A murine homologue of the epithelial membrane protein 2(EMP2) gene was identified in a search for genes associated with B-cell lymphoma tumorigenicity by using suppression subtractive hybridization. Expression of EMP2 messenger RNA in primary mouse tissues was limited to certain epithelial cell types and the peritoneal lymphoid compartment. EMP2 was expressed in the poorly tumorigenic DAC B-lymphoma cell line but was significantly down-regulated in a subline selected for in vivo tumor formation in Balb/c mice. Recombinant restoration of EMP2 expression in the subline suppressed its tumorigenicity, suggesting that loss ofEMP2 was a causal factor in the malignant phenotype. Recombinant overexpression of EMP2 was studied in B lymphoma and NIH3T3 cells. EMP2 in both cell types induced cell death on serum deprivation. EMP2-induced cell death correlated with the expression level of EMP2 protein and was prevented by caspase inhibitors Z-VAD and Z-DEVD. These findings for the first time describe an apoptotic effect of a GAS3family gene in lymphocytes. They also suggest that EMP2 may influence B-lymphoma tumorigenicity through a functional tumor suppressor phenotype.
Introduction
B-cell lymphoma is among the more common classes of human malignancies, with the number of cases doubling over the past 20 years. Lymphomas are generally multifocal at the time of diagnosis and progress over time to more aggressive phenotypes, including invasion of extranodal sites and resistance to therapy.1 A number of molecular targets have been identified in natural B-cell lymphomagenesis (eg, c-myc, bcl-2,bcl-6) with important roles in the molecular pathophysiology of corresponding classes of lymphomas (Burkitt, follicular, and diffuse large cell lymphomas, respectively).2,3 However, in vivo and in vitro experimentation has shown that activation of these genes individually is insufficient for a malignant phenotype. Relatively few collaborating genes have been identified for the progression to malignancy, notably those involving checkpoint control and genomic stability.4-7
The malignant phenotype involves the roles of several biologic traits in addition to growth control.8 For example, in tumors arising in breast and prostate, invasion and metastasis are associated with altered expression of molecules affecting tumor-stromal and -endothelial interactions: matrix proteinases and proteinase inhibitors, and the integrin receptors.9,10 In B-cell lymphomagenesis, there is also evidence for host-tumor interactions involving immune function either directly or through its action against lymphomagenic microbial infection.11
To characterize host-tumor interaction in B-cell lymphomagenesis, our laboratory previously established a murine model system.12-14 The DAC cell line was generated from the spontaneous in vitro outgrowth of splenic lymphocytes. Thus, DAC cells were not selected for in vivo growth and generally failed to form tumors in syngeneic immunocompetent mice. From the exceptional DAC tumors that did occur, malignant variant sublines of DAC (termed MV) were established with a highly tumorigenic phenotype. Both parent and daughter cell lines harbored rearranged and activated forms of c-myc, which presumably contributed to immortalization in vitro. However, additional events must have occurred to account for the tumorigenicity displayed by MV cells. We therefore hypothesized that MV and DAC would be distinguished by differentially expressed genes that either promoted or suppressed traits required in tumor formation.
In this study, we employed suppression subtractive hybridization (SSH) to identify the differentially expressed genes in DAC and MV cells. We isolated Emp2, a murine gene preferentially expressed in DAC cells, and a member of the growth arrest specific 3 (GAS3) family. Expression of Emp2 in the mouse is tissue restricted and is generally absent in lymphoid cell types. Gene transfer ofEMP2 into MV cells inhibited tumor formation, indicating that EMP2 acts as a functional tumor suppressor.EMP2 gene transfer experiments demonstrated thatEMP2 rendered cells susceptible to cell death under stress conditions. These findings implicate EMP2 as a new type of gene capable of affecting tumorigenicity in B lymphoma.
Materials and methods
Cell culture
The isolation of DAC and MV cell lines has been previously described.12-14 DAC and MV cells were cultured in RPMI 1640 medium (Biowhitaker, Walkersville, MD) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT), 2 mM L-glutamine (GIBCO BRL, Grand Island, NY), 1 mM sodium pyruvate (GIBCO BRL), 100 U/mL penicillin (GIBCO BRL), 100 μg/mL streptomycin (GIBCO BRL), and 5 × 10−5 M 2-mercaptoethanol (2-ME; Sigma, St Louis, MO). NIH3T3 cells were grown in Dulbecco modified Eagle medium (GIBCO BRL) supplemented with 10% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were passaged every 3 days.
Suppression subtractive hybridization
Differentially expressed genes were identified by using SSH.15 Briefly, messenger RNA (mRNA) was isolated from DAC and MV cells by using the Fast Track kit (Invitrogen Corp, San Diego, CA). Subtractive hybridization was performed between parental DAC and daughter MV cell lines using 2 cycles of complementary DNA (cDNA) subtraction (Clontech Laboratories, Palo Alto, CA). Both cell lines were used alternatively as testers and drivers to produce libraries of candidate genes that were selectively expressed in MV or DAC cells, respectively.
We isolated the full-length EMP2 cDNA from DAC1 cells by using the Marathon cDNA amplification kit (Clontech) according to the manufacturer's instructions. Two specific primers were designed, based on the sequence of a 246-base pair (bp) EMP2 fragment isolated from the subtracted DNA library. Polymerase chain reaction (PCR) was used to amplify the 5′ and 3′ termini of the cDNA that was then sequenced. Subsequently, the full-length cDNA was isolated by PCR.
Analysis of RNA expression
Total RNA was prepared by using an RNA purification kit (QIAGEN, Valencia, CA). Total RNA (10 μg) was fractionated by agarose-formaldehyde gel electrophoresis (1% agarose/formaldehyde). The RNA was transferred to a nylon membrane (Amersham-Pharmacia, Piscataway, NJ) by capillary action and cross-linked by UV irradiation (Stratalinker; Stratagene, San Diego, CA). The complete cDNA of EMP2 was used to generate a 32P-labeled probe using random primer synthesis (Amersham-Pharmacia). Membranes were pre-hybridized with Rapid-Hyb buffer (Amersham-Pharmacia) for 1 hour and then hybridized with labeled probe for 2 hours at 65°C. Blots were washed with a high stringency buffer (60°C, 0.1× sodium chloride/sodium citrate, and 0.5% sodium dodecyl sulfate [SDS]) and exposed to x-ray film. Murine tissue dot blots were obtained from Clontech and probed as above.
For reverse transcriptase (RT)-PCR, total RNA was prepared using a RNA purification kit (QIAGEN, Valencia, CA). Resident peritoneal cells were isolated by peritoneal lavage as previously described16and by flow cytometry; this population was 40% to 60% CD5+ B cells. RT-PCR was performed using One Step RT-PCR Beads (Amersham-Pharmacia). cDNA was prepared using 0.5 μg total RNA using a random poly(T)12-16 primer for 30 minutes at 42°C. The RT was inactivated at 95°C for 2 minutes at which pointEMP2-specific primers were added. PCR was performed using 50 cycles as follows: denaturation for 30 seconds, annealing for 30 seconds, and extension for 1 minute. The primers used wereEMP2 forward 185 (ATGTTGGTGATTCTTGCCTTC) and EMP2reverse 699 (TTACGCTTCCTCAGGATCATGT).
EMP2 retrovirus expression vector
The EMP2 open reading frame was constructed with the Flag peptide in frame at the 3′ end using the following primers: CGGAATTCACTGCCCTGTGAACATGTTGG (Eco R1 and translational start underlined) and CCGGAATTCCACTTACTTGTCGTCATCGTCTTTGTAGTCTTTACGCTTCCTCAGGATCA (EcoRI and Flag peptide are underlined).
The PCR products were EcoRI digested and subsequently cloned into the retroviral expression vector pSRα in the EcoRI site.19 20 The nucleotide sequence was verified by DNA sequencing (Davis Sequencing Facility, UC Davis). Human 293T cells were co-transfected with the pSRα vector, and a retroviral helper using a standard calcium phosphate transfection to produce virus. NIH3T3 cells or MV cells were infected with viral supernatant in the presence of 8 μg/mL polybrene. Stable transfectants were obtained and maintained under constant selection by Geneticin (G418; 1 mg/mL; GIBCO BRL). NIH3T3/EMP2 refers to NIH3T3 cells that stably overexpress EMP2.
Glutathione-S-transferase–EMP2 fusion protein and anti-EMP2 polyclonal antibodies
Rabbit antibodies were generated against the first extracellular region of the gene (from amino acid 16 to 64) constructed as a glutathione-S-transferase (GST)-EMP2 fusion protein. The EMP2 peptide was cloned by PCR using the following primers: CGCGGATCCTCTACCATTGACAATGCCTGG (forward; BamH1 underlined); CCGGAATTCTTACGCCTGCATCACAGAATAACC (reverse, EcoR1 underlined). The PCR product was directionally cloned into the BamHI and EcoRI sites of the pGEX-4T-1 vector that contains GSTgene (Pharmacia). The EMP2 fragment was cloned in frame with the GST to create a fusion protein. The insert was confirmed by sequencing.
The GST fusion protein was produced as previously described.21 Bacteria in log phase (OD600 0.6 to 0.9) were induced for 2.5 to 3 hours at 37°C with 1 mM isopropyl-1-thio-β-D-galactopyranoside. Bacteria were lysed, and the soluble fraction was loaded onto a glutathione-Sepharose column (Pierce, Rockford, IL). The columns were washed with 10 bed volumes of phosphate-buffered saline (PBS)/EDTA. The fusion protein was eluted from the column using 20 mM reduced glutathione (Sigma, St Louis, MO) in 50 mM Tris-Cl, pH 8.0. For antibody preparation, rabbits were immunized twice with the GST-EMP2 fusion protein, and serum was collected, starting 2 weeks after the last immunization (Research Genetics, Huntsville, AL).
Western blot analysis
Samples were normalized based on cell number and lysed by boiling for 5 minutes in Laemmli buffer (62.5 mM Tris-Cl, pH6.8, 10% glycerol, 2% SDS, 0.01% Bromophenol blue, 2% βME). The lysate was separated on a 12% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Amersham Pharmacia). Membranes were stained with Ponceau S (Sigma) to determine transfer efficiency. Membranes were incubated with 5% low-fat milk in PBS containing 0.1% Tween-20. A primary antibody (anti-Flag M2 mouse monoclonal antibody, 2 ng/μL final concentration [Eastern Kodak] or anti-EMP2 rabbit serum, 1:10 000 dilution) was added and incubated for 1 hour. The membrane was washed 3 times with PBS/Tween-20 and then incubated for 45 minutes with a horseradish peroxidase-labeled secondary antibody (goat anti–mouse immunoglobulin G [IgG] or goat anti–rabbit IgG, 1:2000 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA). Proteins were detected by chemiluminescence (Amersham Pharmacia).
Glycosylation treatment
NIH3T3 cells were plated in 24-well plates. Two-fold serial dilutions of tunicamycin (0.15 to 10 μg/mL; Roche Molecular Biochemicals) were added to the cells the following day and incubated for 24 hours. Freshly diluted tunicamycin was then added again. Cells were harvested after an additional 4 hours for Western blot analysis. Alternatively, NIH3T3 cells and the B-cell lymphoma cell lines were lysed in Laemmli buffer and boiled as described above. Cell lysates were treated with PNGase (New England Biolabs) overnight at 37°C to remove all N-linked glycans as per manufacturer's instructions.
Tumorigenicity and cell death analysis
Exponentially growing MV cells infected with a vector control or with EMP2 were washed and resuspended in sterile PBS. Cells (5 × 105) were injected intraperitoneally into Balb/c mice (JAX Mice, Bar Harbor, ME) at 8 to 15 weeks of age. Animals were monitored daily for 2 months for signs of tumor development and morbidity. Mice were autopsied to confirm tumor formation.
For cell death analysis, cells (1 × 105) were incubated in 24-well plates in 10% FCS medium. After 24 hours, cells were washed with serum-free medium 3 times. Medium containing 0.5% FCS was added to the cultures for 48 hours at 37°C; cell viability was determined by trypan blue exclusion.
To determine the rate of apoptosis in NIH3T3 cells, cells were stained with annexin (Becton Dickinson Biosciences, Torrey Pines, CA) and propidium iodide. The cells were incubated at room temperature for 15 minutes and analyzed on a flow cytometer (Becton Dickinson Biosciences). Cells were harvested at 0, 20, or 40 hours after being placed in a low-serum environment.
In some experiments, cells were prepared in media containing 0.5% FCS plus one of the following supplements: caspase inhibitor Z-VAD-FMK (30 μM; Enzyme Systems Products, Livermore, CA), caspase inhibitor Z-DEVD-FMK (30 μM; Pharmingen), or a dimethyl sulfoxide (DMSO)-negative control. Cells were incubated for 48 hours at 37°C and later harvested. Cell viability was quantitated using trypan blue exclusion.
Results
Isolation of murine homologue of EMP2 in DAC B-cell line
DAC1 and MV1 cells are parent and daughter cell lines distinguished by nontumorigenic and tumorigenic phenotypes, respectively. A molecular screen, SSH, was used to identify genes that may contribute to this phenotypic difference. Sequence analysis of the various clones was used to prioritize genes for further examination.22 The present study explores the role of one of the candidate genes from this panel. The full-length 915-nucleotide cDNA was amplified by rapid amplification of cDNA ends from a DAC cDNA library.23 The cDNA contained an open reading frame of 516 nucleotides, predicting a polypeptide of 172 amino acids with an estimated molecular weight of 20 kDa.
A homology search by BLAST-P analysis showed that this clone had 100% amino acid identity with the murine Xmpgene24 and 78% amino acid identity withEMP2.25 Our analysis also includes 5′ and 3′-untranslated sequences (184 and 214 nucleotides, respectively) not previously reported (GenBank accession no. pending). Accordingly, we construe the gene to be murine Emp2. EMP2 shared significant homology to other members of the murine GAS3 family26: murine Pmp22 (44%), Emp1(44%), and Emp3 (39%) (data not shown).
EMP2 exhibits a restricted tissue distribution
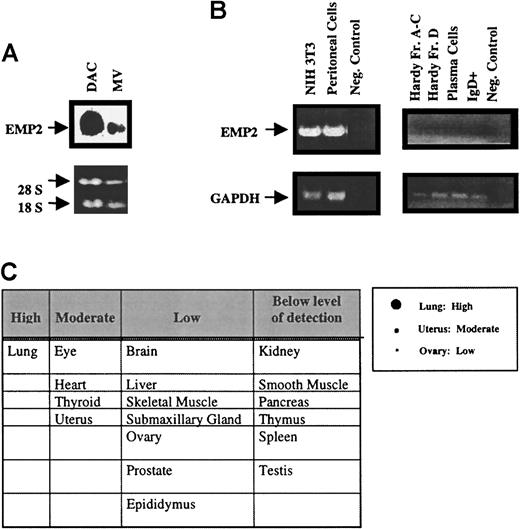
The differential expression of EMP2 in DAC versus MV cells was confirmed by Northern analysis, showing that EMP2 mRNA was significantly down-regulated in MV cell (Figure1A). We evaluated the expression ofEMP2 in primary mouse tissues (Figure 1B,C). EMP2 mRNA was not detectable in spleen and thymus or in purified subpopulations of immature and mature B-cell populations (Hardy fractions of pre-B cells, splenic B cells, and plasma cells). However, it was expressed in peritoneal cells, a population highly enriched for the B1 subset of B lymphocytes. EMP2 was undetected in peritoneal macrophages, the other major component of this population (data not shown).EMP2 was most prominently expressed in murine lung, with intermediate expression in the uterus, eye, and prostate (Figure 1C).
EMP2 has a discrete cell type and tissue distribution.
(A) Northern blot analysis of EMP2 in total DAC and MV RNA. The 4.8-kb EMP2 transcript was significantly higher in DAC than MV. rRNA bands indicate that the samples were equally loaded. (B) RT-PCR of EMP2 expression in NIH3T3 cells, peritoneal cells, Hardy fraction B cells, and plasma cells. PCR yielded the expected 514-bp EMP2 fragment in peritoneal and NIH3T3 fibroblasts. No EMP2 expression was detectable in Hardy fractions or plasma B cells. Glyceraldehyde phosphate dehydrogenase was used as a positive control to normalize expression between the different cell types. (C) EMP2 expression in murine tissue was evaluated by Northern dot blot probed with a 32P-labeledEMP2 fragment. Criteria for semiquantitative assessment of tissue expression are shown in the right panel; results are tabulated in the left panel.
EMP2 has a discrete cell type and tissue distribution.
(A) Northern blot analysis of EMP2 in total DAC and MV RNA. The 4.8-kb EMP2 transcript was significantly higher in DAC than MV. rRNA bands indicate that the samples were equally loaded. (B) RT-PCR of EMP2 expression in NIH3T3 cells, peritoneal cells, Hardy fraction B cells, and plasma cells. PCR yielded the expected 514-bp EMP2 fragment in peritoneal and NIH3T3 fibroblasts. No EMP2 expression was detectable in Hardy fractions or plasma B cells. Glyceraldehyde phosphate dehydrogenase was used as a positive control to normalize expression between the different cell types. (C) EMP2 expression in murine tissue was evaluated by Northern dot blot probed with a 32P-labeledEMP2 fragment. Criteria for semiquantitative assessment of tissue expression are shown in the right panel; results are tabulated in the left panel.
Expression of EMP2 at the protein level
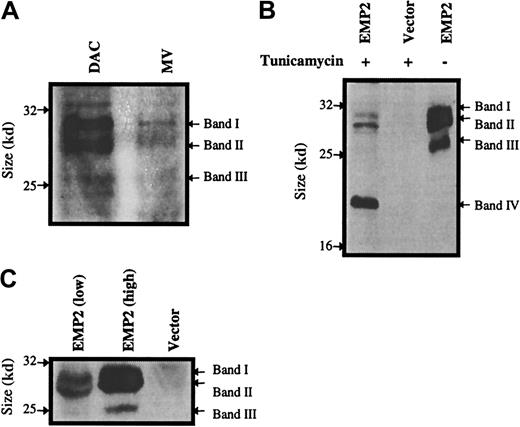
To confirm that there was differential expression ofEMP2 at the protein level between DAC and MV cells, an antibody against the first extracellular domain of EMP2 was generated using an EMP2-GST fusion protein. Polyclonal rabbit antisera verified that EMP2 is differentially expressed at the protein level (Figure 2A).
Protein levels and glycosylation isoforms ofEMP2.
(A) DAC and MV cells were compared for EMP2 protein expression by Western blot using an anti-EMP2 rabbit serum. DAC cells express significantly higher levels of EMP2 than MV cells. (B) Effect of tunicamycin treatment on EMP2 protein. Cells were incubated with tunicamycin, and a Western blot was performed using an anti-Flag M2 antibody. Bands I-III designate glycosylated EMP2 isoforms; Band IV represents the nonglycosylated protein with the predicted molecular weight of 20 kDa. (C) NIH3T3 cells infected with the pSRαEMP2 expression vector express 3 size isoforms ofEMP2.
Protein levels and glycosylation isoforms ofEMP2.
(A) DAC and MV cells were compared for EMP2 protein expression by Western blot using an anti-EMP2 rabbit serum. DAC cells express significantly higher levels of EMP2 than MV cells. (B) Effect of tunicamycin treatment on EMP2 protein. Cells were incubated with tunicamycin, and a Western blot was performed using an anti-Flag M2 antibody. Bands I-III designate glycosylated EMP2 isoforms; Band IV represents the nonglycosylated protein with the predicted molecular weight of 20 kDa. (C) NIH3T3 cells infected with the pSRαEMP2 expression vector express 3 size isoforms ofEMP2.
To further study the biological function of EMP2 protein in vitro, we infected NIH3T3 cells with the pSRα retrovirus bearing full-lengthEMP2 (with a C-terminal Flag peptide) or with an empty vector control. The expression of recombinant EMP2 protein in infected cells was analyzed by Western blot using an anti-Flag antibody (Figure2C). Three protein bands were observed in the cells containing the EMP2-Flag construct. As expected, anti-Flag antibody failed to detect protein in wild-type (uninfected) NIH3T3 cells or cells infected with the vector control. Similar results were obtained using the rabbit antisera (data not shown).
We postulated that the occurrence of multiple EMP2 bands was the result of glycosylation, as the protein contains 3 putative N-linked glycosylation sites. To assess this hypothesis, NIH3T3/EMP2 cells were treated with tunicamycin, an agent that interrupts an early stage in the assembly of N-linked oligosaccharides. NIH3T3/EMP2 cells treated with tunicamycin diminished their expression of the 3 major EMP2 bands and acquired a single 20-kDa protein by the anti-Flag antibody (Figure2B, band IV). The size of this band is consistent with the predicted molecular weight of the minimally glycosylated 172-amino acid EMP2 protein. This finding indicates that EMP2 is glycosylated in vivo and that the EMP2 size heterogeneity is due to distinct EMP2 glycans.
Overexpression of EMP2 suppresses tumor growth
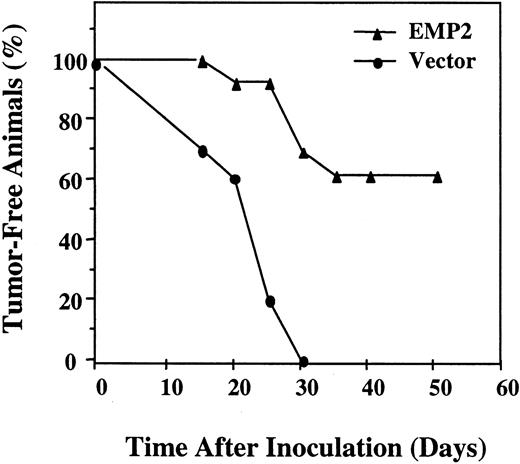
Using the retroviral EMP2 expression construct, we next sought to determine whether there was a causal relationship between elevated EMP2 expression and tumorigenicity in the B-cell lymphoma. EMP2MV cells were stably infected with the retrovirus bearing EMP2 or empty vector. Western analysis and anti-Flag antibody confirmed strong expression of recombinant EMP2 protein in infected cells (data not shown). Balb/c mice were injected intraperitoneally with 5 × 105 B-lymphoma cells and monitored for up to 50 days. Figure 3 demonstrates that recombinant restoration of EMP2 expression dramatically reduced tumor frequency in MV cells.
EMP2 suppresses tumor formation.
EMP2 was recombinantly reintroduced into MV cells using a pSRα retroviral expression vector. Balb/c mice were injected intraperitoneally with 5 × 105 vector control or mEMP2 overexpressing cells. Mice were monitored for up to 50 days for tumor formation.
EMP2 suppresses tumor formation.
EMP2 was recombinantly reintroduced into MV cells using a pSRα retroviral expression vector. Balb/c mice were injected intraperitoneally with 5 × 105 vector control or mEMP2 overexpressing cells. Mice were monitored for up to 50 days for tumor formation.
EMP2 promotes cell death in low serum
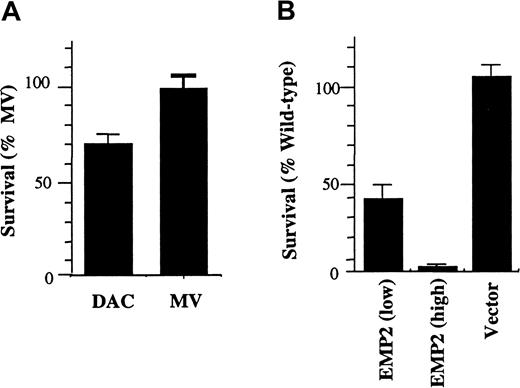
The GAS3 family member PMP22 has been shown to induce cell death under low-serum conditions. To evaluate if the differential expression of EMP2 could also affect cell survival, viability of DAC and MV cells was determined using trypan blue exclusion under low-serum conditions. Indeed, DAC cell survival was reduced approximately 35% compared with MV cells in 0.5% FCS (Figure4A).
EMP2 overexpression induces cell death on growth factor withdrawal.
(A) DAC or MV cells (3 × 105) were cultured in 0.5% FCS for 2 days, and viable cells were enumerated by trypan blue exclusion. MV cells had a 30% higher survival rate than DAC cells. (B) NIH3T3 cells (3 × 105) overexpressing EMP2 at various levels, or infected with empty vector, were plated overnight in 10% FCS, washed, and then incubated for 2 days in medium containing 0.5% FCS. Cells were harvested, and viable cells were enumerated using trypan blue exclusion. EMP2 expression inversely correlated with survival in fibroblasts.
EMP2 overexpression induces cell death on growth factor withdrawal.
(A) DAC or MV cells (3 × 105) were cultured in 0.5% FCS for 2 days, and viable cells were enumerated by trypan blue exclusion. MV cells had a 30% higher survival rate than DAC cells. (B) NIH3T3 cells (3 × 105) overexpressing EMP2 at various levels, or infected with empty vector, were plated overnight in 10% FCS, washed, and then incubated for 2 days in medium containing 0.5% FCS. Cells were harvested, and viable cells were enumerated using trypan blue exclusion. EMP2 expression inversely correlated with survival in fibroblasts.
This effect was further evaluated in NIH3T3 cells under the stress of low serum. Wild-type NIH3T3 cells (control vector infectants) are resistant to apoptosis in low serum.27 However, we observed striking cell death within 48 hours in NIH3T3/EMP2 cells. This phenotype was dose dependent: 100% cell death was observed with highEMP2, whereas there was only 55% and 4% cell death with intermediate and endogenous EMP2 levels, respectively (Figure 4B).
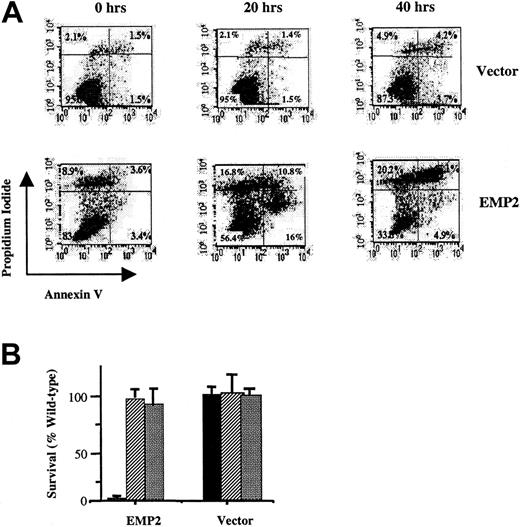
Two independent methods were employed to verify that these cells underwent programmed cell death. First, NIH3T3/EMP2 cells were evaluated by flow cytometry with annexin V and propidium iodide (Figure5A). After 20 hours, annexin V expression was increased more than 8-fold in these EMP2 versus vector control cells (16% and 1.5%, respectively). Increased total cell death (propidium iodide, annexin V++ cells) was also observed and predominated by 48 hours (41% and 4% double-positive cells, respectively). Second, serum-deprivation apoptosis in fibroblasts is a caspase-dependent process.27 28 Two caspase inhibitors Z-VAD-FMK and Z-DEVD-FMK completely protected NIH3T3/EMP2 from serum deprivation cell death (Figure 5B).
EMP2-induced cell death has features of apoptosis.
EMP2 or vector control infectant NIH3T3 cells were cultured for 20 or 40 hours in 0.5% serum and assayed for cell death. (A) Propidium iodide and annexin V staining. Time 0 represents cells before transfer to 0.5% serum conditions. EMP2 infectants after 20 hours express a more than 8-fold increase in annexin V staining compared with vector control infectants. (B) Cells were cultured in 0.5% serum supplemented with either 0.5% DMSO (▪), Z-VAD (30 μM in 0.5% DMSO) (▨), or Z-DEVD (30 μM in 0.5% DMSO) (░). Two days later, cells were harvested, and viable cells were enumerated using trypan blue exclusion. Inhibitors reversed EMP2-mediated serum deprivation apoptosis.
EMP2-induced cell death has features of apoptosis.
EMP2 or vector control infectant NIH3T3 cells were cultured for 20 or 40 hours in 0.5% serum and assayed for cell death. (A) Propidium iodide and annexin V staining. Time 0 represents cells before transfer to 0.5% serum conditions. EMP2 infectants after 20 hours express a more than 8-fold increase in annexin V staining compared with vector control infectants. (B) Cells were cultured in 0.5% serum supplemented with either 0.5% DMSO (▪), Z-VAD (30 μM in 0.5% DMSO) (▨), or Z-DEVD (30 μM in 0.5% DMSO) (░). Two days later, cells were harvested, and viable cells were enumerated using trypan blue exclusion. Inhibitors reversed EMP2-mediated serum deprivation apoptosis.
Discussion
In this study, we report the isolation by SSH of a murine gene that appears to play a role in the malignant progression of the DAC B-lymphoma cell line. Expression of EMP2 was down-regulated in the fully malignant DAC subline, MV, at both the mRNA and protein levels. Recombinant reintroduction of EMP2 into MV cells reversed their tumorigenicity, suggesting that EMP2 acted as a tumor suppressor. EMP2 had a restricted tissue distribution in mice and was not expressed in most lymphoid cell types. In vitro, EMP2 overexpression resulted in a serum deprivation cell-death phenotype. Here, we discuss these findings and their possible functional relationship.
EMP2 belongs to the GAS3 family of tetraspan proteins. All of the family members have similar primary and secondary structures, with 4 relatively conserved transmembrane regions and 2 significant extracellular loops.29,30 Although the endogenous functions of this family remain enigmatic, on disruption, many of the genes yield dramatic phenotypes. For example, EMP1 was initially isolated as a gene expressed in brain tumors but not in normal brain.31PMP22, another GAS3 family member, has gained attention in recent years as alternations inPMP22 expression have been linked to human demyelinating heredity neuropathies, including Charcot-Marie Tooth-1A, hereditary neuropathy (with liability to) pressure palsies, and Dejerine Sottas syndrome, as well as the Trembler phenotype in mice.32-34 To our knowledge, this study provides the first experimental evidence for the role of a GAS3 family protein as a tumor suppressor.
EMP2 exhibited a discrete tissue distribution that is consistent with that reported for human EMP2.25The highest expression was observed in the lung, and EMP2was also expressed throughout the female reproductive system, with expression in both the uterus and the ovary. In our laboratory,EMP2 was originally isolated from the DAC CD5+B-cell line. CD5+ B cells (and related members of the B1 subset of B cells) are the predominant cell type in the peritoneal cavity, where they represent 30% to 60% of the total cells.35 36 Correspondingly, EMP2 expression was detected in B1 cells isolated from a peritoneal lavage but was not detected in major lymphoid tissues or in fractionated Hardy fraction or plasma cell-stage B cells.
What biochemical functions are performed in these cell types by the GAS3 family? As previously noted, no studies have yet established the roles played by this protein family. The tetraspan superfamily includes the connexins, tetraspanins, and GAS3 families. Connexins are the central components of gap junctions, through their formation of hexameric membrane pores with distinctive intercellular-docking properties and regulated permeabilities.37 In this context, it is notable that certain connexins, notably CX32, are close structural homologues and display missense phenocopies ofPMP22.34 Because connexons can form with heteromeric connexin subunits, it is conceivable that GAS3 family members may be included in the formation of connexons with distinctive properties.
Alternatively, GAS3 proteins may have properties analogous to tetraspanins. Interestingly, many of the 4 transmembrane proteins, such as CD9, CD63, and CD82, were first cloned in screens relating to tumorigenesis, including correlative and experimental evidence for tumor suppressor phenotypes.38-41 Although the mechanistic basis for this relationship is unresolved, several tetraspanins have been shown to selectively associate with various integrin isoforms.39,42-45 Integrins are critical mediators of diverse cell functions, including cytoskeleton organization, cell growth, gene expression, survival, and migration. Accordingly, integrin function has been intensively studied for its roles in carcinogenesis and malignant progression,9,46 including a tumor suppressor phenotype for certain isoforms.47
The present study indicates that EMP2 modifies cell survival under serum deprivation conditions, as observed with altered expression or ligation of certain integrins.46 Overexpression of the related GAS3 protein, PMP22, also induces apoptosis of NIH3T3 cells in low serum.32 The mechanism of apoptosis in the PMP22 study has not been defined. In the present study, reversal of cell death by peptide inhibitors suggests that cell death involves a caspase-related pathway. This effect may be interpreted in 2 different contexts. First, ectopically expressed EMP2 may aberrantly act as a caspase inducer. Second, EMP2 under normal conditions may participate in a physiologic apoptotic growth regulatory pathway. We speculate that EMP2 acts in the latter fashion, facilitating signaling by integrin isoforms responsible for growth regulation by distal caspase activation. Validation of this idea will require further delineation of EMP2-integrin interaction, and the reciprocal action of subnormal EMP2 expression. Indeed, ifEMP2 is an authentic tumor suppressor, formal demonstration will entail the observation of augmented lymphomagenesis inEMP2-deficient mice.
CD5+ B cells are implicated in the pathogenesis of chronic lymphocytic leukemia and certain autoimmune diseases.35 Interestingly, the CD5+ population of B cells has been found to be more susceptible to apoptosis than the CD5− population and are susceptible to distinct activation and regulatory pathways.12,36,48 Programmed cell death plays a critical role in the maintenance of the immune system, and disruption of genes associated with apoptosis has been associated with various cancers.8 It is thus possible that differential expression of EMP2 and other tetraspan proteins may play a role in the distinctive properties of CD5+ B cells. The present findings support a further evaluation of the biochemical function of this protein class in B-cell biology and lymphomagenesis.
We thank Drs K. A. Dorshkind and M. G. McHeyzer-Williams for gifts of fractionated B-cell populations.
Supported by National Institutes of Health grants AI38545 and CA12800; the Lymphoma Research Foundation of America; the Jonsson Comprehensive Cancer Center; the Gustavus and Louise Pfeiffer Research Foundation; the John Lloyd Research Award; and a UCLA Dissertation Year Fellowship.
C.-X. W. and M. W. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jonathan Braun, Dept of Pathology and Laboratory Medicine, UCLA School of Medicine, CHS 13-222, 10833 Le Conte Ave, Los Angeles, CA 90095-1732; e-mail: jbraun@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal