Genetic variations in the CC chemokine receptor (CCR5) leading to reduced or absent expression are associated with resistance to human immunodeficiency virus infection and delayed onset of acquired immunodeficiency syndrome. Similarly, lack of the red-cell chemokine receptor Duffy confers protection against malarial infection byPlasmodium vivax. Investigators have previously described a missense mutation (R89C) in the first intracellular loop of Duffy that results in reduced protein expression. The present study shows that the lower Duffy expression is due to loss of the positive charge at this position, resulting in protein instability. Moreover, R60S, a mutation in the first intracellular loop of CCR5 noted in a recent cohort study, likewise results in reduced surface expression and function of CCR5. The presence of a homologous, naturally occurring mutation that may be protective against disease thus defines a novel mechanism accounting for the decreased expression of these receptors in some individuals.
Introduction
Chemokine receptors are 7 transmembrane, G-protein–coupled proteins that mediate a number of functions, including leukocyte trafficking for immune surveillance and inflammatory responses.1,2 Of the cellular chemokine receptors, CCR5 and Duffy are the only 2 receptors that have been unequivocally proved to act as coreceptors for cell entry of pathogens: Duffy for the entry of malarial parasite, Plasmodium vivax, and CCR5 for the entry of M-tropic strains of human immunodeficiency virus (HIV). Although CXCR4 and other chemokine receptors can also function as HIV coreceptors, their association with disease has not yet been established.1 Thus, the Duffy-negative phenotype, prevalent in individuals of African ancestry, confers resistance to infection by P vivax.3,4 Similarly, with few exceptions, individuals with the delta-32 null allele of CCR5 are resistant to HIV infection,5-7 and HIV-1–infected individuals heterozygous for the delta-32 allele have delayed progression of acquired immunodeficiency syndrome.8-10
We and others have recently described a Duffy allele in approximately 3.5% of the population that, because of a single amino acid substitution (R89C) in the first cytoplasmic domain, results in reduced levels of protein, lower antigen expression, and reduced ability to bind chemokines.11-13 In a cohort study by Carrington et al,14 a CCR5 allele that carries a single amino acid substitution (R60S) in the first intracellular domain of the protein was reported to be present in the heterozygous state in one HIV-exposed, uninfected individual.
To assess the structural significance of R89C mutation on Duffy protein expression and to investigate whether R60S mutation in CCR5 might have an analogous reduced expression phenotype, we performed in vitro expression analysis of both Duffy and CCR5 mutant alleles. We found that in both mutant alleles, the loss of a positive charge from the intracellular loop leads to reduced surface expression, thereby identifying a novel mechanism that may be protective against disease.
Study design
Site-directed mutagenesis
Site-directed mutagenesis of the wild-type Duffy (Fyb allele) gene was performed by overlap polymerase chain reaction (PCR) mutagenesis and subsequent fragment replacement of an 800-bp NheI-AccI fragment, as described previously.11 The plasmid encoding the full-length wild-type CCR5 cDNA in pcDNA (from Dr T. Sakmar, Rockefeller University) has a C-terminal 9–amino acid sequence tag from rhodopsin.15 Overlap PCR mutagenesis followed by fragment replacement of a 380-bp HindIII-ClaI was performed for generation of the mutant CCR5 constructs. All mutants were confirmed by sequencing.
Expression analysis of Duffy and CCR5 constructs
293T cells were transiently transfected and analyzed for surface expression by flow cytometry, as described previously.11 To control and normalize for transfection efficiency, we cotransfected an unrelated plasmid (pRC/CMV-Kell) with every construct.11 For detection of CCR5 surface expression by flow cytometry, 2D7 monoclonal antibody (PharMingen, San Diego, CA) was used. For Western blot analysis of CCR5 protein, whole-cell lysates of transfected cells were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under denaturing conditions11 and immunoblotted with monoclonal antibody 1D4 (gift from Dr Sakmar), which recognizes a 9-residue rhodopsin tag15 present in the carboxy-terminal of both constructs.
Calcium mobilization assay
Transiently transfected 293T cells (0.5-1.0 × 106cells/mL) were loaded with 4 μM Fluo-3, am (Molecular Probes, Eugene, OR), and fluorescence due to intracellular calcium was measured at least 3 times on independent samples in a Hitachi F-2000 fluorescence spectrophotometer (Hitachi Instruments, San Jose, CA), as described previously.16 17
HIV-infection assay
Twenty-four hours after transfection of duplicate 239T cultures with expression vectors for CD4, CCR5 (from Dr N. Landau, Salk Institute), and β-galactosidase,18 the cells were infected by adding 400 μL (corresponding approximately to 200 ng of p24) of virus-containing culture supernatant of 293T cells cotransfected with an infectious HIV-1 clone NL4-3-Luc-R−E− and a vector for an M-tropic envelope (JR-FL), as described previously19 (from Dr Landau). Three days after infection, the cells were harvested and the lysates were analyzed for luciferase (indicative of HIV infection) and β-galactosidase (a measure of transfection efficiency of the HIV receptors), as described previously.18
Results and discussion
Role of R89C substitution in reduced expression of mutant Duffy protein
We have shown previously that, despite equal transcript levels, surface expression of mutant Duffy R89C protein in transiently transfected cells is 5-fold reduced compared with wild-type Duffy because the mutant protein is unstable.11 To examine the nature of the defect caused by C89, we replaced this residue with a number of amino acids and analyzed surface expression in transiently transfected 293T cells with 3 different antibodies to separate epitopes in Duffy by flow cytometry (Figure 1A,B). We found that only the substitution to positively charged K fully rescued surface expression to wild-type levels, consistent with previous studies showing that loss of positively charged residues (R and K but not H) flanking the transmembrane segment impedes protein translocation.23 Thus, the loss of a positive charge of R89, rather than incorrect disulfide bonding or other such effects, appears to account for the reduced surface expression of Duffy R89C protein.
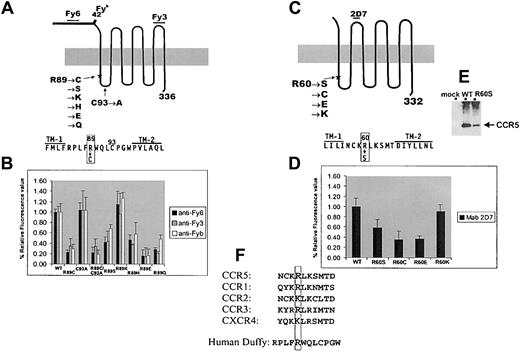
Expression analysis of Duffy and CCR5 mutant constructs.
(A) Schematic diagram of Duffy protein showing the known positions of Fy620 and Fy321 epitopes and the position of the single amino acid polymorphism associated with the Fybantigen.4 The sequence of the first cytoplasmic domain and the flanking sequences in the transmembrane (TM) domains 1 and 2 are also depicted. The introduced mutations are listed. To test whether the C residue at position C93, by forming an abnormal disulfide bond with R89C, can be responsible for weak expression, the double mutant R89C/C93A and the single C93A control mutation were constructed. R89 was also mutated to S to introduce a relatively minor sulfur-to-oxygen side-chain substitution; to K, a positively charged residue; to H, which is positively charged only depending on its surrounding microenvironment; to a negatively charged E; and to Q, which is similar in structure to E but is neutral. (B) Flow cytometric analysis of Duffy mutants expressed in 293T cells using murine monoclonal anti-Fy6 (clone K60), anti-Fy3 (clone CRC-512-1), and the human polyclonal reagent anti-Fyb. Values on the y-axis depict percentage of the median fluorescence intensity relative to wild-type Duffy expression after normalizing for transfection efficiency. As reported previously,11 R89C was expressed weakly as compared with wild-type (WT) Duffy. Substitution of C93 with A resulted in wild-type Duffy expression levels, but with the double mutation R89C/C93A, a low-level expression comparable to that of R89C was seen, indicating that the reduced expression of R89C mutant is not caused by disulfide bond formation with the C93 residue. Only the conservative substitution to K rescued expression to wild-type levels. (C) Schematic diagram of CCR5 with the list of mutations introduced into R60 and the sequence of the first cytoplasmic domain and the flanking TM 1 and TM 2. (D) Flow cytometric analysis of CCR5 constructs expressed in 293T cells using 2D7, which recognizes epitopes on the second extracellular loop of CCR5.22 Values on the y-axis depict percentage of the median fluorescence intensity relative to wild-type CCR5 after normalizing for transfection efficiency. (E) Western blot analysis of 293T cells transfected with wild-type and R60S CCR5 expression vectors using monoclonal antibody 1D4, which recognizes a 9-residue rhodopsin tag15 present in the carboxy-terminal of both constructs. CCR5 protein, indicated by the arrow, is expressed more weakly in R60S CCR5 whole-cell extracts than in wild-type CCR5 and is absent in mock-transfected lysates. (F) Sequence comparison of the predicted first intracellular loop of several members of the chemokine receptor superfamily, highlighting the conservation of the positively charged R60 residue.
Expression analysis of Duffy and CCR5 mutant constructs.
(A) Schematic diagram of Duffy protein showing the known positions of Fy620 and Fy321 epitopes and the position of the single amino acid polymorphism associated with the Fybantigen.4 The sequence of the first cytoplasmic domain and the flanking sequences in the transmembrane (TM) domains 1 and 2 are also depicted. The introduced mutations are listed. To test whether the C residue at position C93, by forming an abnormal disulfide bond with R89C, can be responsible for weak expression, the double mutant R89C/C93A and the single C93A control mutation were constructed. R89 was also mutated to S to introduce a relatively minor sulfur-to-oxygen side-chain substitution; to K, a positively charged residue; to H, which is positively charged only depending on its surrounding microenvironment; to a negatively charged E; and to Q, which is similar in structure to E but is neutral. (B) Flow cytometric analysis of Duffy mutants expressed in 293T cells using murine monoclonal anti-Fy6 (clone K60), anti-Fy3 (clone CRC-512-1), and the human polyclonal reagent anti-Fyb. Values on the y-axis depict percentage of the median fluorescence intensity relative to wild-type Duffy expression after normalizing for transfection efficiency. As reported previously,11 R89C was expressed weakly as compared with wild-type (WT) Duffy. Substitution of C93 with A resulted in wild-type Duffy expression levels, but with the double mutation R89C/C93A, a low-level expression comparable to that of R89C was seen, indicating that the reduced expression of R89C mutant is not caused by disulfide bond formation with the C93 residue. Only the conservative substitution to K rescued expression to wild-type levels. (C) Schematic diagram of CCR5 with the list of mutations introduced into R60 and the sequence of the first cytoplasmic domain and the flanking TM 1 and TM 2. (D) Flow cytometric analysis of CCR5 constructs expressed in 293T cells using 2D7, which recognizes epitopes on the second extracellular loop of CCR5.22 Values on the y-axis depict percentage of the median fluorescence intensity relative to wild-type CCR5 after normalizing for transfection efficiency. (E) Western blot analysis of 293T cells transfected with wild-type and R60S CCR5 expression vectors using monoclonal antibody 1D4, which recognizes a 9-residue rhodopsin tag15 present in the carboxy-terminal of both constructs. CCR5 protein, indicated by the arrow, is expressed more weakly in R60S CCR5 whole-cell extracts than in wild-type CCR5 and is absent in mock-transfected lysates. (F) Sequence comparison of the predicted first intracellular loop of several members of the chemokine receptor superfamily, highlighting the conservation of the positively charged R60 residue.
Expression of CCR5 proteins with mutations affecting residue R60
Carrington et al14 recently reported, but did not further analyze, a naturally occurring mutation (R60S) in the first cytoplasmic loop of CCR5. Because no patient sample homozygous for this variant is available for functional analysis, we mutated R60 in CCR5 to S and found that the mutant protein is expressed at 60% of wild-type CCR5 levels,24 analogous to Duffy R89S expression levels (Figure 1B,D). Analysis of additional R60 replacement constructs revealed that, as with the Duffy variants, only the substitution to K, conserving the positive charge at position 60, produces a wild-type expressed receptor (Figure 1D).
Immunoblotting of whole-cell extracts showed that the lower surface expression of R60S mutant was accompanied by a similarly reduced protein level (Figure 1E), analogous to that with the Duffy R89C phenotype.11 Interestingly, sequence alignment of the first intracellular domain of a number of the members of the chemokine receptor family shows that the R60 is indeed a conserved residue (Figure 1F).
Decreased expression of CCR5 R60S protein is associated with diminished CCR5 receptor function
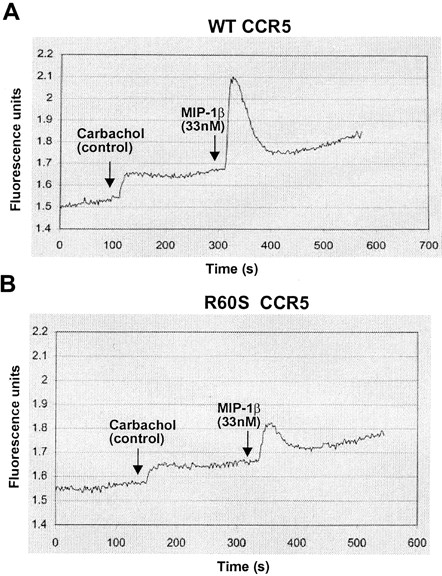
To examine the capacity of the mutant R60S CCR5 to signal, we determined the ability of wild-type and R60S CCR5 to trigger calcium fluxes in 293T cells in response to MIP-1β. Transiently transfected cells were loaded with Fluo-3, am, a dye that exhibits increased fluorescence in the presence of calcium. As determined by fluorometry, the affinity constant for chemokine binding of the mutant receptor was identical to that of wild-type CCR5 (data not shown), but the net calcium flux was consistently approximately 60% of wild-type receptor after normalization (Figure2). Because the mutant protein expression level is also 60% of wild-type CCR5, the impaired signaling ability of R60S receptor can be attributed to reduced amounts of CCR5 in the membrane.
Functional analysis of CCR5 R60S protein in 293T cells.
The ability of transiently transfected wild-type (A) and R60S CCR5 (B) cells to stimulate increases in intracellular calcium concentration in response to MIP-1β was measured using calcium-sensitive dye Fluo-3,am, which is converted to Fluo-3 by intracellular esterases. Representative traces of fluorescence (λex 505 nm, λem 525 nm) (y-axis) from fluo-loaded cells in response to injection of 1 mM carbachol (control) and 33 nM MIP-1β as a function of time (x-axis) are depicted. To control for efficient loading of the dye to the cells and thereby standardize the response, the carbachol-dependent calcium flux associated with ligand binding to endogenous muscarinic receptors was also measured. The activity of the wild-type CCR5 receptor was measured as the ratio of the height of the peak for MIP-1 β (0.42 arbitrary fluorescence units) to that of the peak for carbachol (0.12 units) and was calculated as 3.5 in this experiment and defined as 100%. The height of the MIP-1 β peak (0.16 units) divided by the carbachol peak (0.08 units) was calculated to be 2, which translated as 57% of the wild-type CCR5 receptor activity in this experiment. The labeled arrows indicate the addition of 1 mM carbachol and 33 nM MIP-1β.
Functional analysis of CCR5 R60S protein in 293T cells.
The ability of transiently transfected wild-type (A) and R60S CCR5 (B) cells to stimulate increases in intracellular calcium concentration in response to MIP-1β was measured using calcium-sensitive dye Fluo-3,am, which is converted to Fluo-3 by intracellular esterases. Representative traces of fluorescence (λex 505 nm, λem 525 nm) (y-axis) from fluo-loaded cells in response to injection of 1 mM carbachol (control) and 33 nM MIP-1β as a function of time (x-axis) are depicted. To control for efficient loading of the dye to the cells and thereby standardize the response, the carbachol-dependent calcium flux associated with ligand binding to endogenous muscarinic receptors was also measured. The activity of the wild-type CCR5 receptor was measured as the ratio of the height of the peak for MIP-1 β (0.42 arbitrary fluorescence units) to that of the peak for carbachol (0.12 units) and was calculated as 3.5 in this experiment and defined as 100%. The height of the MIP-1 β peak (0.16 units) divided by the carbachol peak (0.08 units) was calculated to be 2, which translated as 57% of the wild-type CCR5 receptor activity in this experiment. The labeled arrows indicate the addition of 1 mM carbachol and 33 nM MIP-1β.
We next studied the ability of CCR5-R60S protein to serve as an HIV-1 coreceptor. 293T cells transiently cotransfected with CD4 and either wild-type, R60S CCR5, or an empty control vector were infected with HIV-luciferase virus pseudotyped with M-tropic JR-FL envelope. Luciferase activity of the lysates, indicative of successful HIV-1 infection, was then measured. When equal amounts of wild-type or R60S CCR5 were used, a trend suggesting a lower (72%) capacity of R60S CCR5 to support HIV-1 entry was noted, which agrees well with expression and calcium signaling capacity. However, the significance of the HIV-infection result remains unclear because attempts to establish proper dose-response curves by altering the amount of CCR5 plasmid input indicated that the performance of the assay remained suboptimal for scoring small differences in CCR5 expression (data not shown), as was also reported by others.25
In conclusion, we have analyzed a homologous, naturally occurring mutation in a conserved residue in the first intracellular domain of CCR5 and Duffy that results in reduced amounts of the protein in the membrane and consequently may be associated with reduced susceptibility to infection by microbes that depend on these molecules as their receptors.
We thank Päivi Huotari (Institute of Medical Technology) for help in the reporter virus infection experiments; Qian Yu and Rostislov Chernomorsky (New York Blood Center [NYBC]) for technical assistance, and Susan Fetics (Microchemistry Laboratory at the NYBC) for sequencing. We also thank Dr Pablo Rubinstein (NYBC) for providing the anti-Fy6 and Dr Makoto Uchikawa (Japanese Red Cross, Tokyo, Japan) for anti-Fy3. We are grateful to Dr Thomas Sakmar and Dr Steve Lin (Rockefeller University) for helpful discussions and thank Dan Stettler (Rockefeller University) for critical reading of the manuscript. We are indebted to Dr Marion Reid (NYBC) for support.
Supported in part by a National Institutes of Health Specialized Center of Research (SCOR) grant in transfusion medicine and biology (HL54459). D.T. is funded by a National Blood Foundation award to K.Y.
D.T. and V.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Karina Yazdanbakhsh, Immunochemistry Laboratory, New York Blood Center, 310 East 67th St, New York, NY 10021; e-mail:kyazdan@nybc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal