Recently, it was shown that interferon consensus sequence binding protein (ICSBP), a member of the interferon regulatory factor (IRF) family, has a potential role in chronic myeloid leukemia (CML). Deletion of ICSBP gene in mice leads to a CML-like syndrome and samples from CML patients exhibited impaired ICSBP expression. The present study found that ICSBP expression correlated with risk features determined by Sokal score in untreated CML (P = .007 for high versus low risk). In addition, analyzing ICSBP expression during interferon-α (IFN-α) therapy in “good” (n = 27) versus “poor” (n = 15) cytogenetic responders, high ICSBP levels were only observed in “good” responders (P = .0002). Together, these data suggest that ICSBP levels are related to initial presentation of CML and the therapeutic response of CML to IFN-α, indicating an important role of ICSBP in CML.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder characterized by bcr-abl gene rearrangement and a typical 3-phase course (chronic, accelerated, and blastic phase) (see Sawyers1 for a review). Treatment with interferon-α (IFN-α), as monotherapy or in combination with other chemotherapeutic drugs, is frequently used for patients with CML and has prolonged survival.2-6 IFNs are a family of multifunctional cytokines known to regulate cell growth, immune response, and antiviral activity in mammals. They act by activating various downstream signal cascades, which leads to regulation of several transcription factors.7-10
One of these factors is the interferon consensus sequence binding protein (ICSBP), a member of the IFN regulatory factor (IRF) family. ICSBP is known to be regulated by IFNs and subsequently binds to IFN-stimulated response elements (ISRE) in IFN-dependent genes.11,12 ICSBP is preferentially expressed in cells of hematopoietic origin.12,13 ICSBP has been implicated in CML, because ICSBP-knockout mice revealed a granulocytic leukemia similar to CML in humans.14 The deletion ofICSBP gene led also to a blastic transformation that was more frequent in ICSBP−/− homozygous compared to ICSBP+/− heterozygous mice.14 Furthermore, a lack of ICSBP expression in CML patients has been described, and stimulation experiments suggested a mechanism of down-regulation.15
To address the question whether ICSBP expression was predictive of the risk profile of untreated patients with CML or later of their cytogenetic response to IFN-α, we correlated the transcriptional level of ICSBP in CML with clinical data.
Study design
Patient samples, RNA isolation, and complementary DNA synthesis
Patient samples were taken from patients enrolled in the ongoing German CML trials and the Charité-Campus Virchow Klinikum, Humboldt Universität, Berlin, Germany. Heparinized peripheral blood was drawn after informed consent was obtained. All investigated patients were positive for bcr-abl.
RNA was extracted from peripheral blood using the commercially available kits (RNAzol, Paesel, Frankfurt, and RNeasy, QIAGEN, Hilde, Germany). Total RNA (1 μg) was used for complementary DNA (cDNA) synthesis as described previously.15
ICSBP messenger RNA expression analysis
Because of the restricted availability of RNA amounts from leukemia patients and the retrospective design of the study, messenger RNA (mRNA) expression analysis was carried out by reverse transcription-polymerase chain reaction (RT-PCR). RT-PCR for ICSBP and β-actin was performed as described elsewhere.15 The PCR products were electrophoresed and the gels were photographed. Integrated optical densities (IntOD) were calculated using the ONE-Dscan 1.0′ software (Scanalytics, Billerica, MA).
Quantitative RT-PCR was performed using a real-time PCR assay with the ABI PRISM 7700 Sequence Detection System (PE Biosystems, Foster City, CA) as described elsewhere.16 In addition to the previously applied primers,15 a labeled ICSBP probe was used (5′-6FAM-TAAGAGCCCAGATTTTGAGGAAG-(TAMRA)-TGACCGG-3′). Theβ-actin was used as reference gene and ΔCTvalues were then calculated (CTβ-actin− CTICSBP).17
Calculation of Sokal score for risk feature discrimination and determination of cytogenetic response to IFN-α
The Sokal score at diagnosis was calculated as the percentage of peripheral blasts, thrombocytes, size of spleen, and age of the patient at diagnosis as described elsewhere.18 A Sokal score below 0.8 comprises a low risk, from 0.8 to 1.2 an intermediate risk, and above 1.2 a high risk.
Cytogenetic response was assessed analyzing at least 10 metaphases and was defined as “good” comprising complete responders (CR, 0% Ph+ metaphases) and partial responders (PR, 1%-34% Ph+ metaphases) or “poor” including patients with a minor (MinR, 35%-94% Ph+ metaphases) or no response (NR, 95%-100% Ph+ metaphases).19 Mean time of IFN-α administration was 30.2 months for “good” and 30.1 months for “poor” responders.
Results and discussion
Pretreatment risk features in CML can be useful for early indication of allogenic transplantation and are commonly determined by Sokal score.18 Because ICSBP expression may play a role in CML, we wanted to study the association of risk profile with ICSBP levels. Thus, we retrospectively analyzed a total of 72 samples from patients with CML in chronic phase without IFN-α treatment for their ICSBP expression (median 0.098; range 0.000-0.622). For 33 of these samples the ICSBP level was previously presented.15
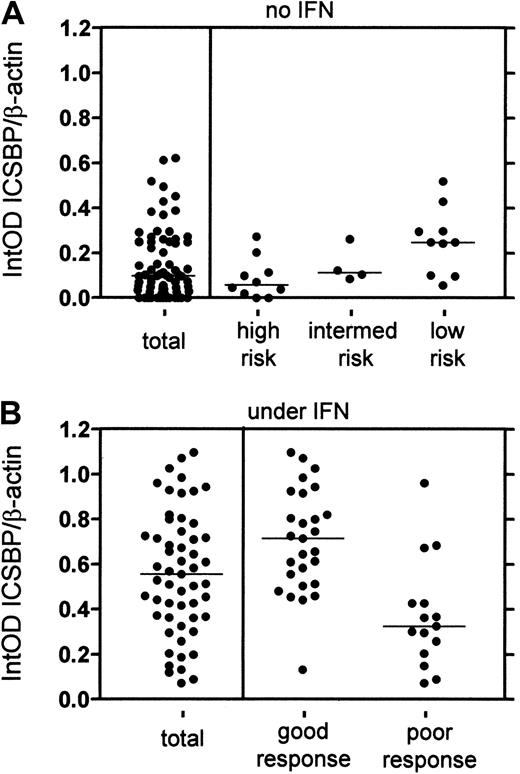
Clinical data for calculation of the Sokal score were available for 24 of the 72 CML samples. Correlating the ICSBP expression with the Sokal score, we detected a significant difference of ICSBP expression in the pretreatment risk groups. Patients from the high-risk group had lower ICSBP levels (n = 10; median 0.059; range 0.000-0.273) than patients from the low-risk group (n = 10; median 0.248; range 0.057-0.519) (P = .007) (Figure 1A). Samples with intermediate risk (n = 4) had no significant difference in ICSBP expression to low (P = .374) or high risk (P = .188). Due to the low amounts of samples and an overlap of ICSBP levels in the risk groups, the indicative value of ICSBP expression must be proved with a larger amount of samples. However, we corroborated our data using a real-time PCR assay. Analysis of each 5 randomly selected samples from the high- and low-risk groups yielded a significant difference (data not shown).
Correlation of ICSBP expression with pretreatment risk groups and cytogenetic response to IFN-α in CML patients.
(A) The ICSBP level of samples without IFN treatment was analyzed (total n = 72). Twenty-four samples were divided into different risk groups by Sokal score: low risk (n = 10; below 0.8), intermediate risk (n = 4; 0.8-1.2) and high risk (n = 10; above 1.2). The data revealed a correlation of low ICSBP level with high risk (low versus high P = .007; low versus intermediateP = .374; intermediate versus high P = 0.188; Mann-Whitney test). (B) The ICSBP level of samples under IFN-α was analyzed (total n = 55). Forty-two samples were divided in groups of “good” (CR/PR; n = 27) and “poor” (MinR/NR; n = 15) responders and revealed a significant difference in ICSBP expression (P = .0002, Mann-Whitney test).
Correlation of ICSBP expression with pretreatment risk groups and cytogenetic response to IFN-α in CML patients.
(A) The ICSBP level of samples without IFN treatment was analyzed (total n = 72). Twenty-four samples were divided into different risk groups by Sokal score: low risk (n = 10; below 0.8), intermediate risk (n = 4; 0.8-1.2) and high risk (n = 10; above 1.2). The data revealed a correlation of low ICSBP level with high risk (low versus high P = .007; low versus intermediateP = .374; intermediate versus high P = 0.188; Mann-Whitney test). (B) The ICSBP level of samples under IFN-α was analyzed (total n = 55). Forty-two samples were divided in groups of “good” (CR/PR; n = 27) and “poor” (MinR/NR; n = 15) responders and revealed a significant difference in ICSBP expression (P = .0002, Mann-Whitney test).
We then sought to delineate the implication of ICSBP in CML with the best known prognostic parameter for survival, cytogenetic response to IFN-α therapy. Because approximately 70% of the patients exhibit only “poor” cytogenetic response to IFN-α therapy, it is useful to identify these resistant patients as early as possible.5 To study the correlation of ICSBP expression and cytogenetic response to IFN-α, we first analyzed a total of 55 patients under IFN-α for ICSBP expression (median 0.556; range 0.072-1.072). The ICSBP level was significantly higher than in the untreated samples above (P < .0001). This is in keeping with data from sorted B cells, showing that ICSBP expression is impaired in B cells from CML patients without IFN-α, whereas B cells from patients under IFN-α exhibit a normal ICSBP level (15, data not shown). For 42 patients, complete clinical data were available. They were specified for their cytogenetic response and were divided into groups of 13 CR, 14 PR, 2 MinR, and 13 NR. The samples were preselected for their response, in an attempt to obtain equal amounts of the respective groups. Interestingly, a significant difference in ICSBP levels between CR versus NR or PR versus NR was found (P = .010 or P = .001, respectively). No significant difference between CR and PR itself was detected (P = .297); however, the ICSBP levels from PR were slightly higher than in CR (Table 1). By analyzing combined groups of “good” (n = 27; CR/PR, 0%-34% Ph+) and “poor” responders (n = 15; MinR/NR, 35%-100% Ph+), we detected an even more striking correlation of “good” response with high ICSBP expression (median 0.715; range 0.132-1.072) and “poor” response with low ICSBP level (median 0.325; range 0.072-0.961) (P = .0002; Figure 1B). Again, we corroborated our data using the real-time PCR assay. Analysis of each 5 randomly selected samples from “good” and “poor” responders gave also a significant difference (data not shown).
Interferon consensus sequence binding protein levels in chronic myeloid leukemia samples from different responders to interferon-α therapy
| Response . | N . | Median* . | Range* . | P value† . |
|---|---|---|---|---|
| CR | 13 | 0.657 | 0.132-0.985 | .010‡ |
| PR | 14 | 0.803 | 0.442-1.072 | .001‡ |
| MinR | 2 | 0.343 | 0.258-0.427 | ND1-153 |
| NR | 13 | 0.325 | 0.072-0.961 | — |
| Response . | N . | Median* . | Range* . | P value† . |
|---|---|---|---|---|
| CR | 13 | 0.657 | 0.132-0.985 | .010‡ |
| PR | 14 | 0.803 | 0.442-1.072 | .001‡ |
| MinR | 2 | 0.343 | 0.258-0.427 | ND1-153 |
| NR | 13 | 0.325 | 0.072-0.961 | — |
N indicates number; CR, complete response; PR, partial response; MinR, minor response; NR, no response; ND, not determined.
Relative ICSBP expression is displayed as arbitrary value, calculated as described.
Mann-Whitney test calculated against NR.
Significant P value (P < .05).
Amount of samples of MinR was too low for calculation of Pvalue. The difference between CR and PR was not significant (P = .297).
Interestingly, analyzing 15 samples during follow-up, the initial ICSBP level before IFN-α treatment of patients becoming “good” responders was significantly higher than that of “poor” responders (data not shown).
Our results suggest that ICSBP expression in untreated CML patients indicates classification into different risk groups. In addition, cytogenetic response to IFN-α therapy in CML correlates to ICSBP expression, leading to normal mRNA levels only in “good” responders. These data suggest a possible usage of ICSBP levels for deciding therapy strategies in CML, maybe by early determination of cytogenetic response to IFN-α, and thus enabling patients to receive a faster allogeneic stem cell transplantation. Current studies with mice support the conclusion of the importance of ICSBP in CML therapy. It was shown that mice transplanted withbcr-abl–transfected bone marrow cells developed a myeloproliferative disease resembling CML.20,21Interestingly, mice transplanted with bone marrow cells coexpressing ICSBP and bcr-abl showed prolonged survival in contrast to the mice transplanted with cells expressing bcr-abl alone, indicating a protective role of ICSBP against bcr-abl.21
Taken together, the data presented here strongly suggest that lack of ICSBP may be involved in chronic myeloid leukemogenesis and an increase of ICSBP may have an important role in the therapeutic effect of IFN-α. Further prospective studies are needed to evaluate the detailed role of ICSBP in CML and a possible use as marker for prognosis or therapy or seperately as a target of gene therapy.
We thank Prof D. Huhn for his help throughout the study. We are grateful to the patients, physicians, and cytogeneticists participating in the German CML trials for sending samples and providing clinical and cytogenetic data.
Supported in part by grants from the Deutsche Forschungsgemeinschaft (to A.N.), the H.W. and J. Hector Stiftung (to M.S. and A.N.), the Deutsche Jose-Carreras-Leukämie-Stiftung e.V. (to M.S., A.N., and A.H.) and the A. and U. Kuhlemann-Stiftung (to M.S. and A.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Neubauer, Klinikum der Philipps-Universität Marburg, Zenrum Innere Medizin, Abteilung Hämatologie/Onkologie/Immunologie, Baldingerstrasse, 35043 Marburg, Germany; e-mail: neubauer@mailer.uni-marburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal