lines generated in a variety of laboratories. In addition, the tumors that arise with theBCL2 transgene mice represent largely high-grade lymphoma, rather than a spectrum from low to high grade, which presents another limitation for studies of possible means of intervention at an early stage. BCLX transgenic mice can also develop tumors, but this occurs only after treatment with tumor-initiating and -promoting agents.25 Extensive studies in which the effects of theBCL2 transgene were evaluated on a variety of genetic backgrounds indicated that the highest incidence of approximately 25% tumor formation occurs in C57BL/6 X SJL hybrid mice.26 A much higher incidence, and greatly accelerated tumor formation, is seen in mice transgenic for both BCL2 and EμMYC.19-21,26 27 However, such a bitransgenic model represents the engineering of 2 rare oncogenic changes into the same target cell. This may circumvent events important early in tumorigenesis, events of particular interest as potential targets for intervention. The lymphoma that develops in BCL2/EμMYC bitransgenic mice is of a rare primitive cell phenotype rather than the subtypes commonly seen in human patients, further suggesting that it may to some extent represent an “engineered” entity. For the above reasons, a transgenic system in which tumors arise in a majority of animals, and recapitulate the spectrum of low- to high-grade disease seen in patients, would facilitate studies on tumorigenesis and its inhibition in the presence of a viability-promoting gene product.
MCL1 is an antiapoptotic BCL2 family member that was discovered as an early response gene in ML-1 human myeloblastic leukemia cells induced to differentiate with 12-O-tetradecanoylphorbol-13-acetate (TPA).28MCL1 is rapidly up-regulated and subsequently down-regulated in this system, expression peaking at an early stage of differentiation.28,29MCL1 has since been found to be highly regulated and expressed in a differentiation stage-specific manner in a variety of tissues, including hematopoietic cells of both the lymphoid and myeloid lineages.30,31Accordingly, MCL1 expression can be induced through a variety of growth and differentiation factor–mediated pathways, such as those stimulated by hemopoietins and interleukins.32-36MCL1 transcription is up-regulated through typical early response gene mechanisms, including extracellular signal-regulated kinase-mediated activation of serum response factor/Elk-1.36-39 In short, MCL1 is normally expressed at specific stages of hematolymphoid cell differentiation and in response to defined growth and differentiation signals.
Endogenous MCL1 expression as stimulated by growth and differentiation factors is associated with the promotion of hematopoietic cell viability.33,34,40,41 Conversely, a loss of MCL1 expression often occurs during cell death and differentiation/developmental arrest.31,33,34,38Accordingly, exogenous introduction of MCL1 prolongs the survival of transfected cells under a variety of apoptosis-inducing conditions.42 43 However, the prolongation seen withMCL1 is of short-term duration (one to several days) and is less pronounced than that seen with BCL2. As a whole, studies of its function and regulation suggest that MCL1functions as a gene that can be rapidly up-regulated for short-term viability-enhancing effects.
We have previously described mice that express MCL1 as a transgene in hematopoietic and lymphoid tissues.44 At up to 6 months of age, these mice display moderate splenomegaly but no gross lymph node enlargement or evidence of lymphoma or other cancers. Hematopoietic cells from these mice exhibit short-term enhancement of cell survival on explantation into tissue culture, providing a parallel to the effect seen in MCL1-transfected cell lines. This moderate effect is seen in both lymphoid (B and T) and myeloid cells at immature as well as mature stages of differentiation. However, when hematopoietic cells from MCL1 transgenic mice are cultured in the presence of interleukin-3 (IL-3) for about a month, immortalized myeloid cell lines invariably arise. This pronounced long-term effect is surprising not only in view of the modest short-term effects ofMCL1 but also because immortalization has not been reported for BCL2 or BCLX except with additional oncogenes.45 46 Thus, although MCL1 may normally be highly regulated for short-term effects, it can lead to a striking long-term effect—immortalization—when introduced into specific target cells placed in a conducive environment.
Because cell immortalization is a component of tumorigenesis,47 we wondered whether MCL1 might have a role in tumor development as does BCL2. MCL1 transgenic mice have now been monitored for up to 2 years to address this question. These mice were found to develop lymphoma with long latency (6 months to 2 years) and at high probability (more than 85%). The disease was of clonal origin and had disseminated to multiple lymphoid tissues as well as other organs in the majority of cases (two thirds). A variety of disease subtypes were seen, prominently follicular B-cell lymphoma and diffuse large-cell lymphoma. The MCL1 transgene thus sets the stage for the development of lymphoma, which recapitulates a spectrum of histologic subtypes as seen in human patients. These findings serve to underscore the importance of the normal, highly regulated pattern ofMCL1 expression. They further suggest the MCL1transgenic system as a model for studying tumorigenesis, and its inhibition, in the presence of a viability-promoting BCL2family member.
Materials and methods
Monitoring of MCL1 transgenic mice for lymphoma
Founder mice (C57BL/6 X SJL-F2) expressing the humanMCL1 transgene under the control of its 5′-genomic flank were described previously.44 These mice were mated with normal C57BL/6 mice for 1 to 3 generations and were then propagated by serial intercrosses. The transgenic line used was that described previously, in which the other line obtained also exhibited tumor formation. Transgenic mice were identified by Southern blotting44 or by polymerase chain reaction (PCR) using primer No. 830F [5′-ACGGCGTAACAAACTGGGGC-3′ (nucleotides 827-846 of human MCL1 cDNA, GenBank No.L08246)] and primer No. 1040R [5′-TGATGCCACCTTCTAGGTCCTC-3′ (nucleotides 1024-1045)]. PCR with Taq polymerase involved 1 cycle at 95°C (1.5 minutes), 55°C (1 minute), and 72°C (1 minute); 25 cycles at 94°C (1 minute), 55°C (1 minute), and 72°C (1 minute); a delay at 72°C (7 minutes) followed by cooling to 4°C. This process results in amplification of an approximate 1-kilobase (kb) fragment representing the humanMCL1 transgene, but it does not amplify endogenous mouseMCL1.
The cohort followed for tumor formation included all mice born between May 24, 1994, and July 6, 1997, that were not used in other studies44 and remained healthy at 6 months of age. This cohort represented 224 MCL1 transgenic and 178 nontransgenic mice. Autopsies were performed on groups of animals on a monthly basis from 6 months to 2 years of age and on all animals remaining alive after that time. The incidence of lymph node disease was calculated for 6-month intervals, and the probability of disease development was estimated by using the Kaplan-Meier method (SAS software; SAS Institute, Cary, NC). A small number of mice were not evaluable or developed other types of tumors. This group included 13 transgenic and 2 nontransgenic mice that died without an autopsy, and 2 transgenic and 3 nontransgenic mice that developed nonlymphoid tumors (hepatocarcinoma or sarcoma). These mice were eliminated from the calculations of tumor incidence and were treated as “censored” without a designation of outcome in the Kaplan-Meier calculations.
Characterization of lymphoma in MCL1transgenic mice
Western blotting to assay expression of the MCL1transgene.
Previous studies demonstrated expression of the protein product of theMCL1 transgene in the spleen and lymph nodes of transgenic mice examined prior to the development of lymphoma.44Western blotting was used to check that the transgene was expressed in the lymphomas reported here. To this end, lymphoma tissue was collected at autopsy, was sliced into small pieces using a razor blade, and was strained through plastic mesh (210 μm; Spectrum, Houston, TX). The cells obtained were then washed with cold phosphate buffered saline (PBS) and were assayed for cell number by hemacytometer.48Gel electrophoresis and immunoblotting were carried out as in previous work,43 except that a monoclonal mouse antihumanMCL1 antibody was used at a dilution of 1:5000 (antibody preparation described below). After probing with the anti–MCL1 antibody, blots were reprobed for expression of Bcl-2 using previously described methods43 or for expression of Bax using an antibody from Santa Cruz Biotechnology (Santa Cruz, CA).
Anti–MCL1 monoclonal antibodies were prepared as described49 by using bacterially produced humanMCL1 as the immunogen. To prepare MCL1 protein for use as an immunogen, a bacterial expression vector was constructed that encoded human MCL1 lacking the carboxyl hydrophobic tail of 23 amino acids (ie, encoding MCL1 amino acid residues 1-32728), linked in frame to an N-terminal His tag. The predicted sequence of the resultant His-MCL1ΔC fusion protein is MGSSHHHHHHSSGLVPRGSHRRASNMFGLKRNAVIGLNLYCGGAGLGAGSGGATRPGGRLLATEKEASARREIGGGEAGAVIGGSAGASPPSTLTPDSRRVARPPPIGAEVPDVTATPARLLFFAPTRRAAPLEEMEAPAADAIMSPEEELDGYEPEPLGKRPAVLPLLELVGESGNNTSTDGSLPSTPPPAEEEEDELYRQSLEIISRYLREQATGAKDTKPMGRSGATSRKALETLRRVGDGVQRNHETVFQGMLRKLDIKNEDDVKSLSRVMIHVFSDGVTNWGRIVTLISFGAFVAKHLKTINQESCIEPLAESITDVLVRTKRDWLVKQRGWDGFVEFFHVEDLEGGS, where the 26th amino acid residue represents the initiator methionine of MCL1.28,39 The vector used for this construct was pET-15b (Novagen, Madison, WI). The antibody produced has been used in previous studies and recognizes the MCL1protein expressed in human ML-1, HL-60, and BL41-3 cells39 50; however, no cross-reacting bands representing endogenous murine MCL1 were detected using spleen or bone marrow from nontransgenic mice.
Flow cytometric analysis of cell surface marker phenotype.
Lymphoma cells, or cells from normal lymph nodes as controls, were harvested as above and assayed for the expression of cell surface markers by flow cytometry (FACSCAN; Becton Dickinson, San Jose, CA) using previously described methods.44 Fluorescently labeled (phycoerythrin [PE] or fluorescein isothiocyanate [FITC]) antibodies (to B220, immunoglobulin M [IgM], CD19, CD3, CD5, CD11b, Sca1, CD34, c-kit) were from Pharmingen (San Diego, CA) or Southern Biotechnologies (Birmingham, AL). Isotype-matched, fluorochrome-conjugated unreactive antibodies were used to set the nonspecific background fluorescence, and single color–positive antibodies were used to adjust the compensation. For each sample, 10 000 cells were assayed. The data obtained were analyzed using CellQuest software (Becton Dickinson).
Assay for immunoglobulin heavy-chain gene rearrangement to assess clonality.
Genomic DNA was isolated from lymphoma samples, spleen or liver, and was subjected to gel electrophoresis and Southern blotting using standard methods.51 The probe used represents the JH region of the murine immunoglobulin heavy-chain gene52 and was obtained from Dr Bonnie Blomberg (University of Miami, Miami, FL). The JH insert was excised from the pGEM4Z plasmid (Promega, Madison, WI) by double digestion with XbaI and EcoRI, was gel purified, and was radiolabeled using the random priming method.53 Southern blotting of lymphoma samples with this probe can be used to assess whether only the germline (unrearranged) immunoglobulin heavy-chain gene locus is detected or whether other rearranged bands are also present; such additional bands indicate the presence of B cells with a uniform rearrangement pattern and is suggestive of a clonal origin.
Results
MCL1-induced enhancement of cell viability sets the stage for lymphomagenesis
Previous studies of MCL1 transgenic mice at younger than 6 months of age did not identify any animals with grossly enlarged lymph nodes or tumors.44 We have now followed a cohort of animals for up to 2 years, because tumorigenesis induced withBCL2 occurs after a long latency period.20,24About 27% of the MCL1 transgenic animals autopsied at 6 to 11 months of age exhibited massive lymph node enlargement (0.5 to 3 cm in length) that was subsequently found to represent lymphoma (see below). Such an effect was not seen in nontransgenic controls examined within this time frame. The incidence of pathologic lymph node enlargement increased with age, reaching values of approximately 50% in transgenic animals examined at 18 to 23 months and approximately 65% at 2 years, as compared with 2% to 12% in nontransgenic controls. In two thirds of affected transgenic mice, multiple disseminated lymph nodes (eg, in the mesenteric, renal, mediastinal, and cervical areas) and the spleen were involved, and lymphoid infiltrates were often found in other tissues (eg, liver, lung, and kidney) (Table 1). In the remaining one third of affected transgenic animals, disease was localized to mesenteric lymph nodes or presented as extranodal lymphoma in the gastrointestinal tract. This latter finding was reminiscent of the localized disease seen in a proportion of BCL2 transgenic mice.24 In the rare nontransgenic animals that were affected, disease was also restricted to mesenteric lymph nodes or to the gastrointestinal tract (Figure 1A). As estimated by using Kaplan-Meier plots (Figure 1A), the overall probability of developing pathologic lymph node disease was 88% in transgenic animals, with the probability of developing disseminated disease being 60%. In contrast, the probability of developing lymphoma was low (approximately 10%) in nontransgenic controls as in other studies,54 55 and these animals did not demonstrate disseminated disease.
MCL1 transgenic mice develop lymphoma of various subtypes
| Type of disease . | No. cases . | Other sites infiltrated* . |
|---|---|---|
| Disseminated disease | 18 total | |
| Follicular lymphoma | 3† | Liver, lung (2), kidney, bone marrow, GI tract, thymus |
| Composite lymphoma: follicular lymphoma and diffuse large-cell lymphoma | 2 | Liver (2) lung, kidney, GI tract, bone marrow, ovary |
| Diffuse large-cell lymphoma DLBCL (5 cases) DLCL, indeterminate lineage‡ (4 cases) | 9 | Liver (5), kidney (2), lung, bone marrow (2), GI tract (2), ovary |
| Follicular and diffuse polymorphic B cell lymphoma | 4 | Liver (3), kidney (3), lung (3), GI tract, bone marrow, thymus |
| Localized disease | 7 total | |
| Diffuse large-cell lymphoma | 3 | None |
| Polymorphic lymphoma | 2 | None |
| Diffuse small lymphocytic lymphoma | 1 | None |
| T-cell lymphoma | 11-153 | None |
| Type of disease . | No. cases . | Other sites infiltrated* . |
|---|---|---|
| Disseminated disease | 18 total | |
| Follicular lymphoma | 3† | Liver, lung (2), kidney, bone marrow, GI tract, thymus |
| Composite lymphoma: follicular lymphoma and diffuse large-cell lymphoma | 2 | Liver (2) lung, kidney, GI tract, bone marrow, ovary |
| Diffuse large-cell lymphoma DLBCL (5 cases) DLCL, indeterminate lineage‡ (4 cases) | 9 | Liver (5), kidney (2), lung, bone marrow (2), GI tract (2), ovary |
| Follicular and diffuse polymorphic B cell lymphoma | 4 | Liver (3), kidney (3), lung (3), GI tract, bone marrow, thymus |
| Localized disease | 7 total | |
| Diffuse large-cell lymphoma | 3 | None |
| Polymorphic lymphoma | 2 | None |
| Diffuse small lymphocytic lymphoma | 1 | None |
| T-cell lymphoma | 11-153 | None |
GI indicates gastrointestinal; DLBCL, diffuse large B-cell lymphoma; DLCL, diffuse large-cell lymphoma.
The number of mice in which a particular site was involved is indicated in parentheses.
In one mouse, the histopathology could not be definitively distinguished as being follicular lymphoma versus florid hyperplasia.
In tumors of indeterminate origin, immunohistochemistry did not reveal unequivocal staining for either B- or T-cell markers, or was not carried out.
Tumor cells were CD4+CD8+ by flow cytometric assay.
Mice expressing MCL1 as a transgene develop massive lymph node enlargement on long-term observation.
(A) Kaplan-Meier probability of remaining free of massive lymph node enlargement. The probability of developing lymph node disease was greater in transgenic (Trans) as compared with nontransgenic (Nontrans) animals (P ≤ .001). Transgenic animals developed either localized or disseminated disease, whereas nontransgenic animals demonstrated localized disease only [a single mass in the mesenteric area (3 animals), 2 mesenteric masses (2 animals), or extranodal lymphoma in the gastrointestinal tract (3 animals)]. The last time point represents animals assayed at 25 to 27 months. (B-C) Expression of the transgene-encoded human MCL1 protein in diseased lymph nodes. Diseased lymph nodes and control tissues were subjected to Western blotting using an antibody directed against human MCL1. The upper photograph shows samples from 3 separate diseased lymph nodes and the spleen from the same transgenic mouse (lanes 1-4), along with lymphoid tissue from a nontransgenic littermate (lane 5). The lower photograph shows diseased lymph nodes from additional transgenic animals with disseminated disease, along with spleen from transgenic animals not exhibiting disseminated disease (lanes 6-7) and ML-1 cells treated with TPA (lane 8). Diseased lymph nodes from transgenic animals with localized disease exhibited levels of MCL1 expression in the same range as the cases with disseminated disease, whereas anMCL1 band was not detected with this antibody in the rare tumors that occurred in nontransgenic animals. Each lane represents 2 × 106 cells in the upper photograph and 5 × 105 cells in the lower photograph.
Mice expressing MCL1 as a transgene develop massive lymph node enlargement on long-term observation.
(A) Kaplan-Meier probability of remaining free of massive lymph node enlargement. The probability of developing lymph node disease was greater in transgenic (Trans) as compared with nontransgenic (Nontrans) animals (P ≤ .001). Transgenic animals developed either localized or disseminated disease, whereas nontransgenic animals demonstrated localized disease only [a single mass in the mesenteric area (3 animals), 2 mesenteric masses (2 animals), or extranodal lymphoma in the gastrointestinal tract (3 animals)]. The last time point represents animals assayed at 25 to 27 months. (B-C) Expression of the transgene-encoded human MCL1 protein in diseased lymph nodes. Diseased lymph nodes and control tissues were subjected to Western blotting using an antibody directed against human MCL1. The upper photograph shows samples from 3 separate diseased lymph nodes and the spleen from the same transgenic mouse (lanes 1-4), along with lymphoid tissue from a nontransgenic littermate (lane 5). The lower photograph shows diseased lymph nodes from additional transgenic animals with disseminated disease, along with spleen from transgenic animals not exhibiting disseminated disease (lanes 6-7) and ML-1 cells treated with TPA (lane 8). Diseased lymph nodes from transgenic animals with localized disease exhibited levels of MCL1 expression in the same range as the cases with disseminated disease, whereas anMCL1 band was not detected with this antibody in the rare tumors that occurred in nontransgenic animals. Each lane represents 2 × 106 cells in the upper photograph and 5 × 105 cells in the lower photograph.
The protein encoded by the human MCL1 transgene was specifically expressed in the lymphoid lesions that developed in transgenic animals (Figure 1B). Expression levels were as high as, and generally higher than, those present in lymphoid tissues of disease-free transgenic animals. However, these levels were not as high as those seen on stimulation of ML-1 cells with TPA and, thus, were not outside the range that can be attained endogenously. Comparable, moderate, endogenously attainable levels have previously been shown to promote cell viability.43,44 In sum, althoughMCL1 does not immediately cause obvious lymph node pathology,44 levels that promote cell viability set the stage for lymphomagenesis, which is overtly manifest as massive lymph node enlargement.
Lymphoma in MCL1 transgenic mice is predominantly of clonal B-cell origin and represents a spectrum of histologic subtypes
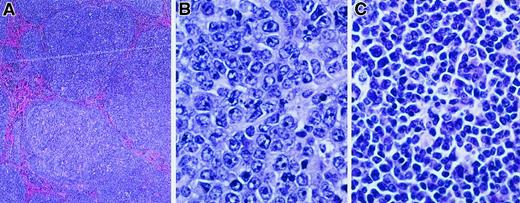
The lymph nodes from diseased MCL1 transgenic mice were assessed for histopathology and the expression of B- and T-cell markers. Examination of 18 cases of disseminated disease revealed a spectrum of histologic subtypes (Figure 2and Table 1). The major subtype observed was diffuse large-cell lymphoma (9 of 18 cases; Figure 2B and Table 1); the morphology of these lesions was similar to the high-grade disease as seen in human patients in which BCL2 is frequently overexpressed.4 Other MCL1 transgenic mice demonstrated follicular lymphoma (Figure 2A), similar in morphology to the human disease associated with BCL2 rearrangement. Some of the latter cases contained areas of diffuse large-cell lymphoma, suggesting disease progression (a total of 5 of the 18 cases had a follicular histology, and 2 of these contained areas with a diffuse histology [Table 1]). The remaining cases of disseminated disease exhibited a polymorphic cytology consisting of a heterogeneous mixture of small and large lymphocytes (4 of 18 cases; Figure 2C and Table 1). Some of these lesions contained expanded, coalescent nodules or remnants of a nodular structure, whereas in others the normal lymph node architecture was largely effaced. These tumors generally exhibited weak immunohistochemical staining for leukocyte common antigen or the B-cell marker CD45R but did not stain for the T-cell marker CD3. Although these tumors remain to be further characterized, they were found to exhibit clonal rearrangements of the JH locus (see below). They thus did not simply represent atypical hyperplasia as can occur in human post-transplant lymphoproliferative disorders that have a somewhat similar appearance.56 In addition to examining disseminated disease as representing the majority of cases, we examined a limited number of transgenic mice with localized disease (Table 1). A variety of disease subtypes were again seen, with diffuse large-cell lymphoma being the major but not the only subtype observed (3 of 7 cases; Table 1). One of these cases consisted of T-cell lymphoma (Table1), whereas all other cases were of B-cell or indeterminate origin. Taken together, these findings in MCL1 transgenic mice suggest parallels to human lymphoma in that a spectrum of disease subtypes arose, follicular lymphoma and diffuse large-cell lymphoma figuring prominently.
MCL1 transgenic mice demonstrate a spectrum of lymphoma subtypes.
Lymphoma specimens from mice exhibiting disseminated disease were fixed in 10% buffered formalin, sectioned with a microtome (4- to 5-μm sections), and stained with hematoxylin and eosin (hematoxylin 2 and eosin progressive staining system, Richard Allan Scientific, Kalamazoo, MI). The lymphoma shown in panel A was photographed at a magnification of × 40 and those in panels B and C at a magnification of × 500. (A) Follicular lymphoma. (B) Diffuse large-cell lymphoma. (C) Polymorphic lymphoma.
MCL1 transgenic mice demonstrate a spectrum of lymphoma subtypes.
Lymphoma specimens from mice exhibiting disseminated disease were fixed in 10% buffered formalin, sectioned with a microtome (4- to 5-μm sections), and stained with hematoxylin and eosin (hematoxylin 2 and eosin progressive staining system, Richard Allan Scientific, Kalamazoo, MI). The lymphoma shown in panel A was photographed at a magnification of × 40 and those in panels B and C at a magnification of × 500. (A) Follicular lymphoma. (B) Diffuse large-cell lymphoma. (C) Polymorphic lymphoma.
Cells from the diseased lymph nodes of MCL1 transgenic mice with diffuse large-cell lymphoma, the major disease subtype observed, were characterized for cell surface marker expression (Figure3A-C). In agreement with the histopathologic diagnosis, cells with increased forward scatter were seen, both in a case of localized disease and—even more prominently—in a case of disseminated disease (Figure 3A, middle and left panels, respectively). B cells (B220+; Figure 3B) were markedly increased relative to T cells in the animal with disseminated disease. In addition, a proportion of the B cells present exhibited reduced staining for cell surface IgM (Figure 3C). These IgM− B cells were prominent on gating on the large-cell population seen on dot plots, and they constituted the majority of the large cells in the case of disseminated disease (Figure 3C, upper left quadrant, percentages in parentheses). Additional tumors examined also exhibited IgM− B cells (Figure 3D). In contrast, no remarkable changes were seen in a variety of other cell surface markers: The B220+ cells present did not express CD5 (Figure 3B) or IgG (not shown), and no significant alterations were seen in markers typical of immature cells (CD34, Sca1, and c-kit; Figure 3D). As in human B-cell lymphoma,57 T cells were present to varying extents, and extensive hyperplasia was seen histologically along with lymphoma in some cases. Overall, the lymphomas that arose in MCL1 transgenic mice contained B cells that lacked expression of cell surface IgM but did not exhibit an increase in immature markers such as CD34.
Lymphomas that develop in MCL1 transgenic mice exhibit alterations in the B-cell compartment.
(A-C) Cell surface marker expression was assayed on cells from a normal nontransgenic lymph node (left column) and from diseased lymph nodes from transgenic animals with diffuse large-cell lymphoma (either localized [middle column] or disseminated [right column] disease). Similar results were obtained when CD19 was used instead of B220. The numbers on the graphs represent the percentage of cells in each quadrant, where those in parentheses were obtained on gating of the population of cells with increased forward scatter. (D) Cells from lymph nodes of MCL1 transgenic mice with disseminated disease (░), or nontransgenic controls (■), were assayed for cell surface markers characterizing lymphoid (B220, IgM, CD3, CD5), myeloid (CD11b), and immature (Sca1, CD34, c-kit) cells. The values shown represent the mean ± SE, where 4 animals per group were tested for the lymphoid and myeloid markers (the animals in the left and right columns along with 3 additional pairs in which the transgenic animals had polymorphic lymphoma) and 3 transgenic and 2 nontransgenic animals were tested for immature cell markers. IgM−B220+ cells represented 64% of the total B220+ population in the diseased transgenic lymph nodes (± 4% SE; n = 4), as compared with a control value of 13% (± 2%). This difference was significant (P < .02; analysis of variance with Sheffee testing), whereas comparisons with the other markers were not.
Lymphomas that develop in MCL1 transgenic mice exhibit alterations in the B-cell compartment.
(A-C) Cell surface marker expression was assayed on cells from a normal nontransgenic lymph node (left column) and from diseased lymph nodes from transgenic animals with diffuse large-cell lymphoma (either localized [middle column] or disseminated [right column] disease). Similar results were obtained when CD19 was used instead of B220. The numbers on the graphs represent the percentage of cells in each quadrant, where those in parentheses were obtained on gating of the population of cells with increased forward scatter. (D) Cells from lymph nodes of MCL1 transgenic mice with disseminated disease (░), or nontransgenic controls (■), were assayed for cell surface markers characterizing lymphoid (B220, IgM, CD3, CD5), myeloid (CD11b), and immature (Sca1, CD34, c-kit) cells. The values shown represent the mean ± SE, where 4 animals per group were tested for the lymphoid and myeloid markers (the animals in the left and right columns along with 3 additional pairs in which the transgenic animals had polymorphic lymphoma) and 3 transgenic and 2 nontransgenic animals were tested for immature cell markers. IgM−B220+ cells represented 64% of the total B220+ population in the diseased transgenic lymph nodes (± 4% SE; n = 4), as compared with a control value of 13% (± 2%). This difference was significant (P < .02; analysis of variance with Sheffee testing), whereas comparisons with the other markers were not.
Peripheral blood and bone marrow samples from transgenic animals were also examined for evidence of leukemia. Peripheral white blood cell (WBC) counts averaged 10 000 WBC/μL in 4 transgenic animals with disseminated lymphoma, as compared to a value of 6000 WBC/μL in 8 nontransgenic controls (coefficient of variation equals 15% to 18%). These values are in the normal range in both cases, and the moderate elevation in transgenic animals was of borderline statistical significance (P = .055). Histopathologic examination of bone marrow sections from the femurs of an additional 7 mice with disseminated disease likewise did not reveal the presence of leukemia, although deposits of lymphoma were seen in 5 cases (Table 1). In previous studies, bone marrow aspirates from nontumor-bearingMCL1 transgenic mice were found to exhibit a modest increase in the ratio of monocytic/B-lymphocytic cells, as assayed by flow cytometric cell surface marker expression (eg, an approximate 1.7-fold increase in the CD11b/B220 ratio44). In conjunction with the present work, bone marrow aspirates were examined from 2 mice with disseminated disease and from 2 nontransgenic controls; beyond the previous finding,44 no further marked differences were seen on flow cytometric assay of B220, CD11b, Gran1, CD3, Thy1.2, CD34, c-kit, and Sca1. Overall, examination of blood or bone marrow samples from a total of 13 transgenic animals did not reveal evidence for leukemia, although further studies are necessary to rule out the possibility that leukemia could have occurred in a proportion of theMCL1 transgenic population but not the sample tested here.
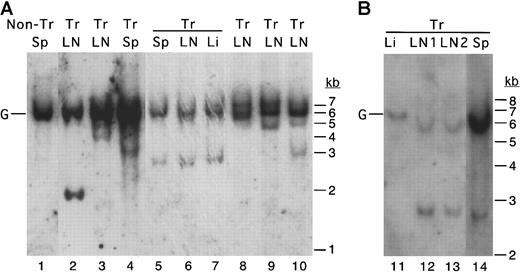
In any particular MCL1 transgenic mouse with disseminated disease, a similar histopathologic process was seen on examination of the diseased lymph nodes from different locations, the spleen, and lymphoid infiltrates in solid tissues. It thus seemed likely that these might represent the outgrowth and dissemination of clonally derived cells. To test for clonal B-cell outgrowth, Southern blotting was performed by using an immunoglobulin heavy-chain JH region probe. This assay detects a band corresponding to the germline heavy-chain gene in polyclonal populations of B cells, and additional rearranged bands arise when cells with specific immunoglobulin rearrangements grow out as clones. Thus, this assay can be used to assess the transition from polyclonal to oligoclonal and monoclonal proliferation that occurs during the progression from hyperplasia to lymphoma.58 59 Along with the unrearranged germline band, the disseminated lesions from MCL1 transgenic mice contained one to several rearranged, clonally derived bands (Figure4). Lesions from different locations within the same animal exhibited the same rearrangement pattern (Figure4, lanes 3-5 and 9-12), whereas lesions from different animals exhibited different patterns as expected. Thus, except for one case of T-cell lymphoma (Table 1), the lymphoma that developed inMCL1 transgenic mice appeared to arise from clones of B cells.
The disseminated lymphoma seen in MCL1 transgenic mice exhibits clonal rearrangement of the immunoglobulin heavy-chain locus.
A probe representing the murine immunoglobulin heavy-chain JH region probe was used in Southern blotting of cases of follicular lymphoma (lanes 3-4), diffuse large-cell lymphoma (lanes 5-7, 9, 11-14), and polymorphic lymphoma (lanes 2, 8, 10). Tissues (LN, lymph node; Sp, spleen; Li, liver) from the same animal are indicated with horizontal overlining. In the animal represented in lanes 5-7, lymphoma was present in the lymph nodes, spleen, and liver. In the animal represented in lanes 11-14, lymphoma was present in the lymph nodes and spleen but had not massively infiltrated the liver. The position of the germline (unrearranged) band is indicated with a G. Tr indicates transgenic.
The disseminated lymphoma seen in MCL1 transgenic mice exhibits clonal rearrangement of the immunoglobulin heavy-chain locus.
A probe representing the murine immunoglobulin heavy-chain JH region probe was used in Southern blotting of cases of follicular lymphoma (lanes 3-4), diffuse large-cell lymphoma (lanes 5-7, 9, 11-14), and polymorphic lymphoma (lanes 2, 8, 10). Tissues (LN, lymph node; Sp, spleen; Li, liver) from the same animal are indicated with horizontal overlining. In the animal represented in lanes 5-7, lymphoma was present in the lymph nodes, spleen, and liver. In the animal represented in lanes 11-14, lymphoma was present in the lymph nodes and spleen but had not massively infiltrated the liver. The position of the germline (unrearranged) band is indicated with a G. Tr indicates transgenic.
Discussion
In monitoring these transgenic mice for tumor formation, we could not anticipate a possible outcome based on previous findings withMCL1. On one hand, transfection with MCL1produces short-term effects less pronounced than those ofBCL2.42 On this basis, MCL1 might be expected to have little effect on tumorigenesis, becauseBCL2 results in tumor formation in only a fraction of transgenic animals.17,18,20,21,24,26 On the other hand, the MCL1 transgene facilitates the immortalization of specific cell types placed in a conducive growth environment.44 On this basis, MCL1 might be expected to have a significant effect on tumorigenesis, which involves immortalization along with changes in oncogenes and tumor suppressor genes.47 The second of these expectations appears to be the case, as lymphoma develops after a long latency withMCL1 as with BCL2 but does so at high probability.
BCL2 and other family members, including MCL1, are increasingly being found to play a role in various types of human leukemia and lymphoma.3-7,60 In fact, follicular lymphoma, in which BCL2 was first identified in the t(14;18) chromosome translocation,7 demonstrates alterations in a variety of family members, specifically an imbalance of antiapoptoticBCL2, MCL1, and BCLX to proapoptoticBAX and BAD.5 It is interesting in this regard that the lymphomas that develop inMCL1 transgenic mice represent a spectrum of histologic subtypes, including follicular lymphoma. Follicular center cell lymphoma is seen in other murine systems, but this frequently exhibits a diffuse rather than a follicular architecture.58,61 62The follicular lymphoma seen in MCL1 transgenic mice should thus be valuable as a model for identifying events important in the conversion from hyperplasia to this low-grade lymphoma (eg, using microarray technology). Because these mice also develop diffuse large-cell lymphoma, this model should similarly be useful for elucidating events that characterize progression to high-grade disease. Finally, this may represent a model system in which viability-promoting gene products can be targeted as an approach to inhibiting disease development or progression.
The high incidence of lymphoma seen with MCL1 probably reflects characteristics of both the gene product itself and other aspects of the transgenic system. In our previous studies of immortalization in the presence of MCL1, both the target cell and the growth environment appeared critical.44 A similar principle may hold for tumorigenesis. Thus, one factor contributing to the high incidence of tumorigenesis in this transgenic model could be the target cells expressing the introducedMCL1 gene, as in other systems.63 We generated these mice with the aim of targeting the transgene to tissues that can normally express MCL1, in particular to myeloid and lymphoid cells,44 as these would be expected to be sensitive to the effects of altered MCL1 expression. Indeed, enhanced survival was seen in mature cells of both these lineages, as well as in various types of immature progenitors.44 In mice expressing a BCL2 transgene targeted to the lymphoid lineage, in contrast, enhanced survival was seen in maturing lymphocytes but not in immature colony-forming cells.17Thus, in the MCL1 transgenic mice, enhanced survival in a broad spectrum of cells, including progenitor cells, may have provided a sizable pool of target cells susceptible to tumorigenesis. Enhanced survival in a spectrum of targets may also account for the fact that a variety of lymphoma subtypes arose. Mice expressing a BCL2transgene throughout the hematopoietic compartment have been described,64,65 and enhanced survival is seen in a variety of cells, including immature progenitors. It will be interesting to learn whether these mice similarly exhibit a high probability of tumorigenesis in a variety of hematopoietic cell types. The levels of transgene expression sustained in the target cells may also contribute to the high incidence of tumorigenesis in the MCL1transgenic mice. These levels were in the range previously found to moderately enhance cell survival and were not increased beyond levels that can be stimulated endogenously. However, in a variety of cell types, MCL1 is normally expressed in a transient fashion, during specific phases in differentiation or in response to defined growth factors. Thus, tumorigenesis could relate to enforced, constitutive expression, in susceptible target cells, ofMCL1 levels that are normally expressed only for defined periods of time and in response to specific signals. Another factor that probably contributes to the high incidence of tumorigenesis is that we used the C57BL/6 X SJL hybrid genetic background previously found to be optimal for the limited tumorigenesis seen withBCL2.26 The environment provided by this genetic background may be particularly conducive to tumorigenesis in the presence of antiapoptotic gene expression in an appropriate target cell. A final factor to be considered is that the tumors that develop in MCL1 transgenic mice occur after a long latency period and represent the outgrowth of lymphocyte clones. It is likely that these cells undergo additional genetic changes that contribute to tumorigenesis. With BCL2, tumorigenesis is thought to result from the complementary actions of antiapoptotic and proliferation-promoting gene products.19-21,24 26 Thus, viability enhancement induced by enforced expression of theMCL1 transgene may act together with endogenous signals or genetic changes that stimulate cell proliferation. In an appropriate target cell and environment, these events may allow the development of lymphoma.
Antiapoptotic gene products are normally under close regulatory control. This control is exemplified by MCL1, which is expressed at specific stages of differentiation and in response to defined signals in a variety of tissues.30MCL1is also subject to rapid turnover and other forms of regulation, and it is postulated to have a role as a viability-promoting gene that can be rapidly induced for short-term effects.66 67 The present studies underscore the importance of the tightly regulated pattern ofMCL1 expression, because alterations in this pattern can contribute to long-term deleterious sequelae such as cell immortalization and cancer. These long-term effects depend on additional conditions, such as the target cell, the growth environment, or additional genetic changes. The complexity of the factors involved may explain why rearrangements and high levels of BCL2expression can occur in the absence of disease. Overall, the fact thatMCL1 transgenic mice exhibit a high probability of developing a spectrum of lymphoma subtypes, including low- and high-grade disease, provides a model for defining the conditions that promote tumor development and evolution in the presence of viability-promoting gene products and for testing means of inhibiting these processes.
We are very grateful to Drs William Wade, Steve Fiering, Julie Vrana, Andreas Strasser, and Aaron Domina for reading the manuscript and for giving very helpful suggestions. The transgenic founder mice were prepared by DNX, Inc (contract no. N01-HD-0-2911).
Supported by grant CA57359 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ruth W. Craig, Department of Pharmacology and Toxicology, Dartmouth Medical School, Hanover, NH 03755; e-mail:ruth.w.craig@dartmouth.edu.

![Fig. 1. Mice expressing MCL1 as a transgene develop massive lymph node enlargement on long-term observation. / (A) Kaplan-Meier probability of remaining free of massive lymph node enlargement. The probability of developing lymph node disease was greater in transgenic (Trans) as compared with nontransgenic (Nontrans) animals (P ≤ .001). Transgenic animals developed either localized or disseminated disease, whereas nontransgenic animals demonstrated localized disease only [a single mass in the mesenteric area (3 animals), 2 mesenteric masses (2 animals), or extranodal lymphoma in the gastrointestinal tract (3 animals)]. The last time point represents animals assayed at 25 to 27 months. (B-C) Expression of the transgene-encoded human MCL1 protein in diseased lymph nodes. Diseased lymph nodes and control tissues were subjected to Western blotting using an antibody directed against human MCL1. The upper photograph shows samples from 3 separate diseased lymph nodes and the spleen from the same transgenic mouse (lanes 1-4), along with lymphoid tissue from a nontransgenic littermate (lane 5). The lower photograph shows diseased lymph nodes from additional transgenic animals with disseminated disease, along with spleen from transgenic animals not exhibiting disseminated disease (lanes 6-7) and ML-1 cells treated with TPA (lane 8). Diseased lymph nodes from transgenic animals with localized disease exhibited levels of MCL1 expression in the same range as the cases with disseminated disease, whereas anMCL1 band was not detected with this antibody in the rare tumors that occurred in nontransgenic animals. Each lane represents 2 × 106 cells in the upper photograph and 5 × 105 cells in the lower photograph.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3902/6/m_h81211196001.jpeg?Expires=1767762633&Signature=aZiVqEYPxkZraQ4REXjySfK38nqZ1EDxrnbL39t8Dr3I9qDwb42j33Vce9YsCAqE1AloOve-nWyfbSWVceRwtO15fPiVs~W7~CBmsBiT~zQIolekp~jr2fZEAWHRhgBwMw2RGczDgHgRA9xBdm7TzwfS6JDAUOgcfsmBvkEzjbYWxBjW7TMdNo1zcVYZc2RMgsQg9d4KQ8Y~-BINIjntBaujQu17zA5reYsNhSss9bdNPqLuzusC1WZXmfcyiYuuny2dpKVS5YXCGwRdJ7VTdTCs6nV9o~ILxNZeF~TI~NJm5DmtwwMS-lHMQxbCxik6sHrQ-8x8jvRg484kZo-Oqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Lymphomas that develop in MCL1 transgenic mice exhibit alterations in the B-cell compartment. / (A-C) Cell surface marker expression was assayed on cells from a normal nontransgenic lymph node (left column) and from diseased lymph nodes from transgenic animals with diffuse large-cell lymphoma (either localized [middle column] or disseminated [right column] disease). Similar results were obtained when CD19 was used instead of B220. The numbers on the graphs represent the percentage of cells in each quadrant, where those in parentheses were obtained on gating of the population of cells with increased forward scatter. (D) Cells from lymph nodes of MCL1 transgenic mice with disseminated disease (░), or nontransgenic controls (■), were assayed for cell surface markers characterizing lymphoid (B220, IgM, CD3, CD5), myeloid (CD11b), and immature (Sca1, CD34, c-kit) cells. The values shown represent the mean ± SE, where 4 animals per group were tested for the lymphoid and myeloid markers (the animals in the left and right columns along with 3 additional pairs in which the transgenic animals had polymorphic lymphoma) and 3 transgenic and 2 nontransgenic animals were tested for immature cell markers. IgM−B220+ cells represented 64% of the total B220+ population in the diseased transgenic lymph nodes (± 4% SE; n = 4), as compared with a control value of 13% (± 2%). This difference was significant (P < .02; analysis of variance with Sheffee testing), whereas comparisons with the other markers were not.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3902/6/m_h81211196003.jpeg?Expires=1767762633&Signature=3p00KASTy9ijufo6l1ZU8mc67JhVyEo9~ACjo-~rsMn2yxikBkZXocqD-htqViNPt~jtvtRZuWCEM5Pc11fhryzs7biI4mSXkuVGYlHIuY0g2I62FfElNmMc-FTyJhOX3pVSk9A14a3-Vg0rephQUVB4abWQcDEsWZvphUAAAFDm0e6~rNxBL5OQZZ-NbxzVe7U5qF6ENzsjZWIZL~-w84ZBJCTEyuyHqhhpQmy55Gd8WDGOC1Ij8wrRbKxIPkV3v0U5pHjtNLsG9gKlP7INeDLGPNPwkXSUYu2JMRoouynmfWVqrXqBEk2f5k-PF7FWF79ZOv~qKMUnLxDQIAoEmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal