Abstract
Childhood T-cell acute lymphoblastic leukemia (T-ALL) is one of the most common childhood cancers. It is reported that preconditioning sublethally irradiated immunodeficient NOD/SCID (nonobese diabetic/X-linked severe combined immunodeficient) mice with human cord blood mononuclear cells facilitates the engraftment, expansion, and dissemination in these mice of primary T-ALL cells obtained from patients at the time of diagnosis. Cells recovered from mouse bone marrow or spleen resembled the original leukemia cells from patients with respect to surface lineage markers and T-cell receptor Vβ gene rearrangements. Moreover, the pattern of leukemia dissemination in mouse tissues, resulting in universally fatal leukemia, is reminiscent of the human clinical disease. In addition, the fidelity of the model to the human disease is documented with regard to the presence of morphologically identifiable human leukemia cells in mouse bone marrow and blood and the maintenance of leukemia-initiating capacity within the leukemia-engrafted mouse. Therefore, several lines of independent approaches are used to suggest that the engrafted cells are of human leukemia origin and are not derived from cord blood. The in vivo model described here should enable the study of the growth properties of primary T-ALL cells obtained from patients and should prove useful in evaluating the potential efficacy of therapeutic strategies directed toward T-ALL.
Introduction
Childhood T-cell acute lymphoblastic leukemia (T-ALL) comprises approximately 15% of the heterogeneous group of ALL cases.1 Currently, more than 70% of children with ALL are disease free 5 years after chemotherapy. However, subgroups of patients remain refractory to treatment. Because T-ALL is an aggressive disease, patients often require intensive chemotherapy; should they relapse, the clinical outcome is dismal.1 Even though a number of leukemia cell lines of T-cell origin have been established from patients,2-5 difficulty in maintaining primary cultures of leukemia cells from patients has impeded study of the development of the disease.
Kamel-Reid et al6 have shown that human non–T-ALL can proliferate in the hematopoietic tissues of immunodeficient X-linked severe combined immunodeficient (SCID) mice. Successful engraftment of T-lineage ALL, however, has until recently been limited to a few samples obtained from patients at the time of relapse.7The availability of nonobese diabetic (NOD)/SCID mice has provided a new and improved milieu for the engraftment of human T-ALL, albeit with modest and slow engraftment.8 We therefore addressed the hypothesis that preconditioning NOD/SCID mice by engrafting human cord blood–derived hematopoietic cells9 10—with the attendant expression of soluble and membrane-bound signals of human origin in mouse bone marrow—would facilitate the subsequent engraftment of primary T-ALL obtained from patients.
We report here that this preconditioning regimen for immunodeficient mice does indeed facilitate the engraftment, expansion, and dissemination in vivo of primary leukemia from patients with T-ALL. We also show that in this system human T-ALL cells recovered from engrafted mouse bone marrow are morphologically identifiable, similar to primary leukemia cells, and maintain the leukemia-initiating capacity for serial transfer to other mouse recipients. Therefore, the experimental approach described here should enable us to study the in vivo growth properties of primary T-ALL cells obtained from patients and should prove useful in evaluating the potential efficacy of therapeutic strategies directed to T-ALL.
Materials and methods
NOD/SCID mice
NOD/SCID mice11 were bred and maintained in a specific pathogen-free environment at The Scripps Research Institute vivarium in sterile Micro-Isolator cages and ventilated mouse racks (Lab Products, Seaford, DE) without antibiotics. Five- to 6-week-old female mice were used in the present study.
Primary leukemia cells
Heparinized peripheral blood or bone marrow samples were obtained from patients with childhood T-ALL who enrolled in protocol 9400 Pediatric Oncology Group (Table 1). Mononuclear cell (MNC) fractions from peripheral blood/bone marrow were isolated by Ficoll-Hypaque density-gradient separation (Pharmacia, Piscataway, NJ). The content of lymphoblasts, as determined by Wright stain, was generally greater than 90%. In some cases, MNCs of T-ALL samples were cryopreserved and stored in liquid nitrogen before use in the studies. Viability on thawing was generally greater than 80%, as determined by trypan blue dye exclusion.
Patient samples
| Patient . | Age (y) . | Sex . | Source of patient's primary T-ALL . | WBC (×109/L) . | Blasts (%) . | Phenotype of patient's primary T-ALL . |
|---|---|---|---|---|---|---|
| 10155 | 4 | F | Peripheral blood | > 700 | 100 | CD2++CD3− CD4++ CD5++CD7++ CD8++ |
| 26710 | 4 | M | Peripheral blood | 797 | 93 | CD2++ CD3−CD4+ CD5++ CD7++CD8+ |
| 27531 | 6 | M | Peripheral blood | 248 | 88 | CD2++ CD3++CD4+ CD5++ CD7++CD8++ |
| 30749 | 2 | M | Peripheral blood | 393 | 94 | CD2++ CD3++CD4+ CD5++ CD7++CD8++ |
| 08916 | 1 | M | Bone marrow | 416 | 87 | CD2++ CD3+CD4− CD5++ CD7++CD8+ |
| 08948 | 15 | M | Peripheral blood | 143 | 73 | CD2++ CD3++CD5++ CD7++ CD8++ |
| 33205 | 13 | F | Peripheral blood | 327 | 94 | CD2+ CD3++CD4++ CD5++ CD7++CD8++ |
| 32564 | 5 | F | Peripheral blood | 350 | 88 | CD2++ CD3+CD4+ CD5++ CD7++CD8+ |
| Patient . | Age (y) . | Sex . | Source of patient's primary T-ALL . | WBC (×109/L) . | Blasts (%) . | Phenotype of patient's primary T-ALL . |
|---|---|---|---|---|---|---|
| 10155 | 4 | F | Peripheral blood | > 700 | 100 | CD2++CD3− CD4++ CD5++CD7++ CD8++ |
| 26710 | 4 | M | Peripheral blood | 797 | 93 | CD2++ CD3−CD4+ CD5++ CD7++CD8+ |
| 27531 | 6 | M | Peripheral blood | 248 | 88 | CD2++ CD3++CD4+ CD5++ CD7++CD8++ |
| 30749 | 2 | M | Peripheral blood | 393 | 94 | CD2++ CD3++CD4+ CD5++ CD7++CD8++ |
| 08916 | 1 | M | Bone marrow | 416 | 87 | CD2++ CD3+CD4− CD5++ CD7++CD8+ |
| 08948 | 15 | M | Peripheral blood | 143 | 73 | CD2++ CD3++CD5++ CD7++ CD8++ |
| 33205 | 13 | F | Peripheral blood | 327 | 94 | CD2+ CD3++CD4++ CD5++ CD7++CD8++ |
| 32564 | 5 | F | Peripheral blood | 350 | 88 | CD2++ CD3+CD4+ CD5++ CD7++CD8+ |
T-ALL indicates T-cell acute lymphoblastic leukemia; WBC, white blood cell; −, < 10%; +, 10%-90%;++, > 90% as determined by flow cytometry.
Human cord blood
Fetal cord blood samples were obtained from umbilical cord scheduled for discard, according to procedures approved by our institutional Human Subjects Committee. After Ficoll-Hypaque density-gradient centrifugation, the MNCs were collected and washed with RPMI 1640 medium containing 2% fetal calf serum. Cord blood was used for injection, as described below.
Mouse injections
Before T-ALL implantation, each mouse received 3.5 Gy total body irradiation from a cesium Cs 137 γ-irradiator. Immediately thereafter, approximately 10 to 25 × 106 fetal cord blood MNCs from healthy newborns was injected in 0.25 mL sterile phosphate-buffered saline through the tail vein. For a given experiment, cord blood from a single donor was used for all mice. Six to 9 days later, approximately 1 to 5 × 106 viable fresh or cryopreserved primary leukemia cells from a patient were suspended in 0.25 mL phosphate-buffered saline and injected through the tail vein. Mice not preconditioned with cord blood were irradiated on the same day as the cord blood–preconditioned mice and injected with T-ALL cells, 9 days after irradiation. For a given experiment, leukemia cells from a single donor were used for all mice. Experiments were set up so that each treatment was typically replicated in 2 to 4 mice. Mice were killed when they became moribund with disseminated leukemia or electively between 5 and 7 weeks after the leukemia cell injection. Necropsies were performed, and the burden of human leukemia cells in mouse tissues was determined by flow cytometry, histocytochemistry, and T-cell receptor (TCR) Vβ gene rearrangement, as described below.
Collection of mouse tissues
Gross examination of various mouse tissues was performed after laparotomy immediately after death. Multiple tissues from mice (including liver, kidney, lung, thymus, lymph node, adrenal gland, and brain) were fixed in aqueous-buffered zinc formalin (Z-Fix; Anatech, Battle Creek, MI), dehydrated, and embedded in paraffin by routine methods. Glass slides with 4-μm tissue sections were prepared and stained with hematoxylin/eosin. Mouse bone marrow was collected from femurs and tibias. Single-cell suspension was prepared by gentle pipetting. Spleen cells were collected by gentle dissociation. Red blood cells within the bone marrow and spleen cell suspensions were lysed using buffered ammonium chloride. Cell debris was removed by filtration through a sterile nylon cell strainer (Becton Dickinson, San Jose, CA). Mouse peripheral blood was collected into heparin by retro-orbital bleeding and cardiac puncture; MNCs were isolated by Ficoll-Hypaque centrifugation. In addition, bone marrow touch preparations and blood smears were made and stained with modified Wright solution.
Flow cytometry
Multiparameter analysis of single-cell suspensions from mouse bone marrow, spleen, and peripheral blood was carried out using a FACScan flow cytometer (Becton Dickinson). Two-color immunofluorescence was used to identify human T-lineage ALL cells. Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse antihuman monoclonal antibodies (mAbs) were obtained from PharMingen (San Diego, CA), with the exception of PE-conjugated anti-TCR Vβ2 (clone MPB2D5; Coulter, Miami, FL). The mAbs used in the work presented here include those directed against human CD5 (clone UCHT2), CD7 (M-T701), CD19 (HIB19), and CD45 (clone HI30). During analysis, residual red blood cells and debris were gated out on the basis of forward angle and 90° side scatter. At least 15 000 events were collected for each sample. Isotype-matched control mAbs (FITC- or PE-conjugated IgG1 [clone MOPC-21]) were used to determine appropriate cursor settings for analysis. Using CellQuest 3.2.1 software (Becton Dickinson), data were analyzed and displayed by 2-dimensional plots and by 1-dimensional histograms. In control experiments, it was shown that none of these mAbs targeting toward human hematopoietic cells cross-reacted with mouse cells (pictures not shown), and their respective isotype controls were used in all analysis reported in this study.
Analysis of human T-cell receptor Vβ expression
To determine human TCR Vβ gene expression by cell samples, semiquantitative polymerase chain reaction (PCR) analysis was performed as described previously.12 Briefly, total RNA was isolated using the RNeasy Total RNA kit (Qiagen, Chatsworth, CA), and RNA from the equivalent of 1 × 106 cells was converted to cDNA in a 25-μL reaction using reverse transcriptase in the presence of an oligonucleotide primer (5′AGGCAGTATCTGGAGTCATTGA3′) complementary to a sequence found in both human TCR Vβ constant region genes Cβ-1 and Cβ-2. Half the cDNA reaction was then transferred to a tube containing Taq polymerase (30 U), Taq buffer, dNTPs (200 μM) and a Cβ primer internal to the primer used for cDNA synthesis, 5′GGGCGGGCTGCTCCTTGAGG3′ (0.6 μM). Fifty microliters mixture was then added to each of 30 individual wells of a microtiter plate that contained individual Vβ-specific oligonucleotide primers (0.6 μM), and the samples were subjected to 28 rounds of PCR amplification consisting of 1-minute denaturation at 94°C, 2-minute annealing at 55°C, and 2-minute extension at 72°C. After amplification, 30 μL was removed from each well, denatured, neutralized, and spotted onto nitrocellulose filters. The filters were hybridized with a32P end-labeled Cβ oligonucleotide corresponding to a sequence 5′ to the one used in the PCR (5′CTCTGCTTCTGATGGCTCAAAC3′), washed, and exposed to x-ray film.
DNA sequences of the Vβ rearrangements were determined by sequencing the PCR products corresponding to the unique Vβ chains expressed with the dideoxy chain termination method using T7 Sequenase (Amersham, Cleveland, OH).12 The reverse transcription (RT)-PCR reaction described above was repeated using fresh RNA and the appropriate Vβ-specific primers. Resultant PCR products were separated on a 1.4% agarose gel, and the appropriate size fragment was purified. A second PCR was performed using a 50-fold excess of the forward Vβ-specific primer. The resultant PCR product was extracted and sequenced directly.12
Results
Engraftment of primary leukemia from patients with T-ALL in NOD/SCID mice
Low-level engraftment of primary human leukemia cells from patients occurs in the traditionally irradiated immunodeficient mouse.6,13 14 We investigated whether preconditioning the NOD/SCID mice with human cord blood would facilitate the subsequent engraftment of primary T-ALL cells. T-ALL engraftment in mouse bone marrow and spleen was determined initially by flow cytometry. CD45 expression is indicative of total human hematopoietic cell engraftment. CD7 is expressed by engrafted human T-lineage ALL. CD19 is indicative of engrafted human cells of the B-cell lineage. In the experiments presented, leukemia samples and data from the 8 patients were collected under protocol 9400 of the Pediatric Oncology Group. Clinical information about the patient donors is presented in Table 1.
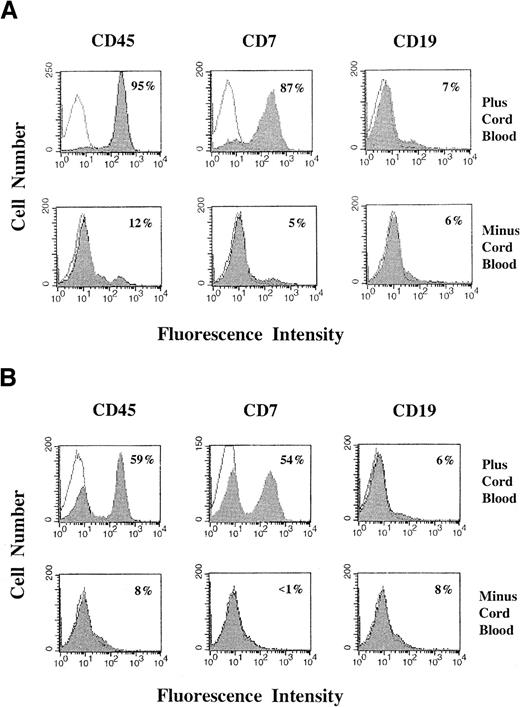
As shown in Figure 1, efficient engraftment of primary T-ALL by 6 weeks depends on preconditioning the mice with fetal cord blood MNCs. In the experiment presented (Figure1), mice were injected either with or without 10 × 106cord blood MNCs and, 9 days later, with 2.3 × 106primary T-ALL cells. Each group of mice showed a remarkable difference in the efficiency of engraftment of human primary leukemia. Mice preconditioned with cord blood showed a high level of leukemia engraftment on day 40 after T-ALL injection. For the mouse presented in Figure 1, 95% of bone marrow cells were human CD45+ and 87% were human CD7+ (Figure 1A); similarly, 59% of spleen cells were CD45+ and 54% were CD7+ (Figure1B). In contrast, the mice without cord blood preconditioning showed little engraftment of human leukemia cells (5% of bone marrow cells for the mouse presented in Figure 1) at this time point (Figure 1A,B). That cord blood preconditioning facilitates T-ALL expansion in NOD/SCID mice is apparent from an analysis of the total number of engrafted T-ALL cells recovered. In this experiment, 1.0 × 108T-ALL cells were recovered from bone marrow plus spleen from the mouse preconditioned with cord blood in Figure 1. From the mouse without cord blood preconditioning, only 0.95 × 106 T-ALL was recovered from bone marrow plus spleen.
Engraftment of human primary T-ALL cells with or without cord blood preconditioning.
Mice were irradiated and injected with or without 10 × 106 cord blood MNCs, 9 days before injection with 2.3 × 106 primary T-ALL cells. They were killed 40 days after T-ALL injection. Bone marrow (A) and spleen (B) cells were analyzed for several phenotypic markers by flow cytometry, as described in “Materials and methods.” For each panel, the filled histogram curve corresponds to the indicated experimental mAb and is superimposed over an open histogram curve corresponding to the isotype control mAb. The fraction of cells staining positively for the experimental mAb was determined by subtraction of the curves, using CellQuest 3.2.1 software. Percentages of human CD45+, CD7+, and CD19+ cells are indicated in the panels.
Engraftment of human primary T-ALL cells with or without cord blood preconditioning.
Mice were irradiated and injected with or without 10 × 106 cord blood MNCs, 9 days before injection with 2.3 × 106 primary T-ALL cells. They were killed 40 days after T-ALL injection. Bone marrow (A) and spleen (B) cells were analyzed for several phenotypic markers by flow cytometry, as described in “Materials and methods.” For each panel, the filled histogram curve corresponds to the indicated experimental mAb and is superimposed over an open histogram curve corresponding to the isotype control mAb. The fraction of cells staining positively for the experimental mAb was determined by subtraction of the curves, using CellQuest 3.2.1 software. Percentages of human CD45+, CD7+, and CD19+ cells are indicated in the panels.
Based on the number of CD5+CD7+ cells recovered from mouse bone marrow and spleen at 5 to 7 weeks after leukemia cell injection, it was found that using different primary leukemia from 9 patients with T-ALL, the engrafted human T-ALL cells comprised 72 × 25% of bone marrow cells and 73 × 21% of spleen cells from mice (Table 2). On average, the total number of T-ALL cells recovered from engrafted bone marrow plus spleen was approximately 30-fold greater than the number of primary T-ALL cells initially injected (not shown). In this study, primary leukemia samples were obtained from patients at the time of diagnosis. In addition, fresh or cryopreserved MNCs from T-ALL patients achieved comparable engraftment in these and other experiments.
Engraftment of primary T-cell acute lymphoblastic leukemia in cord blood preconditioned nonobese diabetic/X-linked severe combined immunodeficient mice
| Patient . | Cord blood MNC (preconditioning) . | Injected T-ALL . | Engraftment . | Metastasis . | Other tissues* . |
|---|---|---|---|---|---|
| Human leukemia cells of mouse bone marrow cells (%) . | Percent human leukemia cells in mouse spleen . | ||||
| 10155 | 25 × 106 | 36 × 106 | 85 | 78 | Yes |
| 26710 | 50 × 106 | 30 × 106 | 89 | 92 | Yes |
| 26710 | 10 × 106 | 5.0 × 106 | 83 | 68 | Yes |
| 26710 | 10 × 106 | 0.5 × 106 | 59 | 26 | Yes |
| 26710 | 10 × 106 | 2.3 × 106 | 87 | 54 | Yes |
| 26710 | 10 × 106 | 2.5 × 106 | 82 | 71 | Yes |
| 27531 | 40 × 106 | 5.0 × 106 | 29 | 55 | Yes |
| 27531 | 50 × 106 | 1.0 × 106 | 29 | 44 | Yes |
| 30749 | 50 × 106 | 3.3 × 106 | 95 | 98 | Yes |
| 30749 | 10 × 106 | 3.3 × 106 | 92 | 95 | Yes |
| 30749 | 10 × 106 | 3.3 × 106 | 91 | 95 | Yes |
| 30749 | 10 × 106 | 1.6 × 106 | 93 | 87 | Yes |
| 30749 | 10 × 106 | 1.6 × 106 | 94 | 92 | Yes |
| 30749 | 10 × 106 | 1.6 × 106 | 93 | 95 | Yes |
| 08916 | 20 × 106 | 4.9 × 106 | 34 | 60 | Yes |
| 08916 | 20 × 106 | 4.9 × 106 | 34 | 49 | Yes |
| 08948 | 20 × 106 | 5.1 × 106 | 38 | 57 | Yes |
| 33205 | 25 × 106 | 5.0 × 106 | 85 | 86 | Yes |
| 32564 | 25 × 106 | 6.0 × 106 | 77 | 87 | Yes |
| Patient . | Cord blood MNC (preconditioning) . | Injected T-ALL . | Engraftment . | Metastasis . | Other tissues* . |
|---|---|---|---|---|---|
| Human leukemia cells of mouse bone marrow cells (%) . | Percent human leukemia cells in mouse spleen . | ||||
| 10155 | 25 × 106 | 36 × 106 | 85 | 78 | Yes |
| 26710 | 50 × 106 | 30 × 106 | 89 | 92 | Yes |
| 26710 | 10 × 106 | 5.0 × 106 | 83 | 68 | Yes |
| 26710 | 10 × 106 | 0.5 × 106 | 59 | 26 | Yes |
| 26710 | 10 × 106 | 2.3 × 106 | 87 | 54 | Yes |
| 26710 | 10 × 106 | 2.5 × 106 | 82 | 71 | Yes |
| 27531 | 40 × 106 | 5.0 × 106 | 29 | 55 | Yes |
| 27531 | 50 × 106 | 1.0 × 106 | 29 | 44 | Yes |
| 30749 | 50 × 106 | 3.3 × 106 | 95 | 98 | Yes |
| 30749 | 10 × 106 | 3.3 × 106 | 92 | 95 | Yes |
| 30749 | 10 × 106 | 3.3 × 106 | 91 | 95 | Yes |
| 30749 | 10 × 106 | 1.6 × 106 | 93 | 87 | Yes |
| 30749 | 10 × 106 | 1.6 × 106 | 94 | 92 | Yes |
| 30749 | 10 × 106 | 1.6 × 106 | 93 | 95 | Yes |
| 08916 | 20 × 106 | 4.9 × 106 | 34 | 60 | Yes |
| 08916 | 20 × 106 | 4.9 × 106 | 34 | 49 | Yes |
| 08948 | 20 × 106 | 5.1 × 106 | 38 | 57 | Yes |
| 33205 | 25 × 106 | 5.0 × 106 | 85 | 86 | Yes |
| 32564 | 25 × 106 | 6.0 × 106 | 77 | 87 | Yes |
Metastasis to other tissues determined by hematoxylin-eosin staining of paraffin-embedded sections of liver, kidney, lung, and so on, as described in “Materials and methods.” See also Figure 4.
MNC indicates mononuclear cell; T-ALL, T-cell acute lymphoblastic leukemia.
The results presented here, along with those obtained using mAbs directed against CD2, CD3, CD5, CD20, CD38, or glycophorin A (below and not shown), indicate that, to the extent studied, phenotypes of cells recovered from engrafted mouse bone marrow and spleen were identical to those of injected primary T-ALL cells obtained from the patient. This was found to be the case for each of 8 patients with T-ALL leukemia.
It is also important to determine whether these CD5+CD7+ cells are derived from the proliferation or expansion of cells in cord blood. In this respect, our recent findings15 demonstrate that 3.5 Gy-irradiated cord blood also facilitates the engraftment of T-ALL cells in NOD/SCID mice in a manner similar to that of fresh cord blood, as presented in Figure1. Such experiments using irradiated cord blood MNCs thus do not support the contention that the CD5+CD7+cells are of cord blood origin. Finally, because human cells expanded in NOD/SCID mice engrafted with cord blood alone are predominantly CD19+ (not shown),9,10 16-18 the paucity of CD19+ cells in mouse bone marrow or spleen in Figure 1suggests that the expansion of these cells from engrafted cord blood used for preconditioning is in large part displaced by the expansion of T-ALL leukemia cells.
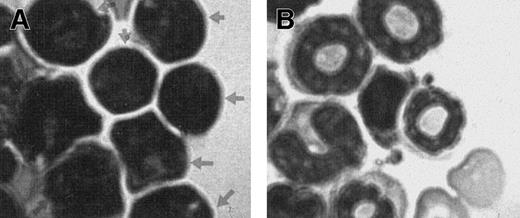
To confirm that the human cells growing in the NOD/SCID mice were leukemic, the modified Wright-stained bone marrow touch preparations harvested from engrafted mice 6 weeks after transplantation were examined. As shown in Figure 2, characteristic morphologic features of human leukemia blasts were clearly identifiable in mouse marrow samples obtained from mice engrafted with T-ALL (indicated with arrowheads in Figure 2A). This is in direct contrast to the unique morphology of leukocytes and other mature cells found in the control mouse (Figure 2B). The notable absence of normal mouse leukocytes in Figure 2A is in line with the predominantly high level of leukemia engraftment in these mice.
Presence of human leukemia cells on mouse bone marrow smear.
(A) Bone marrow smear from a mouse 6 weeks after injection with cells from a patient with T-ALL showing the characteristic morphology of leukemia blasts (arrowheads). (B) Bone marrow smear from a control NOD/SCID mouse showing the characteristic leukocytes. Bone marrow smears were stained with modified Wright stain.
Presence of human leukemia cells on mouse bone marrow smear.
(A) Bone marrow smear from a mouse 6 weeks after injection with cells from a patient with T-ALL showing the characteristic morphology of leukemia blasts (arrowheads). (B) Bone marrow smear from a control NOD/SCID mouse showing the characteristic leukocytes. Bone marrow smears were stained with modified Wright stain.
Analysis of TCR Vβ gene rearrangement in engrafted T-ALL
It was important to establish, as another independent approach, that the engrafted CD7+ cells in bone marrow and spleen of cord blood–preconditioned mice were derived from the injected primary T-ALL cells obtained from patients rather than from the human cord blood. To this end, we took advantage of the fact that the extensive recombinatorial diversity of TCR β variable region gene (TCR Vβ) rearrangement19 allows this rearrangement to be used as a clonal marker for primary leukemia. For each of 2 patients, total RNA isolated from primary T-ALL cells and from engrafted mouse bone marrow and spleen was analyzed for human TCR Vβgene expression.
In the following experiment, mice were injected with 10 × 106 cord blood MNCs and, 6 days later, with 3.3 × 106 primary T-ALL cells. These T-ALL cells from the patient had been shown in the laboratory to have a CD3+αβTCR+ phenotype (not shown). The mice were killed on day 38 after T-ALL injection. Results of semiquantitative RT-PCR analysis using individual Vβ oligonucleotide primers, as described in “Materials and methods,” revealed the expression of a single predominant Vβ2 rearrangement in all 3 samples (ie, primary T-ALL, mouse bone marrow, and spleen), strongly suggesting that the engrafted CD7+ cells recovered from the preconditioned NOD/SCID mice were in fact derived from the primary leukemia sample.
Furthermore, DNA sequencing of the Vβ2 rearrangement revealed a single homogeneous sequence that was identical in all 3 samples (primary T-ALL, mouse bone marrow, and spleen). This TCR Vβ2 rearrangement was shown by our sequencing to be in frame, to use the Dβ1.1 segment rearranged to Jβ1.1, and to encode the amino acid sequence CAREYRHEIVNTEAFFGQGTRLTVV.
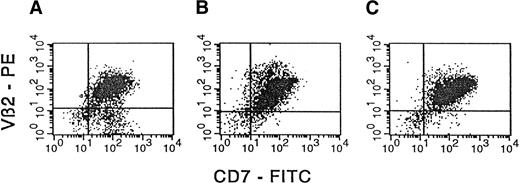
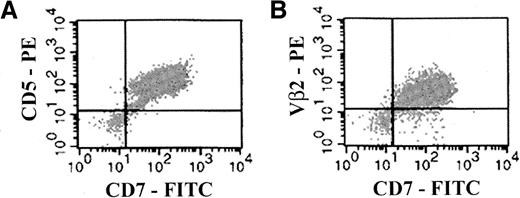
Based on these findings, cells from the same 3 samples were analyzed by flow cytometry after they were stained with a specific anti-Vβ2 antibody.20 It is apparent from Figure3 that for each primary T-ALL sample from the patient, engrafted mouse bone marrow, and engrafted mouse spleen, more than 85% of cells expressed Vβ2, consistent with RT-PCR findings that engrafted CD7+ cells in mouse bone marrow and spleen were derived from the primary leukemia.
Concordant expression of TCR Vβ2 by the primary T-ALL sample and engrafted NOD/SCID bone marrow and spleen.
MNCs from primary T-ALL obtained from a patient (A) and from bone marrow and spleen cells (B and C, respectively) harvested from a NOD/SCID mouse preconditioned with cord blood MNCs (10 × 106) and implanted with T-ALL leukemia from the patient (3.3 × 106 cells) were analyzed by flow cytometry for surface expression of TCR Vβ2. In the panels presented, the expression of Vβ2 was determined simultaneously with the expression of the T-ALL marker CD7. The CD7 and Vβ2 mAbs were used as FITC and PE conjugates, respectively. Isotype-matched control mAbs (FITC- or PE-conjugated IgG1) were used to determine appropriate cursor settings for analysis. Percentages of human Vβ2+CD7+ double-positive cells were 80%, 91%, and 93%, respectively, for samples A, B, and C.
Concordant expression of TCR Vβ2 by the primary T-ALL sample and engrafted NOD/SCID bone marrow and spleen.
MNCs from primary T-ALL obtained from a patient (A) and from bone marrow and spleen cells (B and C, respectively) harvested from a NOD/SCID mouse preconditioned with cord blood MNCs (10 × 106) and implanted with T-ALL leukemia from the patient (3.3 × 106 cells) were analyzed by flow cytometry for surface expression of TCR Vβ2. In the panels presented, the expression of Vβ2 was determined simultaneously with the expression of the T-ALL marker CD7. The CD7 and Vβ2 mAbs were used as FITC and PE conjugates, respectively. Isotype-matched control mAbs (FITC- or PE-conjugated IgG1) were used to determine appropriate cursor settings for analysis. Percentages of human Vβ2+CD7+ double-positive cells were 80%, 91%, and 93%, respectively, for samples A, B, and C.
Finally, analogous TCR Vβ PCR analysis was performed on primary T-ALL leukemia from another patient and on cells harvested from the corresponding engrafted mouse bone marrow. These results also confirmed that the engrafted human cells recovered from mice were of leukemic origin. Specifically, both the primary T-ALL cells and the cells recovered from mouse bone marrow presented with the same pair of productively rearranged Vβ genes, namely Vβ7.3 and Vβ14. Recently it was reported that approximately 1% of normal human T cells simultaneously express 2 productively rearranged Vβ genes.21 22
In vivo dissemination of human T-ALL in preconditioned NOD/SCID mice
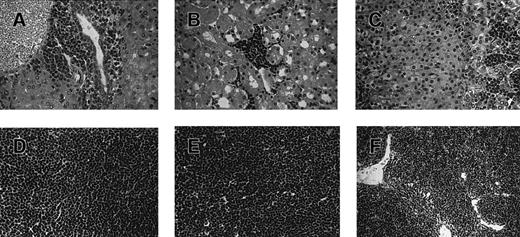
To further assess the fidelity of this mouse model of T-ALL to the human disease, we investigated the dissemination of the leukemia cells to other tissues (summarized in Table 2) and in peripheral blood. The dissemination of human T-ALL in mice preconditioned with human cord blood is illustrated in Figure 4, resulting in universally fatal leukemia. In liver, there were notable infiltrations of leukemia cells in portal areas and sinusoidal spaces (Figure 4A). Leukemia cells showed a characteristic chromatin pattern in the nuclei of cells with high nuclear–cytoplasm ratios. In kidney, human leukemia cells aggregated in perivascular and periglomerular interstitial spaces (Figure 4B). Immunohistochemical analysis of liver and kidney indicated that the infiltrating cells observed here expressed human CD5 and CD7 (not shown), consistent with the phenotype of the primary T-ALL cells injected (Table 1).
Histologic analysis of leukemic infiltrates in engrafted NOD/SCID mice.
Tissue sections (A-E) were obtained from a mouse preconditioned with 25 × 106 cord blood MNCs 7 days before the injection of 36 × 106 primary T-ALL cells. The mouse was killed 40 days after the T-ALL injection. Tissues were fixed in aqueous-buffered zinc formalin and embedded in paraffin; 4-μm sections were prepared and stained with hematoxylin and eosin. (A) Liver, (B) kidney, (C) adrenal gland, (D) lymph node, and (E) thymus from the engrafted mouse. For comparison, a tissue section (F) was obtained from the normal thymus of an unmanipulated, age-matched NOD/SCID mouse. Human T-ALL engraftment levels in bone marrow and spleen for this mouse were found by flow cytometry to be 85% and 78%, respectively (not shown).
Histologic analysis of leukemic infiltrates in engrafted NOD/SCID mice.
Tissue sections (A-E) were obtained from a mouse preconditioned with 25 × 106 cord blood MNCs 7 days before the injection of 36 × 106 primary T-ALL cells. The mouse was killed 40 days after the T-ALL injection. Tissues were fixed in aqueous-buffered zinc formalin and embedded in paraffin; 4-μm sections were prepared and stained with hematoxylin and eosin. (A) Liver, (B) kidney, (C) adrenal gland, (D) lymph node, and (E) thymus from the engrafted mouse. For comparison, a tissue section (F) was obtained from the normal thymus of an unmanipulated, age-matched NOD/SCID mouse. Human T-ALL engraftment levels in bone marrow and spleen for this mouse were found by flow cytometry to be 85% and 78%, respectively (not shown).
Leukemia infiltration was also found in the cortex region of the adrenal gland (Figure 4C). Normal lymph node architecture was totally effaced by a diffuse infiltration of immature lymphoid cells similar to those seen in liver (Figure 4D). Numerous leukemia cells (Figure4E) destroyed the architecture seen in the normal thymus (Figure 4F). In the lung, leukemia cells were detected in the visceral pleura (not shown). In brain, leukemia cells were observed in the leptomeninges but not in the parenchyma (not shown). Therefore, the engrafted human leukemia in cord blood–preconditioned NOD/SCID mice disseminated to various tissues in a pattern similar to human disease. In contrast, at the same time point, there was no sign of widespread dissemination of human leukemia for the mice injected with primary T-ALL without cord blood preconditioning (not shown).
In this in vivo model of human T-ALL, we also observed leukemia cells in the peripheral blood of mice. For example, for the mouse in Figure5, approximately 90% of the peripheral blood MNCs were human T-ALL cells. In this mouse, T-ALL was present in peripheral blood at 14 × 106 T-ALL cells per milliliter with CD5+CD7+ and CD7+Vβ2+ phenotypes (Figure 5), identical to that of the primary leukemia obtained from the patient. Additionally, to confirm that human cells in the blood were leukemic, modified Wright-stained blood smears were prepared from mice previously engrafted with human T-ALL. Cell morphology in these blood smears clearly shows the presence of human leukemia cells in large numbers (picture not shown). Engraftment of other patients' primary T-ALL in cord blood–preconditioned mice also led to the findings of human leukemia cells in the peripheral blood of mice.
Presence of leukemia cells in the peripheral blood of mice engrafted with primary T-ALL.
Mice were preconditioned with 10 × 106 cord blood MNCs before injection with 1.6 × 106 primary T-ALL cells. For the mouse presented here, the levels of T-ALL engraftment in bone marrow and spleen were 93% and 87%, respectively, followed both as CD5+CD7+ cells and Vβ2+CD7+ cells (not shown). Peripheral blood obtained from this mouse was analyzed by flow cytometry for the presence of human T-ALL cells. As indicated, T-ALL in the peripheral blood was similarly followed both as CD5+CD7+cells and as Vβ2+CD7+ cells. Isotype-matched control mAbs were used to determine appropriate cursor settings for analysis. Percentages of human CD5+CD7+ (A) and Vβ2+CD7+ (B) double-positive cells in mouse peripheral blood were determined to be 95% and 92%, respectively.
Presence of leukemia cells in the peripheral blood of mice engrafted with primary T-ALL.
Mice were preconditioned with 10 × 106 cord blood MNCs before injection with 1.6 × 106 primary T-ALL cells. For the mouse presented here, the levels of T-ALL engraftment in bone marrow and spleen were 93% and 87%, respectively, followed both as CD5+CD7+ cells and Vβ2+CD7+ cells (not shown). Peripheral blood obtained from this mouse was analyzed by flow cytometry for the presence of human T-ALL cells. As indicated, T-ALL in the peripheral blood was similarly followed both as CD5+CD7+cells and as Vβ2+CD7+ cells. Isotype-matched control mAbs were used to determine appropriate cursor settings for analysis. Percentages of human CD5+CD7+ (A) and Vβ2+CD7+ (B) double-positive cells in mouse peripheral blood were determined to be 95% and 92%, respectively.
Transfer of engrafted T-ALL to a secondary recipient
We then addressed whether human T-cell leukemia in mice can be transferred to a secondary mouse recipient and whether T-ALL engraftment in the secondary recipient was also facilitated by preconditioning with human cord blood. To this end, we injected T-ALL cells from the engrafted spleen of a primary mouse recipient into secondary recipients that had or had not been preconditioned with cord blood.
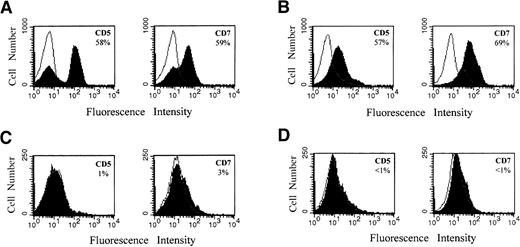
For the experiment presented in Figure 6, mice were (Figure 6A,B) or were not (Figure 6C, D) preconditioned with 25 × 106 human cord blood MNCs 8 days before the injection of 2.4 × 106 T-ALL cells recovered previously from the engrafted spleen of a primary mouse recipient (human T-ALL constituted 92% of these injected spleen cells). Five weeks after T-ALL injection, the mice receiving cord blood were killed, and the fraction of human CD5+ and CD7+ (T-ALL) cells in bone marrow and spleen was determined. Because the primary T-ALL cells of the patient also expressed TCR Vβ2, engraftment of T-ALL in the secondary recipient was also followed using this marker. For the representative mouse presented in Figure 6, on the basis of CD5, CD7, and Vβ2 expression, there was approximately 60% T-ALL engraftment both in bone marrow (Figure 6A) and in spleen (Figure 6B) of the secondary recipients. Similarly, the cells harvested from the second NOD/SCID recipients were also uniquely identified as CD7+Vβ2+ cells (picture not shown). Studies based on these phenotypic markers and analysis of TCR Vβ2 gene usage suggest that leukemia cells recovered from primary mouse recipients have leukemogenic potential in secondary mouse recipients.
Engraftment of primary T-ALL cells in secondary NOD/SCID recipients with or without cord blood preconditioning.
Representative results are presented for a mouse preconditioned with cord blood (A, B) and for a mouse that received no cord blood (C, D). One group of mice was injected with 25 × 106 human cord blood MNCs 8 days before injection with 2.4 × 106T-ALL cells from engrafted spleen. A second group of mice received no cord blood MNCs before injection of T-ALL cells. Human T-ALL constituted 92% of the injected engrafted mouse spleen cells on the basis of CD5 and CD7 expression (not shown). Mice in the first group were killed 5 weeks after T-ALL injection, whereas mice in the second group were killed 6 weeks after T-ALL injection. Bone marrow (A, C) and spleen (B, D) cells were analyzed by flow cytometry for several T-ALL markers, as described in “Materials and methods.” Percentages of human CD5+ and CD7+ cells were determined using CellQuest 3.2.1 software and are indicated in the panels.
Engraftment of primary T-ALL cells in secondary NOD/SCID recipients with or without cord blood preconditioning.
Representative results are presented for a mouse preconditioned with cord blood (A, B) and for a mouse that received no cord blood (C, D). One group of mice was injected with 25 × 106 human cord blood MNCs 8 days before injection with 2.4 × 106T-ALL cells from engrafted spleen. A second group of mice received no cord blood MNCs before injection of T-ALL cells. Human T-ALL constituted 92% of the injected engrafted mouse spleen cells on the basis of CD5 and CD7 expression (not shown). Mice in the first group were killed 5 weeks after T-ALL injection, whereas mice in the second group were killed 6 weeks after T-ALL injection. Bone marrow (A, C) and spleen (B, D) cells were analyzed by flow cytometry for several T-ALL markers, as described in “Materials and methods.” Percentages of human CD5+ and CD7+ cells were determined using CellQuest 3.2.1 software and are indicated in the panels.
In marked contrast, analysis of a representative mouse not receiving cord blood revealed that at 6 weeks after T-ALL injection only approximately 3% T-ALL engraftment in bone marrow (Figure 6C) and negligible T-ALL engraftment in the spleen (Figure 6D) occurred. Therefore, results also suggest that engraftment in a secondary recipient also is facilitated by preconditioning the mouse with human cord blood.
Discussion
In this study, we report that preconditioning NOD/SCID mice with fetal cord blood facilitates the engraftment, expansion, and dissemination in mice of primary human T-ALL cells obtained from patients. Cells recovered from engrafted mouse bone marrow or spleen resembled the original cells from patients not only with respect to the expression of various lineage markers but also with respect toTCR Vβ gene rearrangement. Moreover, the pattern of leukemia dissemination in mouse tissues is reminiscent of the human clinical disease from primary T-ALL samples. In addition, we document the fidelity of the model to the human disease with regard to the presence of morphologically identifiable human leukemia cells in mouse bone marrow and blood and with regard to the maintenance of the leukemia-initiating capacity within the leukemia-engrafted mouse.
In this study, several independent approaches have been used to provide evidence for the human leukemia origin in cells harvested from engrafted mice. These include (1) phenotypic analysis using more than 9 different markers (including the lack of CD19+cells), (2) gene usage of TCR Vβ in cells harvested from mice, (3) presence of morphologically identifiable human leukemia cells in bone marrow and in blood, and (4) progression/dissemination of human leukemia to other tissues, resulting in universally fatal leukemia. In addition, recent studies of the pattern of cDNA expression in microarray analysis of the cells harvested from engrafted mouse marrow confirmed that the harvested cells from mice are derived from primary leukemia samples from the patient rather than from injected human cord blood (JY et al, unpublished observations, 2000).
It has also been suggested that injected T-ALL cells may alter the growth or lead to the expansion of subsets of human cord blood cells in NOD/SCID mice. Although intriguing, this contention does not gain support from our recent findings15 that 3.5 Gy-irradiated cord blood also facilitates the engraftment of T-ALL cells in NOD/SCID mice in a manner similar to that of fresh cord blood. In addition, it was reported recently that CD5+ cells were found among CD19+ B lymphoid cells in NOD/SCID mice injected with only cord blood.23 This observation is in line with numerous reports that human cells expanded in NOD/SCID mice engrafted with cord blood alone are predominantly CD19+.9,10,16-18The paucity of CD19+ cells in the engrafted NOD/SCID mice suggests that the demonstration of CD5+CD7+cells in these mice is less likely be attributed to the expansion of this subset of CD5+CD19+ double-staining cells, as observed in Novelli et al.23 Instead, the results suggest that the expansion of the cells from engrafted cord blood used for preconditioning has, in large part, been displaced by the expansion of T-ALL leukemia cells.
Efficient engraftment in mouse bone marrow was typically observed when more than 1 × 106 primary T-ALL cells were injected and was also dependent on the number of cord blood cells used for preconditioning (D.P.D. et al, manuscript submitted). The capacity of cord blood preconditioning to facilitate the subsequent engraftment in NOD/SCID mice of primary human T-ALL is interesting in light of previous in vivo work in SCID mice indicating that human cord blood manifests antileukemia activity directed against leukemia cell lines.24 A number of significant differences between the 2 in vivo systems—including the order and route of cord blood and leukemia cell injections, the interval between injections, and the use of primary T-ALL cells obtained from patients versus leukemia cell lines—could account for the discordant effects of cord blood observed.
Furthermore, we achieved approximately 30-fold expansion of human leukemia cells in primary mouse recipients. Transfer into secondary mouse recipients expanded the patient's primary leukemia approximately another 30-fold. These expanded leukemia cells can be stored and used subsequently for an increased number of in vivo preclinical tests of new therapies directed against childhood T-ALL. Therefore, this in vivo mouse model of human leukemia will enable not only the study of the growth properties of primary leukemia and the analysis of the so-called leukemia-initiating cell,25 it will also enable the evaluation of the potential efficacy of therapeutic strategies in vivo.
Whereas in previous studies only subgroups of patients with more aggressive T-ALL or patients at relapse had successful engraftment of primary leukemia in immunodeficient mice,7 14 the present studies achieved significant engraftment, expansion, and dissemination of T-ALL cells obtained from patients at the time of diagnosis. In addition, both fresh and cryopreserved MNCs from patients with T-ALL achieved comparable engraftment in these and other experiments. Moreover, we have shown that this mouse model leads to engraftment of the leukemia in secondary mouse recipients that also were preconditioned with human cord blood.
Present studies also indicated that the leukemia-initiating capacity was maintained within the leukemia-engrafted mouse. Leukemic progenitor cells have been implicated in the maintenance and expansion of leukemic blast populations.26,27 Until recently, clonogenic blast cells could only be identified based on their ability to proliferate and form colonies in semisolid media in response to specific growth factors,28,29 with the colony-forming blasts assumed to represent the in vitro counterparts of the in vivo ALL blast progenitors.26 Only recently have leukemia-initiating cells been demonstrated in vivo.25,30-32 The ongoing development of in vivo mouse models for leukemia is expected to expedite characterization of these leukemia-initiating cells.30 The phenotype of the leukemia-initiating cell for T-ALL is presently unknown; in particular, there is no specific marker to distinguish these clonogenic blasts from nonprogenitor blasts in the primary leukemia. We show here, by serial transfer of the leukemia, that in our mouse model the T-ALL leukemia-initiating capacity is maintained within the leukemia-engrafted mouse. Our mouse model should therefore facilitate the identification of signals driving in vivo expansion of these leukemic clonogenic cells and the identification of progenitor-specific markers.
It is unknown how preconditioning cord blood facilitates the engraftment of human T-ALL in NOD/SCID mice. It is conceivable that the engraftment of primary leukemia cells is supported by multiple mechanisms, including the release of cytokines, the differences in homing of the “adherent stromal cells,” and the proliferation of mature accessory cells in the cord blood used here for preconditioning. For example, studies by Ildstad et al33 suggest that mouse and primate lymphocytes contain “facilitating cells” that can increase allogeneic engraftment, but only when cotransplanted with the major histocompatibility complex-matched hematopoietic cells. Alternatively, accessory cells in the cord blood could release cytokines that facilitate engraftment of the subsequently injected primary T-ALL cells. This interpretation seems to be consistent with recent observations that preconditioning with the irradiated cord blood also enhanced the leukemia engraftment in NOD/SCID mice.15Recently, the chemokine stromal derived factor-1 (SDF-1) has been implicated in the homing of normal human hematopoietic stem cells to bone marrow in the NOD/SCID mouse.34 Although it is intriguing to find expression of the receptor for SDF-1, namely CXCR4, in primary leukemia from patients with T-ALL, it is also shown that the presence of these CXCR4 receptors does not result in the transmigration of T-ALL cells induced by SDF-1 (D.P.D. et al, manuscript submitted). Therefore, the functionality of the expressed CXCR4 and the importance of cord blood–derived SDF-1 to the enhancement of leukemia engraftment are unknown.
Collectively, the results presented here support the thesis that engrafted human cells of cord blood origin provide important microenvironmental signals that in the NOD/SCID mouse are otherwise limiting with respect to engraftment of human primary T-ALL. The identity of the putative limiting signal(s) and the cord blood cell(s) from which T-ALL is derived is under investigation in our laboratory. We have shown, for example, that a cord blood–conditioned medium significantly increases the number and the burst size of primary T-ALL colonies in methylcellulose culture.35,36 In addition, it was recently found that after reconstitution with human cord blood alone, a significant fraction of mouse bone marrow cells was of human cord blood cell origin and that cells harvested from mice indeed secreted factor(s) into conditioned medium that enhanced human leukemia colony formation in culture by at least 2-fold.15 However, much work will have to be performed to prove any mechanism(s) by which cord blood cells facilitate T-ALL engraftment observed in this study. In addition, it is also an unsettled, but important, issue to determine how these laboratory observations will translate for transplantations using cord blood.
In conclusion, the significance of the work presented here is several-fold. It establishes a novel and possibly a more clinically relevant experimental avenue for delineating signals critical in vivo for T-ALL engraftment, expansion, and metastasis of primary leukemia obtained from patients. For example, the mouse model described here should lend itself to the characterization of signals driving engraftment and expansion of the putative leukemia-initiating cell. Finally, our work establishes a robust in vivo system for the evaluation of newly developed therapeutic strategies directed against T-ALL.
We thank Judith Preston for secretarial assistance in the preparation of this manuscript. We thank the investigators from the Pediatric Oncology Group for providing primary T-ALL samples, the staff at PharMingen for generously providing antibodies, and the staff at Scripps GCRC for flow cytometry support.
Supported by the Leukemia Society of America (grant 6226) and the National Institutes of Health (grants CA 79951 and MO1-RR00833). D.P.G. is a Scholar of the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John Yu, Department of Molecular and Experimental Medicine, MEM265, The Scripps Research Institute, 10550 North Torrey Pines Rd, La Jolla, CA 92037.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal