Abstract
This work aims to demonstrate that CD4+CD56+ malignancies arise from transformed cells of the lymphoid-related plasmacytoid dendritic cell (pDC) subset. The analysis of malignant cells from 7 patients shows that in all cases, like pDCs, leukemic cells are negative for lineage markers CD3, CD19, CD13, CD33, and CD11c but express high levels of interleukin-3 receptor α chain (IL-3Rα), HLA-DR, and CD45RA. Tumor cells produce interferon-α in response to influenza virus, while upon maturation with IL-3 they become a powerful inducer of naive CD4+T-cell proliferation and promote their T-helper 2 polarization. As pDCs, leukemic cells also express pre-Tα and lambda-like 14.1 transcripts, arguing in favor of a lymphoid origin. In addition, malignant cells express significant levels of CD56 and granzyme B. Overall, those observations suggest that CD4+CD56+ leukemic cells could represent the malignant counterpart of pDCs, both of which are closely related to B, T, and NK cells.
Introduction
Several reports in the literature1-7describe unusual and rare hematopoietic tumors expressing CD4 and CD56, without CD3, CD19, CD13, and CD33 conventional lineage markers, and no T- or B-cell receptor gene rearrangement. Clinically, these cases are characterized by a rapid aggressive course, an extranodal and notable skin involvement, and a frequent evolution toward an overt leukemia. The possible existence of a new entity has been raised, but the origin of the tumor cells has not yet been resolved, even if they have occasionally been classified as T,3 natural killer (NK),3-5 or myeloid5 precursors. In fact, after comparison of their phenotype with that of all known hematopoietic cells, we postulated that these cells are related to the newly characterized plasmacytoid dendritic cell (pDC).8
The pDCs are members of the heterogeneous dendritic cell family that could be derived from human bone marrow9 and thymus10,11 lymphoid-restricted progenitors. They have been identified in peripheral blood12-14 and in T-cell areas of tonsils,8 lymph nodes,15 and thymus.16,17 The pDCs not only have a typical morphology,8,12 but they also have characteristic phenotypic features such as expression of CD4, HLA-DR, and CD45RA while they are lacking myeloid-related antigens CD11c, CD13, and CD33.8,15,18 They are devoid of lineage-associated markers such as CD3, CD14, CD19, and CD56, but CD2, CD5, and CD7 have occasionally been observed.15,16 Noteworthy, they express a low level of granulocyte-macrophage colony-stimulating factor receptor α chain (GM-CSFRα) (CD116) and high level of interleukin-3 receptor α chain (IL-3Rα) (CD123),15,19and this latter marker has been used to identify them in situ.15,16 Costimulatory molecules CD80 and CD86 are absent or low.8 Recently, pDCs have also been demonstrated to contain messenger RNA (mRNA) for pre-Tα 16 and lambda-like 14.1,17 further arguing in favor of a lymphoid origin. They also are highly prone to spontaneous apoptosis, with no surviving cells after 3 days in culture medium.8However, they can be partly rescued by IL-3 or GM-CSF.8,13,14 At an immature stage, they have been recognized as the most powerful interferon (IFN)-α–secreting cells in response to viruses,15,20 suggesting a primary role in innate immunity. Furthermore, they display poor endocytic capacity8,14 and poor ability to stimulate allogeneic T lymphocytes.13 However, in vitro–induced IL-3, CD40L, or virus maturation of pDCs confer them the ability to stimulate T-lymphocyte proliferation.8,13,21,22 This characteristic property results from an increased expression of markers involved in antigen presentation like HLA class II and costimulation (CD40, CD80, CD86).8,21,22 Matured pDCs display typical dendrites upon CD40L activation.8,13 Depending on the activation mode, pDCs can either cause naive CD4+ T cells to preferentially produce T helper (Th)1 21,22 or, with a more or less pronounced polarization, Th2 cytokines.15,19 They could potentially be implicated in the induction of an antiviral immunity or in peripheral tolerance either against self23 or allogeneic24 antigens.
In the present work, we identify CD4+, CD56+, CD3−, CD13−, CD33−, and CD19− tumor cells from 7 patients as members of the pDC family, and we demonstrate that they retained many phenotypic and functional properties of their normal counterpart. We also show that they share some markers with T, B, and NK cells, suggesting a relation to lymphoid lineage.
Patients, materials, and methods
Patients and isolation of tumor cells
Seven patients (6 men and 1 woman from 8 to 86 years of age, mean = 63 years) with a CD4+CD56+ leukemia are included in this study. They all reveal massive bone marrow infiltration, either at diagnosis or during follow-up, and evolution toward an overt leukemia within a few months. Extranodal localizations are always observed (mainly skin, but also lung, mucosa, or central nervous system), whereas lymph node or spleen are involved in 4 cases. At the time of diagnosis, cytopenia was observed in 6 of 7 cases (anemia 4 of 7, thrombopenia 4 of 7, and neutropenia 4 of 7). These 7 cases are part of a larger series of 19 patients sharing the same clinical and biological characteristics (J. Feuillard, et al, unpublished data, 2000). Tumor cells were obtained from peripheral blood, bone marrow, or lymph nodes at diagnosis or relapse, as previously described,25 and cryopreserved. They represented from 50% to 98% of the mononuclear cells. Thus, they were purified by immunomagnetic negative selection in 3 cases (patients CAD, CAT, and GUE, the initial percentages of tumor cells being 50%, 60%, and 80%, respectively) with anti-CD20, -CD14, and -CD3 monoclonal antibodies (mAbs) (Beckman Coulter Immunotech, Marseille, France) and beads (Dynal, Olso, Norway) according to the manufacturer's instructions. Depletion of beadbound cells resulted in 90% to 99% purity as determined by flow cytometry.
Effect of cytokines on cell viability
A total of 105 purified tumor cells were seeded in 200 μL RPMI 1640 (Gibco Life Technology, Cergy Pontoise, France) supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, nonessential amino acids (referred to as complete medium), and 10% fetal calf serum. The effect of IL-2 (50 U/mL, gift from Roussel Uclaf, Romainville, France), IL-3 (10 ng/mL; PeproTech, London, England), IL-4 (10 ng/mL; PeproTech), and GM-CSF (500 U/mL; Schering-Plough, Levallois Perret, France) was analyzed after a 72-hour culture. The Annexin V-FITC/PI kit (Beckman Coulter Immunotech) was used according to the manufacturer's instructions to determine on a FACScan (Becton Dickinson, Mountain View, CA) the percentage of viable cells.26
Differentiation of tumor cells into mature DCs
Purified tumor cells from 5 patients were cultured at 106/mL for 3 or 6 days in complete 10% fetal calf serum medium supplemented or not with IL-3 (10 ng/mL). In 2 cases, maturation with CD40 ligation was analyzed: Irradiated (70 Gy) CD40L-transfected L cells (gift from P. Garonne, Schering-Plough) were added on day 4 to the IL-3 tumor cell culture (CD40L cells: tumor cell ratio = 1:5). On day 6, phenotypic and functional analyses were performed. Culture supernatants were cryopreserved for cytokine measurements.
Flow cytometry and immunofluorescence
Immunophenotype was analyzed by flow cytometry, using direct or indirect labeling, with the antibodies listed in Table1. For comparison of expression levels, the mean fluorescence intensity ratio between specific mAb and isotypic control was calculated. Intracytoplasmic flow cytometric staining of perforin and granzyme B was performed after paraformaldehyde fixation (2%) and permeabilization of the cells (saponin 0.3%). Cytospins of cell suspensions were dehydrated, fixed with acetone (−20°C, 10 minutes), and cryopreserved. After thawing and rehydratation, the incubation with primary antibodies (perforin or CD83) was followed by incubation with fluorescein isothiocyanate (FITC)–goat antimouse immunoglobulin (Beckman Coulter Immunotech) and counterstaining with Evan's blue dye (Sigma, Saint Quentin Fallavier, France).
Surface phenotype of purified fresh tumor cells
| AntigenPatients: . | CAD . | CAT . | GEN . | GUE . | LAI . | PER . | VOI . | Clone . | Source . |
|---|---|---|---|---|---|---|---|---|---|
| T cells | |||||||||
| CD2 | 7 | 2 | 4 | 37 | 3 | 5 | 1 | RPA-2.10 | PH |
| CD3 | 4 | 8 | 1 | 0 | 3 | 3 | 10 | UCHT-1 | BCI |
| CD4 | 37 | 68 | 89 | 70 | 95 | 88 | 92 | 13B8.2 | BCI |
| CD7 | 73 | 53 | 67 | 0 | 1 | 46 | 0 | M-T701 | PH |
| CD8 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | B9.11 | BCI |
| Monocyte | |||||||||
| CD13 | 2 | 1 | 4 | 1 | 0 | 3 | 0 | WM-47 | Dako |
| CD14 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | RMO52 | BCI |
| CD33 | 1 | 3 | 4 | 0 | 2 | 7 | 0 | WM-54 | Dako |
| CD64 | 2 | 3 | 3 | 0 | 2 | 2 | 1 | 22 | BCI |
| Natural killer cells | |||||||||
| CD16 | 1 | 2 | 4 | 0 | 3 | 3 | 1 | 3G8 | BCI |
| CD56 | 49 | 78 | 15 | 79 | 98 | 96 | 70 | N901 | BCI |
| CD57 | 0 | nd | 0 | 0 | 0 | 0 | 0 | HNK1 | BD |
| CD94 | 5 | nd | 2 | 0 | nd | nd | nd | HP-3D9 | Ancell |
| CD161 | 0 | nd | 1 | 1 | nd | 1 | nd | DX12 | BD |
| Granzyme B* | nd | nd | 95 | 63 | 68 | nd | nd | CLB-GB11 | Pelicluster |
| Perforin*,† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | γG9 | PH |
| B cells | |||||||||
| CD19 | 3 | 4 | 1 | 2 | 0 | 1 | 0 | J4.119 | BCI |
| Cytokine receptor | |||||||||
| CD25 | nd | 32 | 0 | 0 | nd | 6 | 6 | B1.49.9 | BCI |
| CD116 | 1 | 4 | 2 | 4 | 50 | 21 | 33 | M5D12 | PH |
| CD122 | 4 | 1 | 1 | 0 | 1 | 4 | 0 | Mik-β2 | PH |
| CD123 | 88 | 95 | 99 | 98 | 95 | 99 | 97 | 9F5 | PH |
| Dendritic cells | |||||||||
| CD1a | 1 | 1 | 0 | 0 | 0 | 2 | 0 | BL6 | BCI |
| CD1c | 9 | 52 | 5 | 2 | 1 | 17 | nd | L161 | BCI |
| CD11c | 9 | 6 | 1 | 1 | 4 | 7 | 1 | BU15 | BCI |
| CD83 | 1 | 0 | 35 | 6 | 2 | 5 | 0 | HB15a | BCI |
| ILT3 | 85 | 89 | 97 | 98 | 93 | 81 | nd | ZM3.8 | D. Jarrossay, BCI |
| Antigen-presenting cells | |||||||||
| CD40 | 3 | 6 | 32 | 65 | 67 | 6 | 1 | mAb89 | BCI |
| CD80 | 1 | 5 | 0 | 0 | 6 | 3 | 3 | MAB104 | BCI |
| CD86 | 21 | 67 | 12 | 1 | 53 | 13 | 4 | 2331 | PH |
| HLA-ABC | 97 | 100 | 100 | 99 | 100 | 100 | 97 | B9.12.1 | BCI |
| HLA-DR | 83 | 95 | 97 | 78 | 99 | 97 | 100 | B8.12.2 | BCI |
| Miscellaneous | |||||||||
| CD10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ALB1 | BCI |
| CD34 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 581 | BCI |
| CD45 RA | 94 | 84 | 99 | 90 | 99 | 98 | 98 | J33 | BCI |
| CD62L | 1 | nd | 64 | 35 | 95 | 33 | nd | DREG56 | BCI |
| CD117 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95C3 | BCI |
| CLA | 89 | nd | 0 | 0 | 14 | 2 | nd | HECA-452 | PH |
| AntigenPatients: . | CAD . | CAT . | GEN . | GUE . | LAI . | PER . | VOI . | Clone . | Source . |
|---|---|---|---|---|---|---|---|---|---|
| T cells | |||||||||
| CD2 | 7 | 2 | 4 | 37 | 3 | 5 | 1 | RPA-2.10 | PH |
| CD3 | 4 | 8 | 1 | 0 | 3 | 3 | 10 | UCHT-1 | BCI |
| CD4 | 37 | 68 | 89 | 70 | 95 | 88 | 92 | 13B8.2 | BCI |
| CD7 | 73 | 53 | 67 | 0 | 1 | 46 | 0 | M-T701 | PH |
| CD8 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | B9.11 | BCI |
| Monocyte | |||||||||
| CD13 | 2 | 1 | 4 | 1 | 0 | 3 | 0 | WM-47 | Dako |
| CD14 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | RMO52 | BCI |
| CD33 | 1 | 3 | 4 | 0 | 2 | 7 | 0 | WM-54 | Dako |
| CD64 | 2 | 3 | 3 | 0 | 2 | 2 | 1 | 22 | BCI |
| Natural killer cells | |||||||||
| CD16 | 1 | 2 | 4 | 0 | 3 | 3 | 1 | 3G8 | BCI |
| CD56 | 49 | 78 | 15 | 79 | 98 | 96 | 70 | N901 | BCI |
| CD57 | 0 | nd | 0 | 0 | 0 | 0 | 0 | HNK1 | BD |
| CD94 | 5 | nd | 2 | 0 | nd | nd | nd | HP-3D9 | Ancell |
| CD161 | 0 | nd | 1 | 1 | nd | 1 | nd | DX12 | BD |
| Granzyme B* | nd | nd | 95 | 63 | 68 | nd | nd | CLB-GB11 | Pelicluster |
| Perforin*,† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | γG9 | PH |
| B cells | |||||||||
| CD19 | 3 | 4 | 1 | 2 | 0 | 1 | 0 | J4.119 | BCI |
| Cytokine receptor | |||||||||
| CD25 | nd | 32 | 0 | 0 | nd | 6 | 6 | B1.49.9 | BCI |
| CD116 | 1 | 4 | 2 | 4 | 50 | 21 | 33 | M5D12 | PH |
| CD122 | 4 | 1 | 1 | 0 | 1 | 4 | 0 | Mik-β2 | PH |
| CD123 | 88 | 95 | 99 | 98 | 95 | 99 | 97 | 9F5 | PH |
| Dendritic cells | |||||||||
| CD1a | 1 | 1 | 0 | 0 | 0 | 2 | 0 | BL6 | BCI |
| CD1c | 9 | 52 | 5 | 2 | 1 | 17 | nd | L161 | BCI |
| CD11c | 9 | 6 | 1 | 1 | 4 | 7 | 1 | BU15 | BCI |
| CD83 | 1 | 0 | 35 | 6 | 2 | 5 | 0 | HB15a | BCI |
| ILT3 | 85 | 89 | 97 | 98 | 93 | 81 | nd | ZM3.8 | D. Jarrossay, BCI |
| Antigen-presenting cells | |||||||||
| CD40 | 3 | 6 | 32 | 65 | 67 | 6 | 1 | mAb89 | BCI |
| CD80 | 1 | 5 | 0 | 0 | 6 | 3 | 3 | MAB104 | BCI |
| CD86 | 21 | 67 | 12 | 1 | 53 | 13 | 4 | 2331 | PH |
| HLA-ABC | 97 | 100 | 100 | 99 | 100 | 100 | 97 | B9.12.1 | BCI |
| HLA-DR | 83 | 95 | 97 | 78 | 99 | 97 | 100 | B8.12.2 | BCI |
| Miscellaneous | |||||||||
| CD10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ALB1 | BCI |
| CD34 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 581 | BCI |
| CD45 RA | 94 | 84 | 99 | 90 | 99 | 98 | 98 | J33 | BCI |
| CD62L | 1 | nd | 64 | 35 | 95 | 33 | nd | DREG56 | BCI |
| CD117 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95C3 | BCI |
| CLA | 89 | nd | 0 | 0 | 14 | 2 | nd | HECA-452 | PH |
Percentages of positive cells were determined by 2- or 3-color flow cytometry.
BCI indicates Beckman Coulter Immunotech; PH, Pharmingen; BD, Becton Dickinson; Ancell, of Bayport, NM; Pelicluster, of Flanders, NJ; nd, not done.
Intracytoplasmic staining flow cytometry.
Immunocytofluorescent analysis.
Reverse transcriptase–polymerase chain reaction
RNA was isolated from FACS-sorted normal blood pDCs, from tumor cells purified from 4 patients, from blood NK cells, and from monocyte-derived DCs obtained after a 6-day culture in GM-CSF and IL-13 (Sanofi, Labège, France) (mo-DCs) using MasterPure RNA purification kit (Epicentre Technologies, Madison, WI) according to the manufacturer's instructions. First-strand complementary DNA was prepared using oligo(dT) primers (Pharmacia, Uppsala, Sweden) and Superscript RNase-H reverse transcriptase (Gibco BRL, Gaithersburg, MD). Polymerase chain reaction (PCR) was performed in a DNA thermal cycler (PE Applied Biosystems, Foster City, CA) for 35 cycles (1 minute of denaturation at 94°C, 1 minute of annealing at 55°C for lambda-like 14.1 and granzyme B, or 60°C for IL-3Rα, FasL, pre-Tα, perforin, CD56, and 2 minutes of elongation at 72°C) with ampliTaq enzyme and buffer (Gene Amp PCR reagents kit, PE Applied Biosystems, Foster City, CA), dNTPS at 10 mM (PE Applied Biosystems, Foster City, CA), and dimethyl sulfoxide at 5% final concentration. The β-actin mRNA amplification was performed for 28 cycles at 60°C on the complementary DNA as positive control of reaction efficiency. The primers used were as follows: IL-3Rα (sense: 5′-ATGCCGACTATTCTATGCCG-3′, antisense: 5′-TGTCTCTGACCTGTTCTGTG-3′); lambda-like 14.1 (sense: 5′-ATGCATGCGGCCGCGGCATGTGTTTGGCAGC-3′, antisense: 5′-ATCCGCGGCCGCATCGATAGGTCACCGTCA-AGATT-3′); pre-Tα (sense: 5′-GGCACACCCTTTCCTTCTCTG-3′, antisense: 5′-GCAGGTCCTGGCTGTAGAAGC-3′); FasL (sense: 5′-GGATTGGGCCTGGGGATGTTTCA-3′, antisense: 5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′); perforin (sense: 5′-CAGGTCAACATAGGCATCCA-3′, antisense: 5′-CGAGTTTACCCAGGCTGAGT-3′); granzyme B (sense: 5′-ACCTCTCCCAGTGTAAATCT-3′, antisense: 5′-GCGGTGGCTTCCTGATACAA-3′); CD56 (sense: 5′-GAGATCAGCGTTGGAGAGTC-3′, antisense: 5′-AAGAGTGACCTGCTCCTCTA-3′); β-actin (sense: 5′-GTCCACCTTCCAGCAGATGT-3′, antisense: 5′-CAATGCTATCACCTCCCCTG-3′). Reverse transcriptase (RT)-PCR products for lambda-like 14.1, pre-Tα, and granzyme B have been cloned and sequenced and corresponded to the sequences reported in public data bank (not shown).
Cytokine production and virus activation
Supernatants cryopreserved at the end of 6-day cultures with IL-3 were tested for IL-12 p70 and IL-8 contents by enzyme-linked immunosorbent assay (ELISA) (Beckman Coulter Immunotech; sensitivity, 5 pg/mL for IL-12 and 8 pg/mL for IL-8). To determine IFN-α production, thawed purified tumor cells and FACS-sorted CD56+ and CD56− cells from 3 patients (LAI, GEN, and GUE) as well as fresh FACS-sorted blood, CD11c−pDCs, and CD11c+ myeloid-related DCs were stimulated with 1 hemagglutinating unit (HAU)/mL formaldehyde-inactivated influenza virus strain Beijing/262/95 (kindly provided by Dr N. Kuehm, Aventis Pasteur, Val de Reuil, France) in duplicate wells (106 cells/mL, in 24- or 96-well culture plate) for 24 hours. IFN-α was measured in supernatant by ELISA that specifically recognizes IFN-α2 (Beckman Coulter Immunotech). The sensitivity of this assay is 0.6 U/mL.
Naive T-lymphocyte stimulation and polarization
Proliferative response of naive T lymphocytes was evaluated in response to tumor cells activated or not. CD4+CD45RA+ lymphocytes were isolated from cord blood by negative immunomagnetic depletion (Stem Cell Technology, Meylan, France), resulting in more than 97% purity. Mixed lymphocyte cultures (MLCs) were conducted in quadruplicate in 200 μL 96-well flat-bottom plates (Falcon) by mixing 25 × 103responding purified CD4+CD45RA+ cells and 5 × 103 to 25 × 103 irradiated (30 Gy) tumor cells (unactivated or after IL-3 treatment). Six-day cultures were performed in complete medium supplemented with 15% heat-inactivated human AB serum; 37 × 103 Bq [3H]thymidine was added to each well and harvested 18 hours later. Mo-DCs obtained from adherent peripheral blood mononuclear cells (normal volunteers) cultured for 6 days with GM-CSF (500 U/mL) and IL-13 (50 ng/mL)27 were used as control.
To evaluate T-lymphocyte polarization, 5 × 103irradiated IL-3–differentiated tumor cells were cocultured with 5 × 104 CD4+CD45RA+ cord blood T cells during 6 days. The proportion of IL-4– and IFN-γ–producing cells was determined by flow cytometry after a further 6-hour stimulation with 5 ng/mL phorbol myristate acetate (Sigma) and 0.5 μg/mL ionomycin (Sigma). During the last 5 hours, monensin (3 μM, Sigma) was added. Cells were washed in cold phosphate-buffered saline, fixed in paraformaldehyde (4%), and permeabilized with saponin (0.1%).28 The cells were then stained with FITC-labeled anti–IFN-γ, phycoerythrin-labeled anti–IL-4 (PharMingen, San Diego, CA), and PC5-labeled CD25 (Beckman Coulter Immunotech).
Results
Identification of a tumoral equivalent for pDCs
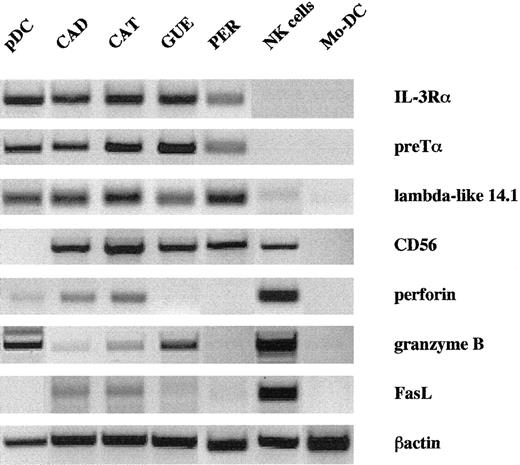
Seven tumor samples were selected on the basis of their expression of CD4 and CD56 in the absence of other lineage markers (CD3, CD19, CD13, and CD33). Malignant cells expressed the panleukocyte antigen CD45 at a low level, similarly to blastic hematopoietic cells, but CD10, CD34, and CD117 that are related to progenitors were negative. Using a large panel of mAbs, we identified them as a homogeneous entity and postulated that they could be related to the pDC subset (Table 1). They were all positive for CD45RA and negative for CD45RO, CD1a, and CD11c (Table 1). CD1c, CD83, and CD116 were inconstantly slightly expressed (2 of 6, 1 of 7, and 3 of 7 cases, respectively). CD36 and CD68 monocytic antigens were always expressed (not shown), but CD14 and CD64 were absent. Regarding NK cell–related markers, besides CD56, granzyme B was detected both at the mRNA and protein levels (Figure1 and Table 1). Intensity of granzyme B staining on leukemic pDCs (and normal pDCs, data not shown) was 10-fold lower than that observed on NK cells. Because perforin labeling was negative on leukemic pDCs (Table 1 and on normal pDCs, data not shown), we could not exclude that the slight mRNA signals for perforin and FasL on a leukemic sample (Figure 1) might be due to contaminating NK cells. Also, CD2 or CD7 could be expressed (1 of 7 and 4 of 7 cases, respectively), but CD16, CD57, CD94, and CD161 were negative (Table 1). These cells were also characterized by the presence of surface molecules associated with antigen-presenting cells (APCs): HLA-ABC and -DR in all cases, CD40 in 3 cases, and/or CD86 in 3 cases. They highly expressed immunoglobulin-like transcript (ILT)3 and IL-3Rα/CD123 (Table 1, Figure 1). Besides CD56, this phenotype is highly similar to that of pDCs isolated from blood, tonsils, or thymus from nonmalignant patients. Like normal pDCs, tumor cells expressed strong levels of pre–T-cell receptor α (pre-Tα)16 and lambda-like 14.117 mRNA (Figure 1), both of which were negative in NK cells and in mo-DCs. Of note, in all instances, no expression of CD3 and CD19 mRNA was found, excluding T- and B-cell contaminations (not shown).
Leukemic cells express pDC- and NK-related transcripts.
Expression of relevant mRNA was analyzed by RT-PCR from pure tumor cells, CD11c− pDCs, NK cells, and mo-DCs. Like normal pDCs, tumor cells expressed high levels of IL-3Rα, pre-Tα, and lambda-like 14.1 mRNA that were absent from NK cells and mo-DCs. In contrast, only weak expression of NK cell–related genes (perforin, granzyme B, FasL) was detected in tumor cells, except for CD56.
Leukemic cells express pDC- and NK-related transcripts.
Expression of relevant mRNA was analyzed by RT-PCR from pure tumor cells, CD11c− pDCs, NK cells, and mo-DCs. Like normal pDCs, tumor cells expressed high levels of IL-3Rα, pre-Tα, and lambda-like 14.1 mRNA that were absent from NK cells and mo-DCs. In contrast, only weak expression of NK cell–related genes (perforin, granzyme B, FasL) was detected in tumor cells, except for CD56.
Cell survival is sustained by IL-3 or GM-CSF
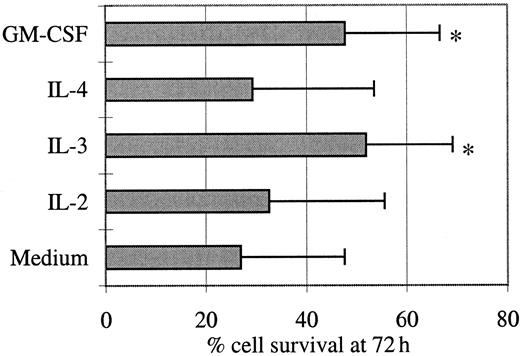
Given the immunophenotypic similarities between these tumor cells and pDCs, we investigated the effect of IL-2, IL-3, IL-4, or GM-CSF on their in vitro survival (Figure2). Day 0 viability of thawed cells was high (mean = 88%, not shown), but these cells died in the absence of cytokines (day 3 mean survival = 27%) even though slower than normal pDCs that undergo rapid spontaneous apoptosis in culture, resulting in 90% of cells displaying apoptotic figures at 16 hours.8 After a few hours in culture with IL-3 or GM-CSF, the tumor cells aggregated and formed large clusters, whereas their aspect remained unchanged with IL-2 or IL-4. IL-3 and GM-CSF improved slightly but significantly viability at day 3 from 27% to 52% and 48%, respectively (P < .05, Wilcoxon test). In contrast, IL-2 and IL-4 were not responsible either for such a significant improvement of cell survival or for an apoptotic signal. In 2 cases, day 3 [3H]thymidine incorporation was measured in the presence of these 4 cytokines, and no proliferation was detected (not shown).
Leukemic cell survival is sustained by IL-3 and GM-CSF.
Purified tumor cells were cultured for 72 hours in the presence of the indicated cytokines. The percentage of viable cells was measured by flow cytometric analysis after an Annexin V/PI staining of dead cells. Mean values ± SD of results obtained with the cells from 5 patients are shown. A significant (*P < .05, Wilcoxon test) increase of cell survival was observed in the presence of IL-3 or GM-CSF.
Leukemic cell survival is sustained by IL-3 and GM-CSF.
Purified tumor cells were cultured for 72 hours in the presence of the indicated cytokines. The percentage of viable cells was measured by flow cytometric analysis after an Annexin V/PI staining of dead cells. Mean values ± SD of results obtained with the cells from 5 patients are shown. A significant (*P < .05, Wilcoxon test) increase of cell survival was observed in the presence of IL-3 or GM-CSF.
Tumor cells differentiate into mature DCs with IL-3 and CD40L
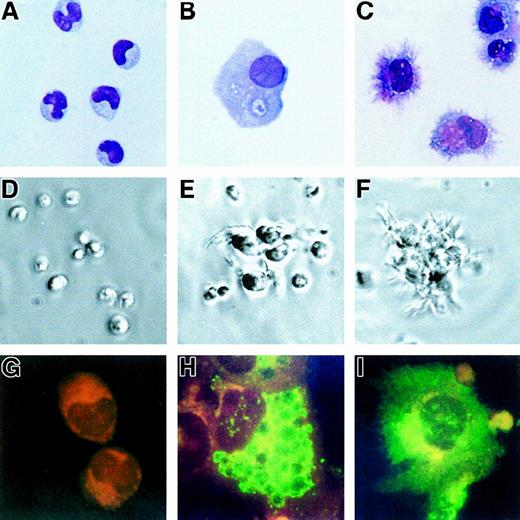
Six-day cultures of purified tumor cells were performed with IL-3 (5 cases) or GM-CSF (3 cases). As for normal pDCs, no increase in cell number was observed at the end of these cultures. In the presence of IL-3, the initial blastic morphology (Figure3A) of the tumor cells was highly modified; the cells enlarged, acquiring an abundant cytoplasm containing many vacuoles (Figure 3B). A mature DC morphology was achieved with many fine dendrites when CD40L-transfected L cells were added on day 4 for 48 hours (Figure 3C). In the absence of cytokines, the cells that remained isolated rapidly died in culture (Figure 3D), whereas they formed large clusters in the presence of IL-3 (Figure 3E), in the periphery of which dendrites were observed upon CD40L-induced activation (Figure 3F). IL-3 induced in most cases an up-regulation of the expression of HLA-ABC and -DR molecules and of costimulatory molecules CD80 and CD86 (Table2, Figure4), which were further increased upon CD40 activation (Figure 4). CD1a, CD1c, and CD83 were also up-regulated in response to IL-3 alone (Table 2). In all cases but one (GEN), CD83 was negative on tumor cells (Table 1, Figure 3G). IL-3 led to a low surface expression of CD83 (Table 2), while high intracytoplasmic levels were detected in response to IL-3 (Figure 3H) and IL-3 plus CD40L (Figure 3I). In 3 of 5 cases, CD13 and/or CD33 appeared with IL-3, and CD11c became highly expressed in all cases. The morphology (not shown) and the immunophenotype of the cells differentiated with GM-CSF (3 cases, Table 2) were highly similar to the phenotype described with IL-3, and no expression of CD14 or CD64 could be detected. In 4 cases, we examined the profile of cytokines produced at the end of 6-day cultures with IL-3. As reported for CD40L-activated IL-3–matured pDCs,19 tumor cells produced IL-8 (mean = 1914 pg/mL), whereas IL-4 and IL-12 p70 were undetectable by ELISA (not shown).
IL-3 induces differentiation of leukemic cells.
The initial morphology of the tumor cells (patient GUE) (A) (× 63) stained with May-Grünwald Giemsa was highly modified after 6-day culture with IL-3: The cells enlarged (B) (× 63) and, when activated during the last 48 hours with CD40L-transfected L cells, they acquired many fine dendrites (C) (× 63). Tumor cells die in culture medium alone (D) (× 10, as shown in phase contrast microscopy). Some dendrites can be seen in culture with IL-3 alone (E) (× 10) and on most of the cells that are either isolated or within clusters after CD40L activation (F) (× 10). CD83 (green fluorescence, red counterstaining) was not detected in fresh cells (G) (× 100) but became apparent after culture with IL-3, being localized in the cytoplasm (H) (× 100) and expressed at the cell surface after CD40L activation (I) (× 100).
IL-3 induces differentiation of leukemic cells.
The initial morphology of the tumor cells (patient GUE) (A) (× 63) stained with May-Grünwald Giemsa was highly modified after 6-day culture with IL-3: The cells enlarged (B) (× 63) and, when activated during the last 48 hours with CD40L-transfected L cells, they acquired many fine dendrites (C) (× 63). Tumor cells die in culture medium alone (D) (× 10, as shown in phase contrast microscopy). Some dendrites can be seen in culture with IL-3 alone (E) (× 10) and on most of the cells that are either isolated or within clusters after CD40L activation (F) (× 10). CD83 (green fluorescence, red counterstaining) was not detected in fresh cells (G) (× 100) but became apparent after culture with IL-3, being localized in the cytoplasm (H) (× 100) and expressed at the cell surface after CD40L activation (I) (× 100).
Phenotype of purified tumor cells after a 6-day culture
| AntigenPatients: . | IL-3 . | GM-CSF . | ||||||
|---|---|---|---|---|---|---|---|---|
| CAT . | CAD . | GEN . | GUE . | LAI . | CAT . | CAD . | GEN . | |
| CD4 | 10 | 91 | 95 | 81 | 73 | 91 | 42 | 86 |
| Monocyte | ||||||||
| CD13 | 80 | 11 | 15 | 9 | 37 | 72 | 30 | 25 |
| CD14 | 2 | 1 | 1 | 0 | 4 | 1 | 3 | 2 |
| CD33 | 61 | 1 | 30 | 19 | 8 | 74 | 20 | 44 |
| CD64 | 3 | 1 | 2 | 4 | nd | 1 | 5 | 4 |
| Natural killer cells | ||||||||
| CD56 | 72 | 6 | 11 | 28 | 77 | 77 | 35 | 21 |
| Dendritic cells | ||||||||
| CD1a | 74 | 8 | 94 | 22 | 11 | 57 | 42 | 88 |
| CD1c | 82 | 16 | 72 | 29 | 5 | 94 | 69 | 93 |
| CD11c | 65 | 47 | 91 | 50 | 55 | 65 | nd | 66 |
| CD83 | 3 | 30 | 65 | 30 | nd | nd | 31 | 35 |
| Antigen-presenting cells | ||||||||
| CD40 | 53 | 88 | 98 | 96 | 81 | nd | 68 | 94 |
| CD80 | 12 | 82 | 72 | 84 | 56 | 33 | 33 | 68 |
| CD86 | 47 | 87 | 81 | 77 | 59 | 76 | 49 | 77 |
| AntigenPatients: . | IL-3 . | GM-CSF . | ||||||
|---|---|---|---|---|---|---|---|---|
| CAT . | CAD . | GEN . | GUE . | LAI . | CAT . | CAD . | GEN . | |
| CD4 | 10 | 91 | 95 | 81 | 73 | 91 | 42 | 86 |
| Monocyte | ||||||||
| CD13 | 80 | 11 | 15 | 9 | 37 | 72 | 30 | 25 |
| CD14 | 2 | 1 | 1 | 0 | 4 | 1 | 3 | 2 |
| CD33 | 61 | 1 | 30 | 19 | 8 | 74 | 20 | 44 |
| CD64 | 3 | 1 | 2 | 4 | nd | 1 | 5 | 4 |
| Natural killer cells | ||||||||
| CD56 | 72 | 6 | 11 | 28 | 77 | 77 | 35 | 21 |
| Dendritic cells | ||||||||
| CD1a | 74 | 8 | 94 | 22 | 11 | 57 | 42 | 88 |
| CD1c | 82 | 16 | 72 | 29 | 5 | 94 | 69 | 93 |
| CD11c | 65 | 47 | 91 | 50 | 55 | 65 | nd | 66 |
| CD83 | 3 | 30 | 65 | 30 | nd | nd | 31 | 35 |
| Antigen-presenting cells | ||||||||
| CD40 | 53 | 88 | 98 | 96 | 81 | nd | 68 | 94 |
| CD80 | 12 | 82 | 72 | 84 | 56 | 33 | 33 | 68 |
| CD86 | 47 | 87 | 81 | 77 | 59 | 76 | 49 | 77 |
Percentages of positive cells were determined by 2- or 3-color flow cytometry after 6-day culture with IL-3 (10 ng/mL) or GM-CSF (500 U/mL).
nd indicates not done.
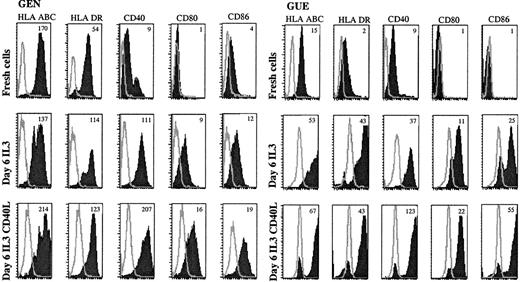
Leukemic cells up-regulate costimulatory molecules upon IL-3 and CD40L activation.
Expression of APC-related molecules on fresh and cultured leukemic cells were shown in patient GEN and GUE CD40, CD80, and CD86 that were either not expressed or were present at a low level on fresh cells (upper panels) were up-regulated after a 6-day culture with IL-3 (middle panels). HLA molecules that were already present on fresh cells were also up-regulated. Addition of CD40L-transfected L cells during the last 48 hours of culture further increased the expression of these molecules (lower panels). Open curves show isotype control; shaded curves show specific staining. Results are representative of 5 experiments of culture with IL-3. Mean fluorescence intensity ratios between specific mAb and isotypic control are indicated in the plot.
Leukemic cells up-regulate costimulatory molecules upon IL-3 and CD40L activation.
Expression of APC-related molecules on fresh and cultured leukemic cells were shown in patient GEN and GUE CD40, CD80, and CD86 that were either not expressed or were present at a low level on fresh cells (upper panels) were up-regulated after a 6-day culture with IL-3 (middle panels). HLA molecules that were already present on fresh cells were also up-regulated. Addition of CD40L-transfected L cells during the last 48 hours of culture further increased the expression of these molecules (lower panels). Open curves show isotype control; shaded curves show specific staining. Results are representative of 5 experiments of culture with IL-3. Mean fluorescence intensity ratios between specific mAb and isotypic control are indicated in the plot.
DCs differentiated from leukemic cells stimulate allogeneic naive T cells and induce their Th2 polarization
In the absence of activation, tumor cells from patients GEN and GUE did not induce the proliferation of naive T cells, whereas cells from patient CAT did. The ability of IL-3–differentiated leukemic DC to induce a primary allogeneic response was analyzed in MLC. In the 5 cases analyzed, a strong (8000 to 40 000 cpm) proliferation of cord blood–purified naive T cells was induced, similar to the proliferation obtained with mo-DCs (Figure 5, upper panels).
Leukemic cells stimulate naive T cells and induce Th2 polarization.
CD45RA+CD4+ allogeneic naive T-lymphocyte response to increasing numbers of irradiated leukemic cells or mo-DCs was measured after a 6-day MLC by 18-hour [3H]thymidine incorporation (upper panels). Fresh tumor cells (closed diamonds) were capable (CAT) or not (GEN and GUE) of stimulating naive CD4+ T-lymphocyte proliferation. Conversely, in all 5 cases analyzed, the day-6 IL-3–cultured tumor cells (open squares) were able to activate the proliferation of allogeneic naive T cells, with the same potency as mo-DCs (stars). IFN-γ and IL-4 production in T cells primed by stimulation with IL-3–cultured leukemic pDCs or mo-DCs was analyzed by flow cytometry (lower panels). The percentages of IL-4– or IFN-γ–producing cells are indicated in the plots and show the Th2 polarizing capacity of leukemic DCs, whereas mo-DCs induced a Th1 polarization.
Leukemic cells stimulate naive T cells and induce Th2 polarization.
CD45RA+CD4+ allogeneic naive T-lymphocyte response to increasing numbers of irradiated leukemic cells or mo-DCs was measured after a 6-day MLC by 18-hour [3H]thymidine incorporation (upper panels). Fresh tumor cells (closed diamonds) were capable (CAT) or not (GEN and GUE) of stimulating naive CD4+ T-lymphocyte proliferation. Conversely, in all 5 cases analyzed, the day-6 IL-3–cultured tumor cells (open squares) were able to activate the proliferation of allogeneic naive T cells, with the same potency as mo-DCs (stars). IFN-γ and IL-4 production in T cells primed by stimulation with IL-3–cultured leukemic pDCs or mo-DCs was analyzed by flow cytometry (lower panels). The percentages of IL-4– or IFN-γ–producing cells are indicated in the plots and show the Th2 polarizing capacity of leukemic DCs, whereas mo-DCs induced a Th1 polarization.
Given the reported ability of normal IL-3–matured pDCs to promote the differentiation of Th2 cells,15 19 we next examined the T-cell polarizing capacity of IL-3–differentiated leukemic DCs. After a 6-day MLC, the cytokine production profile was analyzed (Figure 5, lower panels). In 4 of 4 cases, the naive cord blood cells primed with IL-3–differentiated leukemic DCs were mainly IL-4–producing cells (9%-21%), whereas only a few IFN-γ–producing cells were detected (4%-6%), demonstrating a Th2 polarization. In contrast, 20% of the naive T cells primed with mo-DCs became IFN-γ–producing cells, and only 3% produced IL-4. The unprimed naive T cells activated with phorbol myristate acetate and ionomycin failed to produce either IFN-γ or IL-4 (not shown).
Leukemic cells produce IFN-α
Normal pDCs are also characterized by their ability to secrete IFN-α in response to virus.15 20 Thus, 4 patients (CAD, LAI, GEN, and GUE) were studied for the ability of either enriched tumor cells or FACS-sorted CD56+ and CD56−cells to produce IFN-α in response to inactivated influenza virus. After a 24-hour incubation with the virus, but not upon CD40 signaling (not shown), significant levels of IFN-α were measured in the supernatants of the culture from patients GEN and GUE that were purified from peripheral blood and lymph nodes, respectively, while no IFN-α production was detectable in those from bone marrow samples of CAD and LAI. To exclude the possible contamination of enriched tumor cells by residual normal pDCs, we FACS-sorted CD56+ and CD56− cells (LAI, GEN, and GUE). Both the CD56+ subset (devoid of normal pDCs) and CD56−subset from patients GEN and GUE produced IFN-α in response to the virus. Of note, (1) CD56 was not expressed homogeneously by leukemic cells (Table 1) and (2) we have shown that CD56 could be modulated in response to cytokines (Table3). Thus, our interpretation is that most of the enriched cells were indeed leukemic. Higher levels of IFN-α were produced by pDCs from healthy volunteers (24 990 IU/mL; Table 3) compared with leukemic cells, which could be due to their malignant status, their different organ origin, and/or an alteration of their reactivity by cryopreservation.
Leukemic cells produce IFN-α when stimulated with influenza virus
| Patients . | IFN-α (U/mL) . | |||||
|---|---|---|---|---|---|---|
| Enriched tumor cells . | Sorted cells . | |||||
| CD56+ . | CD56− . | |||||
| Medium . | Influenza virus . | Medium . | Influenza virus . | Medium . | Influenza virus . | |
| CAD (bone marrow) | < 0.6 | 3 | nd | nd | nd | nd |
| LAI (bone marrow) | nd | nd | < 0.6 | 30 | < 0.6 | < 0.6 |
| GEN (blood) | ||||||
| Experiment 1 | 9 | 1302 | nd | nd | nd | nd |
| Experiment 2 | < 0.6 | 1695 | < 0.6 | 912 | < 0.6 | 2026 |
| GUE (lymph node) | ||||||
| Experiment 1 | 6 | 1099 | nd | nd | nd | nd |
| Experiment 2 | nd | nd | < 0.6 | 1146 | < 0.6 | 1235 |
| Patients . | IFN-α (U/mL) . | |||||
|---|---|---|---|---|---|---|
| Enriched tumor cells . | Sorted cells . | |||||
| CD56+ . | CD56− . | |||||
| Medium . | Influenza virus . | Medium . | Influenza virus . | Medium . | Influenza virus . | |
| CAD (bone marrow) | < 0.6 | 3 | nd | nd | nd | nd |
| LAI (bone marrow) | nd | nd | < 0.6 | 30 | < 0.6 | < 0.6 |
| GEN (blood) | ||||||
| Experiment 1 | 9 | 1302 | nd | nd | nd | nd |
| Experiment 2 | < 0.6 | 1695 | < 0.6 | 912 | < 0.6 | 2026 |
| GUE (lymph node) | ||||||
| Experiment 1 | 6 | 1099 | nd | nd | nd | nd |
| Experiment 2 | nd | nd | < 0.6 | 1146 | < 0.6 | 1235 |
The production of IFN-α by enriched and sorted tumor cells was measured by ELISA after a 24-hour stimulation with influenza virus. In parallel, sorted CD11c− pDCs and CD11c+ DCs were found to produce 24 990 and 57 IU/mL IFN-α, respectively, in response to virus, while in the absence of virus the values were below 0.6 IU/mL.
nd indicates not done.
Discussion
In the present work, we analyzed unusual tumor cells in 7 patients. In light of recent knowledge from the literature, we confirmed that they belong to the pDC lineage, because they (1) produce IFN-α upon virus stimulation, (2) express markers characteristics of this DC subset, (3) survive and differentiate into functional DCs in response to IL-3, and (4) induce Th2 polarization upon IL-3 activation.
The link between those tumor cells and pDCs has first been postulated according to their unique phenotype: conventional T, B, and myeloid lineage markers are absent, except CD7 or CD2 in some cases. T-cell and B-cell receptors are in germline configuration, and no myeloperoxidase enzymes are observed (not shown). Expression of costimulatory molecules such as CD40 or CD86 in several cases is evocative of an APC. Indeed, expression of CD4, HLA-DR, CD123, ILT3, and CD45RA but absence of CD45R0 and CD11c is highly reminiscent of the phenotype of pDCs.8,13,14 Moreover, despite the expression of some monocytic antigens like CD36 and CD68 (also reported on blood CD11c− DCs29), these cells do not differentiate into monocytes or macrophages in presence of GM-CSF. The link with pDCs is also strongly emphasized by the demonstration of characteristic functional properties of this cell type. In contrast to the lack of IFN-α secretion by bone marrow malignant cells from 2 patients, tumor cells from peripheral blood and lymph nodes of 2 other patients produce IFN-α in response to viruses, a distinct feature of pDCs and as such referred to as “natural interferon-producing cells.”15,20,30,31 Like normal pDCs,13,14malignant cells are partly rescued from spontaneous apoptosis by IL-3 or GM-CSF. Conversely, they differ from normal pDCs by their resistance to IL-4–induced apoptosis,19 suggesting that those leukemic cells might be less sensitive to apoptotic signals. They also form aggregates in the presence of IL-3 and acquire typical cytoplasmic dendrites upon CD40 signaling.8,13 Spontaneously, they are not or only poorly stimulatory for naive cord blood allogeneic T lymphocytes, but upon IL-3 treatment, the cells from all patients studied became powerful inducers of T-cell proliferation, as reported for normal pDCs in the same culture condition.8,13 This newly acquired capacity parallels the up-regulation of markers related to antigen presentation and costimulation CD80, CD86, CD40, HLA-I and -II. It should be pointed out that this same mature DC phenotype, together with the acquisition of CD1a and CD1c, is also observed following culture in GM-CSF. Furthermore, IL-3–treated malignant DCs induce a Th2 polarization of the immune response, as reported for their normal counterpart.19,32 It would be important to determine whether, as recently reported for normal pDCs,21 22 virus-treated leukemic cells could also induce a Th1 polarization. Considering the APC function of the tumor cells and the potential of their normal equivalent to prime either Th1 or Th2 response, it will be important to decipher the mechanisms responsible for the lack of tumor rejection.
Lymphoid-restricted progenitors exist in human bone marrow, thymus, and fetal liver, the most immature one being able to differentiate into T, B, and NK cells and DCs.10,11,33,34 However, those progenitors express CD34, which is not the case for leukemic pDCs. The capacity of the malignant cells to differentiate into T, B, or NK cells has to be tested, but at present we have shown that they are not precursors of monocytes or macrophages. As for pDCs,13,14culture in GM-CSF or IL-3 induced CD13, CD33, and CD11c myeloid antigens, but the interpretation with regard to the myeloid lineage is not clear at present.8,35 Due to the expression of CD56 on malignant cells, an NK origin has been evoked in many reports despite the negativity of most NK differentiation markers and the absence of azurophilic granulations. In fact, although classical for NK cells, CD56 has often been described in malignant pathologies outside this lineage, such as in myeloma36 and acute myeloid leukemia.37,38 Because of the positivity of granzyme B, further functional assays are ongoing to assess the NK potential of those leukemic cells. The down-regulation of CD56 expression observed after IL-3 activation in some cases suggests that the precursor involved in the leukemic transformation might be more immature than the blood pDCs. We also consistently found genes associated with early T or B precursors such as pre-Tα and lambda-like 14.1. In fact, these observations suggest that these leukemic cells, like pDCs, share common precursors with NK, T, and B cells. Indeed, mice lacking flt3 ligand have been shown to have a profound deficiency in both DCs and NK cells, in addition to myeloid and lymphoid progenitors.39Leukemic cells being in an immature stage is also supported by their blastic morphology and the low expression of CD45 by analogy to normal or leukemic cells from other hematopoietic lineages.40Based on the latter criteria, the leukemic population appears homogeneous; however, a maturation asynchronism may be suspected. Indeed, in some patients, in the absence of activation one can observe low levels of CD1c, CD86, or CD40 and stimulation of naive T lymphocytes that is in favor of a more advanced degree of differentiation for part of the tumor cells. Noteworthy, in vivo as in vitro, the blockade of maturation might not be absolute, contrary to most hematopoietic malignancies.
In conclusion, we demonstrate that a new hematologic entity has been identified involving a malignant equivalent of plasmacytoid DCs. The CD4+, CD56+, CD3−, CD13−, CD33−, CD19− profile that is defined with commonly used antibodies in routine phenotyping is highly evocative of this pathology. Because malignant cells usually mimic their normal equivalent, this disease is of particular interest for the understanding of pDC behavior in vivo. Importantly, these cells can be obtained in large amounts, which might be precious for prospective studies of pDCs.
We thank Dr Xavier Ronot for help with contrast phase microscopy and Dr David Jarrossay for anti-ILT3 mAb. We thank Myriam Brachet, Patricia Cheron, Agnès Colomer, Ghislaine Del Vecchio, Richard Di Schiena, Michel Drouin, Jean-Paul Molens, Christine Vallet, and Mireille Favre for technical assistance. We thank Drs Dominique Masson and Agnès Moine for HLA typing of the cells. We thank Marina Cella, Marco Colonna, and Giorgio Trinchieri for advice and helpful discussions. We thank Christophe Caux for critically reading the manuscript and Isabelle Durand for FACS sorting.
M.C.J. and J.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laurence Chaperot or Marie-Christine Jacob, Dept of Research and Development, EFS Rhône-Alpes Grenoble, BP35, F38701 La Tronche Cedex, France; e-mail:laurence.chaperot@efs.sante.fr ormarie-christine.jacob@efsrhonealpes.org.

![Fig. 5. Leukemic cells stimulate naive T cells and induce Th2 polarization. / CD45RA+CD4+ allogeneic naive T-lymphocyte response to increasing numbers of irradiated leukemic cells or mo-DCs was measured after a 6-day MLC by 18-hour [3H]thymidine incorporation (upper panels). Fresh tumor cells (closed diamonds) were capable (CAT) or not (GEN and GUE) of stimulating naive CD4+ T-lymphocyte proliferation. Conversely, in all 5 cases analyzed, the day-6 IL-3–cultured tumor cells (open squares) were able to activate the proliferation of allogeneic naive T cells, with the same potency as mo-DCs (stars). IFN-γ and IL-4 production in T cells primed by stimulation with IL-3–cultured leukemic pDCs or mo-DCs was analyzed by flow cytometry (lower panels). The percentages of IL-4– or IFN-γ–producing cells are indicated in the plots and show the Th2 polarizing capacity of leukemic DCs, whereas mo-DCs induced a Th1 polarization.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/10/10.1182_blood.v97.10.3210/6/m_h81011038005.jpeg?Expires=1769144948&Signature=tcho7hq0aAVcz7OnkSydh4-dqSXPIuyVZrIGkA5tViKn9071fxza~DMHwsrnWq6O8o4Uc3FZIG7~BviMWST8oO4wSwI4F67YHO56L5YaC4BN~bFyxhPDsy8oJ0zVIRVQUeUymolGTFXsr9lgsSHwrpadZk8s9RuTxYFh3xvPW41jiseABSUOuwz~hfSAvyXzdTiE-fE2tyyOYgB~9rteJa2l8H04ujptnVaah5GG54Ufrvv14AKtpHBUmvjVYUUvNN5Cy7UqrjSMa50b-GV61ExGbPsoB9ddetZ4VjUOn~Sb6BffXwrppjFfaDoVygWr0AMXv-Zv~9uLOXS2e7eTcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal