Abstract
In the absence of arterial recanalization, thrombolytic agents induce a dose-related extension of focal cerebral ischemic injury (FII) in experimental animals. However, FII is smaller in mice lacking α2-antiplasmin (α2-AP), the physiologic inhibitor of plasmin, suggesting its depletion might reduce FII in the absence of reperfusion. Therefore, the effect of human plasmin (Pli), human miniplasmin (mPli), and an Fab fragment neutralizing murine α2-AP (Fab-4H9) on FII after middle cerebral artery (MCA) ligation was studied in mice and in hamsters. In BALB/c mice, the median FII after 24 hours was 28 μL (range, 20-34) (n = 10) with saline and 23 μL (range, 17-26) (n = 9) with a single bolus of 0.07 mg Pli, given after MCA ligation (P = .010), which reduced α2-AP to 44% and fibrinogen from 0.75 to 0.44 g/L. FII was 20 μL (range, 13-26) (n = 6, P = .025) with 0.2 mg mPli and was 24 μL (range, 20-27) (n = 6,P = .020) with 1.7 mg Fab-4H9. Neuronal atrophy and reduction of laminin immunoreactivity were comparably observed in the infarct area after saline and Pli. In hamsters, a single bolus injection of 1 mg Pli, after MCA ligation, depleted α2-AP and fibrinogen and reduced FII at 24 hours from 20 μL (range, 9.9-38) (n = 6) to 7.0 μL (range, 0.44-31) (n = 7,P = .032). Thus, reduction of circulating α2-AP, with a single bolus of plasmin or of a neutralizing antibody fragment, significantly reduced FII after MCA ligation in mouse and hamster models, suggesting that, provided these observations can be extrapolated to human beings, transient depletion of circulating α2-AP might reduce ischemic stroke in the absence of reperfusion.
Introduction
Thrombolytic therapy of ischemic stroke with alteplase improves clinical outcome at 3 months.1,2However, alteplase infusion increased infarct volume after focal cerebral ischemic injury (FII) induced by occlusion of the middle cerebral artery (MCA) in mice,3 possibly as a result of plasmin-mediated degradation of laminin, a component of basal lamina playing a role in neuronal survival.4 The deleterious effects of alteplase on FII in mice were confirmed and extended using alteplase, streptokinase, or staphylokinase in hamsters,5indicating that this phenomenon is neither species specific nor agent specific and that it occurs in the absence of systemic fibrinogen breakdown. Thus, one could hypothesize that thrombolytic therapy for ischemic stroke might cause infarct expansion in cases with persistent cerebral arterial occlusion and indeed be harmful to a subgroup of patients. This would, however, imply that the clinical benefits of alteplase in stroke were due to recanalization (which was not measured) and increases in infarct volume were due to a lack of recanalization. The development of specific conjunctive strategies to counteract the potential deleterious effects of thrombolytic agents or of alternative nonthrombolytic treatments appears to be warranted.
FII induced by MCA occlusion is increased in mice with a genetic deficiency of plasminogen, a substrate of thrombolytic agents6 and, conversely, reduced in mice with genetic deficiency of α2-antiplasmin (α2-AP). The α2-AP is the physiologic inhibitor of plasmin; it reacts extremely rapidly with plasmin, and its congenital deficiency causes a mild bleeding tendency.7 Furthermore, FII is increased after infusion of human α2-AP in α2-AP–deficient mice and again reduced to baseline by in vivo immunoneutralization of the infused human α2-AP.6 These results indicate that thrombolytic agents expand FII via another pathway than plasmin generation and suggest that depletion of circulating α2-AP might reduce FII.
In the present study, the effects on FII of reduction of circulating α2-AP by infusion of human plasmin (Pli) or the Fab fragment of a monoclonal antibody neutralizing murine α2-AP were tested in mice. Significant reduction of infarction size was observed with a single bolus injection of Pli or the Fab fragment, suggesting that either agent might constitute an alternative to thrombolytic therapy of FII. The effect of plasmin on FII reduction was confirmed in a hamster model of MCA ligation, demonstrating that the effect was not species specific. No differences in immunostaining for laminin or fibrin(ogen) in the injured region were observed in association with plasmin infusion.
Materials and methods
Mice and hamsters
BALB/c, C57Bl/6, and SV129 mice, bred at the Specific Pathogen Free Facility of the Molecular Cardiovascular Medicine Group, Campus Gasthuisberg, KU Leuven, Belgium, weighing 25 to 30 g, and Pfd Gold hamsters, bred at the KU Leuven laboratory animal facility, weighing 100 to 120 g, were used.
Human plasmin and human miniplasmin
Human plasminogen was prepared from fresh frozen human blood bank plasma, essentially as described previously.8Briefly, human plasma (6 L) was absorbed batchwise with lysine-Sepharose, washed, and eluted with 0.05 M 6-aminohexanoic acid in phosphate-buffered saline (PBS). The pool was concentrated and gel filtered on Ultrogel AcA44 equilibrated with PBS, yielding approximately 590 mg protein. Sodium dodecyl sulfate gel electrophoresis revealed a single main band (> 95% of total protein).
Pli was prepared by adding lysine-Sepharose to human plasminogen (200 mg) solution. The mixture was stirred, washed, and resuspended in 0.1 M NaH2PO4 buffer, pH 7.4, and urokinase (1 μM final) was added overnight at 4°C. The gel was then washed to remove urokinase, eluted with 0.1 M NaH2PO4 buffer containing 0.05 M 6-aminohexanoic acid, glycerol was added to 10%, and the pool was dialyzed at 4°C against 0.1 M NaH2PO4 buffer containing 10% glycerol. The final recovery was 25 mL solution with a protein concentration of 2.8 mg/mL and an active plasmin concentration of 33 μM, comprising less than 1% residual plasminogen.
Human miniplasminogen was prepared by digestion of purified human plasminogen with insolubilized elastase, followed by gel filtration and chromatography on lysine-Sepharose, as described elsewhere.9 Briefly, purified human plasminogen (900 mg) was dissolved in 0.1 M NH4HCO3, pH 7.8, containing 700 KIU aprotinin per milliliter. Insolubilized elastase (7.5 g wet gel) was added and the mixture stirred for 24 hours at room temperature. After removal of the elastase gel by filtration, the mixture was gel-filtered on a Sephadex G75 equilibrated with NH4HCO3, pH 7.8, and passed through a lysine-Sepharose column.
The mPli was prepared by digestion of 650 μL miniplasminogen (1.1 mg/mL) dissolved in 0.05 M phosphate buffer, pH 7.4, containing 10% glycerol with insolubilized urokinase (1% final concentration) for 5 minutes at 37°C. The final solution had a protein concentration of 0.9 mg/mL and an active mPli concentration of 18.5 μM.
Fab fragments of murine monoclonal antibodies neutralizing murine α2-antiplasmin
Monoclonal antibodies against murine α2-AP were produced essentially as previously described10,11 but using mice with inactivated α2-AP genes.12The mice were immunized by subcutaneous injection of 50 μg murine α2-AP purified as described elsewhere.13Spleen cells were isolated and fused with P3X63-Ag8.653 myeloma cells. Hybridomas secreting monoclonal antibodies were screened for neutralizing activity against murine α2-AP. Positive clones were used for production of ascitic fluid in pristane-primed mice. The monoclonal antibodies were purified from ascites by affinity chromatography on protein A–Sepharose14 and again tested for neutralizing activity against purified murine and human α2-AP. A total of 63 hybridomas produced antibodies against murine α2-AP, of which 14 neutralized murine α2-AP and 4 also neutralized human α2-AP. Following purification, monoclonal antibody MAP-4H9 inhibited 60% of murine α2-AP in equimolar mixtures.
Fab fragments were produced from 1 mg/mL solutions of MAP-4H9 by digestion with 1% (wt/wt) papain in the presence of 50 mM cysteine and 1 mM ethylenediaminetetraacetic acid for 2 hours at 37°C. The reaction was arrested by addition of 75 mM iodoacetamide. After dialysis, the mixture was passed over a protein A–Sepharose column, which binds an Fc fragment and uncleaved immunoglobulin G (IgG). From 21 mg MAP-4H9, 14 mg Fab-4H9 was obtained, which neutralized murine α2-AP for 50% in equimolar mixtures.
The α2-antiplasmin in plasma
Levels of α2-antiplasmin in murine or hamster plasma were measured by a chromogenic substrate assay based on its rapid inhibition of plasmin.15 Briefly, 10 μL murine or hamster plasma (diluted 1:10 in 0.05 M NaH2PO4buffer, pH 7.4, containing 0.01% Tween 20) was mixed at 37°C with 420 μL 0.05 M Tris-HCl, 0.1 M NaCl buffer, pH 7.4, containing 0.01% Tween 20, and with 20 μL 0.125 μM Pli (final concentration 5 nM). After 10 seconds of incubation, 50 μL 3 mM S2403 (Chromogenics, Antwerp, Belgium) was added and the change in absorbance measured at 405 nm. The change in absorbance is approximately 0.18 per minute-1 with buffer and 0.09 per minute-1 with pooled murine or hamster plasma, which was used for the construction of a calibration curve.
Physiologic parameters
Animal experiments were conducted according to the guiding principles of the American Physiological Society. Hemodynamic parameters were measured in mice and hamsters under anesthesia with 1 mL/kg of a mixture of ketamine (75 mg/mL; Apharmo, Arnhem, The Netherlands) and xylazine (5 mg/mL; Bayer, Leverkusen, Germany).
Blood pressure was measured by insertion of a pressure transducer (SPR-671, Millar Instruments, Houston, TX) in the left carotid artery before and during 15 minutes after injection of 0.2 mg Pli in mice or 1 mg Pli in hamsters. Blood pH, Pco2, Po2, and hemoglobin (Hb) concentration were measured using a blood gas analyzer (ABL4, Radiometer, Copenhagen, Denmark) before and 15 minutes after injection of saline or 0.2 mg Pli in mice or 1 mg Pli in hamsters.
Murine cerebral ischemic infarction model
FII was produced by persistent occlusion of the MCA, as described in detail elsewhere.6 16 Anesthesia was routinely performed with 1 mL/kg of a mixture of ketamine (75 mg/mL; Apharmo) and xylazine (5 mg/mL; Bayer). Alternatively, to establish that these drugs did not affect FII size, anesthesia was performed with inhalation of 2% isoflurane in oxygen. During the surgery and until recovery from anesthesia, the animals were kept on a heated pad that was maintained at 37 ± 0.5°C.
The following experimental groups of BALB/c mice were studied: (1) Pli as a bolus intravenous dose of 0.07 mg or 0.2 mg either 15 minutes before or 15 minutes after ligation of the MCA; (2) mPli as a bolus of 0.1 mg or 0.2 mg 15 minutes before ligation of the MCA; or (3) Fab-4H9 as a bolus of 1 mg given 15 minutes before or 1.7 mg given 15 minutes after MCA ligation. Each experimental group was matched by a saline control group of equal size.
To determine whether plasmin activity was necessary to affect FII, 0.2 mg Pli, inactivated with 1500 KIU aprotinin (Trasylol, Bayer, Leverkusen, Germany) that had less than 1% residual plasmin activity, was given after 15 minutes of ligation of the MCA and compared with groups receiving Pli or aprotinin alone. Pli at a dose of 0.07 mg was given to BALB/c, C57Bl/6, or SV129 mice to evaluate the effect of genetic background.
The animals were allowed to recover and were then returned to their cages. After 24 hours, the mice were anesthetized and a blood sample was taken by heart puncture to determine α2-AP and fibrinogen levels. The animals were then killed with an overdose of Nembutal (500 mg/kg; Abbott Laboratories, North Chicago, IL), and decapitated. The brain was removed and placed in a matrix for sectioning in 1 mm segments. The sections were immersed in 2% 2,3,5-triphenyltetrazolium chloride (TTC) in saline,17incubated for 30 minutes at 37°C, and placed in 4% formalin in PBS. The sections were photographed and subjected to planimetry. Infarct area (I), ipsilateral viable area (A), and contralateral viable area (C) were directly measured by planimetry on sequential sections in groups of mice given either saline or 0.2 mg Pli. Volumes were determined as the sums of the areas multiplied by the thickness of the sections. Necrotic volume (N) was derived as N = C−A and edema volume (E) as E = I−N.18
Immunohistochemical analysis
Immunostaining for laminin and for fibrinogen was carried out in mice given saline or 0.2 mg Pli 15 minutes after MCA occlusion, followed by perfusion with 1% paraformaldehyde in PBS at 24 hours. As a control, mice with stereotactic injection (Model 900, David Kopf Instruments, Tujunga, CA) of 1.5 nM kainic acid in the CA1 region in the hippocampus,19 followed by perfusion with 1% paraformaldehyde in PBS at 24 hours, were used. The coordinates of the injection were as follows: bregma −2.0 mm, mediolateral 1.3 mm, dorsoventral 1.5 mm.
Brains were removed, embedded in paraffin, and sectioned in 5 μm slices. For immunostaining of laminin, sections were incubated with a primary rabbit antilaminin antibody (Sigma Chemical, St. Louis, MO) diluted 1:50, followed by a peroxidase-labeled swine antirabbit IgG (Dako, Glostrup, Denmark) diluted 1:50. Fibrin(ogen) was stained via a 3-step procedure with a goat antimouse fibrinogen (Nordic Immunologies, Tilburg, The Netherlands) diluted 1:200, followed by rabbit antigoat IgG (Dako) diluted 1:100 and goat peroxidase-antiperoxidase complex (Dako) diluted 1:50. Peroxidase activity was developed by incubating sections in 0.05 M Tris-HCl buffer, pH 7.0, containing 0.06% 3,3′-diaminobenzidine and 0.01% H2O2 with enhancement of laminin staining by 7 mM ammonium nickel(II) sulfate. Counterstaining with hematoxylin was used to identify neuronal atrophy.
Hamster FII model
FII was produced by a combination of persistent occlusion of the left MCA and the left common carotid artery (CCA) and a transient occlusion of the right CCA, as described in detail elsewhere.5 The MCA was occluded by ligation with 10-0 nylon thread (Ethylon, Neuilly, France), after which the artery was transsected distally and the temporal muscle and skin were sutured back in place. A vertical incision was then made above the sternum, and both CCAs were exposed. The left CCA was ligated with 2 7-0 nylon threads and transsected in between. The right CCA was occluded with an arterial clamp during 30 minutes and then released. During the clamping, an incision was made in the right groin, and the femoral vein was cannulated with a 2FG catheter for study drug administration. Finally, the skin wound was closed. Hamsters were given a saline or 1 mg Pli bolus injection 15 minutes after release of the right CCA occlusion. The animals were then allowed to recover and were returned to their cages. After 24 hours, the animals were killed with an overdose of Nembutal (500 mg/kg; Abbott) and decapitated. The brain was removed and processed for planimetry as described above. One animal in the saline group died during the 24 hours observation period and was omitted from the analysis.
Statistical analysis
The data are represented as median and range (for cerebral infarct size) or as mean ± SEM (for coagulation and hemodynamic parameters) of the number of determinations. The significance of differences was determined using Mann-Whitney test or Student t test, as appropriate.
Results
Effects of injection of human plasmin on hemodynamic variables
Bolus intravenous injection of 0.2 mg Pli in mice or 1 mg Pli in hamsters did not affect arterial pH, Pco2, Po2, Hb concentration, or arterial blood pressure (Table 1).
Hemodynamic parameters after intravenous bolus injection in mice or hamsters
| Species . | Saline group . | Pli group . | ||
|---|---|---|---|---|
| Baseline . | 15 min after injection . | Baseline . | 15 min after injection . | |
| Mouse | ||||
| Parameter | ||||
| pH | 7.2 ± 0.02 | 7.2 ± 0.02 | 7.2 ± 0.02 | 7.2 ± 0.01 |
| pco2 | 53 ± 2.7 | 55 ± 3.8 | 53 ± 2.7 | 53 ± 1.1 |
| po2 | 100 ± 9.2 | 110 ± 9.0 | 100 ± 9.2 | 110 ± 7.1 |
| Hb | 18 ± 0.2 | 18 ± 0.2 | 18 ± 0.2 | 19 ± 0.2 |
| MAP | 90 ± 3.4 | 90 ± 6.9 | 90 ± 3.4 | 90 ± 3.7 |
| Hamster | ||||
| Parameter | ||||
| pH | 7.3 ± 0.03 | 7.3 ± 0.02 | 7.3 ± 0.03 | 7.2 ± 0.05 |
| pco2 | 52 ± 2.8 | 52 ± 5.3 | 51 ± 3.6 | 54 ± 2.6 |
| po2 | 95 ± 8.4 | 98 ± 4.0 | 110 ± 11 | 120 ± 16 |
| Hb | 22 ± 1.5 | 21 ± 2.2 | 22 ± 0.3 | 22 ± 0.7 |
| MAP | 98 ± 6.7 | 98 ± 8.1 | 100 ± 3.7 | 100 ± 5.0 |
| Species . | Saline group . | Pli group . | ||
|---|---|---|---|---|
| Baseline . | 15 min after injection . | Baseline . | 15 min after injection . | |
| Mouse | ||||
| Parameter | ||||
| pH | 7.2 ± 0.02 | 7.2 ± 0.02 | 7.2 ± 0.02 | 7.2 ± 0.01 |
| pco2 | 53 ± 2.7 | 55 ± 3.8 | 53 ± 2.7 | 53 ± 1.1 |
| po2 | 100 ± 9.2 | 110 ± 9.0 | 100 ± 9.2 | 110 ± 7.1 |
| Hb | 18 ± 0.2 | 18 ± 0.2 | 18 ± 0.2 | 19 ± 0.2 |
| MAP | 90 ± 3.4 | 90 ± 6.9 | 90 ± 3.4 | 90 ± 3.7 |
| Hamster | ||||
| Parameter | ||||
| pH | 7.3 ± 0.03 | 7.3 ± 0.02 | 7.3 ± 0.03 | 7.2 ± 0.05 |
| pco2 | 52 ± 2.8 | 52 ± 5.3 | 51 ± 3.6 | 54 ± 2.6 |
| po2 | 95 ± 8.4 | 98 ± 4.0 | 110 ± 11 | 120 ± 16 |
| Hb | 22 ± 1.5 | 21 ± 2.2 | 22 ± 0.3 | 22 ± 0.7 |
| MAP | 98 ± 6.7 | 98 ± 8.1 | 100 ± 3.7 | 100 ± 5.0 |
The data represent mean ± SEM 15 minutes after injection in a group of 5 animals. No significant differences existed between the control group, saline group, or Pli group (0.2 mg in mice, 1 mg in hamsters) between before and after Pli treatment.
Pli indicates human plasmin; Hb, hemoglobin; MAP, mean arterial pressure.
Effects of human plasmin or Fab-4h9 on α2-antiplasmin and fibrinogen levels
The effects of intravenous bolus injection of the study compounds in BALB/c mice on plasma α2-AP and fibrinogen levels are summarized in Table 2. In all experimental groups, α2-AP levels had normalized within 24 hours, whereas levels of fibrinogen, an acute phase reactant, had increased to 1.2 to 1.9 g/L. Thus, α2-AP depletion was transient and only persisted during the first hours after the single intravenous bolus injection of the depleting agent.
Effect of human plasmin, miniplasmin, or Fab fragment of a monoclonal antibody neutralizing murine α2-antiplasmin (α2-AP) (Fab-4H9) on plasma α2-AP and fibrinogen levels in mice or hamsters
| Species . | Compound . | Dose, mg . | α2-AP, % . | Fibrinogen, g/L . | ||
|---|---|---|---|---|---|---|
| 15 min . | 24 hr . | 15 min . | 24 hr . | |||
| Mouse | Saline | — | — | 120 ± 2 [51] | 0.75 ± 0.14 [5] | 1.0 ± 0.02 [36] |
| Pli | 0.07 | 44 ± 4 [3] | 110 ± 3 [15] | 0.44 ± 0.14 [6] | 1.6 ± 0.02 [6] | |
| 0.2 | 14 ± 4 [3] | 110 ± 10 [6] | 0.09 ± 0.0 [3] | 1.7 ± 0.11 [6] | ||
| mPli | 0.1 | 33 ± 7 [3] | 97 ± 9 [6] | 0.23 ± 0.02 [3] | 1.2 ± 0.06 [6] | |
| 0.2 | 15 ± 3 [6] | 110 ± 2 [6] | 0.22 ± 0.04 [6] | 1.6 ± 0.11 [6] | ||
| Fab-4H9 | 1 | 38 ± 8 [3] | 96 ± 2 [6] | 0.46 ± 0.01 [2] | 1.3 ± 0.13 [6] | |
| 1.7 | — | 110 ± 2 [6] | — | 1.9 ± 0.14 [6] | ||
| Hamster | Saline | — | 100 ± 4 [3] | 130 ± 7 [6] | 0.33 ± 0.01 [3] | 1.7 ± 0.04 [6] |
| Pli | 1 | < 10 [5] | 140 ± 6 [5] | < 0.08 [5] | 1.7 ± 0.06 [6] | |
| Species . | Compound . | Dose, mg . | α2-AP, % . | Fibrinogen, g/L . | ||
|---|---|---|---|---|---|---|
| 15 min . | 24 hr . | 15 min . | 24 hr . | |||
| Mouse | Saline | — | — | 120 ± 2 [51] | 0.75 ± 0.14 [5] | 1.0 ± 0.02 [36] |
| Pli | 0.07 | 44 ± 4 [3] | 110 ± 3 [15] | 0.44 ± 0.14 [6] | 1.6 ± 0.02 [6] | |
| 0.2 | 14 ± 4 [3] | 110 ± 10 [6] | 0.09 ± 0.0 [3] | 1.7 ± 0.11 [6] | ||
| mPli | 0.1 | 33 ± 7 [3] | 97 ± 9 [6] | 0.23 ± 0.02 [3] | 1.2 ± 0.06 [6] | |
| 0.2 | 15 ± 3 [6] | 110 ± 2 [6] | 0.22 ± 0.04 [6] | 1.6 ± 0.11 [6] | ||
| Fab-4H9 | 1 | 38 ± 8 [3] | 96 ± 2 [6] | 0.46 ± 0.01 [2] | 1.3 ± 0.13 [6] | |
| 1.7 | — | 110 ± 2 [6] | — | 1.9 ± 0.14 [6] | ||
| Hamster | Saline | — | 100 ± 4 [3] | 130 ± 7 [6] | 0.33 ± 0.01 [3] | 1.7 ± 0.04 [6] |
| Pli | 1 | < 10 [5] | 140 ± 6 [5] | < 0.08 [5] | 1.7 ± 0.06 [6] | |
The data represent mean ± SEM of the number of observations, which are given in brackets.
Pli indicates human plasmin; mPli, human miniplasmin.
Injection of 1 mg Pli in hamsters depleted the α2-AP and fibrinogen levels, measured 15 minutes after the administration (Table2). After 24 hours, α2-AP levels were normalized and fibrinogen levels had increased to 1.7 g/L.
Effect of human plasmin on FII in mice
Ligation of the MCA induced FII with a volume of 28 μL (range, 20-34) (n = 10) in inbred BALB/c mice (Figure1A), 6.9 μL (range, 0.3-9.0) (n = 8) in SV129 mice, and 15 μL (range, 8.0-23) (n = 12) in C57BL/6 mice (Table 3). Injection of 0.07 mg Pli in BALB/c mice reduced FII to 23 μL (range, 17-26) (n = 9,P = .01 vs saline). Similar decreases were observed whether the Pli injection was given 15 minutes before or 15 minutes after ligation of the MCA. In C57Bl/6 mice, injection of 0.07 mg Pli reduced FII to 11μL (range, 1.8-16) (n = 12, P = 0.008 vs saline). In SV129 mice, with much smaller infarcts, injection of 0.07 mg Pli produced a similar 30% reduction of FII. Injection of 0.2 mg Pli in BALB/c mice either 15 minutes before or 15 minutes after MCA ligation induced a 20% reduction of FII (Table 3). Injection of 0.1 mg mPli in BALB/c mice reduced FII after MCA ligation from 26 μL (range, 20-27) (n = 5) to 24 μL (range, 18-25) (n = 6,P = .14), whereas 0.2 mg mPli reduced FII from 27 μL (range, 23-32) (n = 6) to 20 μL (range, 13-26) (n = 6,P = .025). Injection of a mixture of 0.2 mg Pli and 1500 KU aprotinin, with a residual plasmin activity of less than 1%, produced a FII volume of 28 μL (range, 25-30) (n = 6) indistinguishable from that of aprotinin alone, at 29 μL (range, 24-30) (n = 6), whereas the fibrinogen and α2-AP levels measured after 15 minutes (when excess aprotinin was cleared from the circulation) remained unchanged (data not shown).
TTC-stained brain sections.
(A) BALB/c mouse. (B) Pfd Gold hamster. The injured area is identified as the unstained (white) area surrounded by stained (brick red) area.
TTC-stained brain sections.
(A) BALB/c mouse. (B) Pfd Gold hamster. The injured area is identified as the unstained (white) area surrounded by stained (brick red) area.
Effect of human plasmin, miniplasmin, or the Fab fragment of a monoclonal antibody neutralizing murine α2-antiplasmin (Fab-4H9) on focal cerebral ischemic injury in mice and hamsters
| Species . | Compound . | Strain . | Dose, mg . | Injection before ligation . | Injection after ligation . | Combined . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline . | Pli . | P . | Saline . | Pli . | P . | Saline . | Pli . | P . | ||||
| Mouse | Pli | SV129 | 0.07 | 6.5 (0.34-7.8) [5] | 1.8 (0.70-7.8) [5] | .53 | 8.0 (3.0-9.0) [3] | 4.8 (3.4-5.3) [3] | .51 | 6.9 (0.34-9.0) [8] | 4.1 (0.70-7.8) [8] | .19 |
| C57Bl/6 | 0.07 | 15 (13-23) [5] | 10 (5.7-16) [6] | .068 | 15 (8.0-20) [7] | 11 (1.8-14) [6] | .063 | 15 (8.0-23) [12] | 11 (1.8-16) [12] | .0079 | ||
| BALB/c | 0.07 | 24 (20-29) [3] | 17 (17-20) [3] | .05 | 28 (23-34) [7] | 23 (22-26) [6] | .038 | 28 (20-34) [10] | 23 (17-26) [9] | .010 | ||
| 0.2 | 26 (24-27) [6] | 22 (20-24) [6] | .007 | 31 (27-32) [6] | 26 (24-28) [6] | .010 | 27 (24-32) [12] | 24 (20-28) [12] | .0027 | |||
| mPli | BALB/c | 0.1 | 26 (20-27) [6] | 24 (18-25) [6] | .14 | — | — | — | — | — | — | |
| 0.2 | 27 (23-32) [6] | 20 (13-26) [6] | .025 | — | — | — | — | — | — | |||
| Fab-4H9 | BALB/c | 1.0 | 26 (21-27) [6] | 22 (15-24) [6] | .055 | — | — | — | — | — | — | |
| 1.7 | — | — | — | 28 (25-30) [6] | 24 (20-27) [6] | .020 | — | — | — | |||
| Hamster | Pli | Pfd Gold | 1.0 | — | — | — | 20 (9.9-38) [6] | 7.0 (0.44-31) [7] | .032 | — | — | — |
| Species . | Compound . | Strain . | Dose, mg . | Injection before ligation . | Injection after ligation . | Combined . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline . | Pli . | P . | Saline . | Pli . | P . | Saline . | Pli . | P . | ||||
| Mouse | Pli | SV129 | 0.07 | 6.5 (0.34-7.8) [5] | 1.8 (0.70-7.8) [5] | .53 | 8.0 (3.0-9.0) [3] | 4.8 (3.4-5.3) [3] | .51 | 6.9 (0.34-9.0) [8] | 4.1 (0.70-7.8) [8] | .19 |
| C57Bl/6 | 0.07 | 15 (13-23) [5] | 10 (5.7-16) [6] | .068 | 15 (8.0-20) [7] | 11 (1.8-14) [6] | .063 | 15 (8.0-23) [12] | 11 (1.8-16) [12] | .0079 | ||
| BALB/c | 0.07 | 24 (20-29) [3] | 17 (17-20) [3] | .05 | 28 (23-34) [7] | 23 (22-26) [6] | .038 | 28 (20-34) [10] | 23 (17-26) [9] | .010 | ||
| 0.2 | 26 (24-27) [6] | 22 (20-24) [6] | .007 | 31 (27-32) [6] | 26 (24-28) [6] | .010 | 27 (24-32) [12] | 24 (20-28) [12] | .0027 | |||
| mPli | BALB/c | 0.1 | 26 (20-27) [6] | 24 (18-25) [6] | .14 | — | — | — | — | — | — | |
| 0.2 | 27 (23-32) [6] | 20 (13-26) [6] | .025 | — | — | — | — | — | — | |||
| Fab-4H9 | BALB/c | 1.0 | 26 (21-27) [6] | 22 (15-24) [6] | .055 | — | — | — | — | — | — | |
| 1.7 | — | — | — | 28 (25-30) [6] | 24 (20-27) [6] | .020 | — | — | — | |||
| Hamster | Pli | Pfd Gold | 1.0 | — | — | — | 20 (9.9-38) [6] | 7.0 (0.44-31) [7] | .032 | — | — | — |
The relative contribution of necrosis and edema to ischemic injury was determined by integration of the infarct areas; the necrotic areas, ie, the difference between the contralateral and the ipsilateral viable areas; and the edema areas, ie, the difference between infarct and necrotic areas (Table 3). In control BALB/c mice, the infarct volume of 31 μL (range, 27-32) consisted of 26 μL (range 22-27) necrotic volume and of 4.6 μL (range 1.4-5.8) edema, representing 14%. In mice given 0.2 mg Pli, the infarct volume of 26μL (range 24-28) (P = .01 vs saline) consisted of 22 μL (range 20-26) necrotic volumes (P = .025 vs saline) and of 3.4 μL (range 0.0-5.9) edema (P = .33 vs saline).
FII in BALB/c mice anesthetized with isoflurane was 28 μL (range 27-31) (n = 6) with saline and 26μL (range 20-27) (n = 6) with 0.2 mg Pli (P = .006).
Effect of Fab-4H9 on cerebral infarct size in mice
Injection of 1 mg of Fab-4H9 15 minutes before MCA ligation reduced FII from 26 μL (range 21-27) (n = 6) to 22 μL (range 15-24) (n = 6, P = .055) (Table 2). Injection of 1.7 mg Fab-4H9 15 minutes after MCA ligation reduced FII from 28 μL (range 25-30) (n = 6) to 24 μL (range 20-27) (n = 6,P = .032).
Effect of human plasmin on cerebral infarct size in hamsters
Injection of 1 mg Pli after release of the transient occlusion of the right CCA reduced FII from 20 μL (range 9.9-38) (n = 6) with saline (Figure 1B, Table 2) to 7.0 μL (range 0.44-31) (n = 7) with Pli (P = .032). In control hamsters, FII of 20 μL (range 9.9-38) contained 2.2 μL (range 0.0-5.6) edema, representing 12%. The reduction in FII was primarily due to reduction of necrosis from 16 μL (range 9.9-33) with saline to 4.6 μL (range 0.44-26) with Pli (P = .015) and not to differences in edema (Table4).
Relative contribution of necrosis and edema to focal cerebral ischemic injury
| Species . | FII . | Necrosis . | Edema . | Number . |
|---|---|---|---|---|
| Mouse | ||||
| Saline | 31 (27-32) | 26 (22-27) | 4.6 (1.4-5.8) | 6 |
| Pli (0.2 g) | 26 (24-28) | 22 (20-26) | 3.4 (0-5.9) | 6 |
| P | .01 | .025 | .33 | |
| Hamster | ||||
| Saline | 20 (9.9-38) | 16 (9.9-33) | 2.2 (0-5.6) | 6 |
| Pli (1 mg) | 7.1 (0.44-31) | 6.4 (0.44-26) | 0.34 (0-5.7) | 7 |
| P | .032 | .015 | .28 |
| Species . | FII . | Necrosis . | Edema . | Number . |
|---|---|---|---|---|
| Mouse | ||||
| Saline | 31 (27-32) | 26 (22-27) | 4.6 (1.4-5.8) | 6 |
| Pli (0.2 g) | 26 (24-28) | 22 (20-26) | 3.4 (0-5.9) | 6 |
| P | .01 | .025 | .33 | |
| Hamster | ||||
| Saline | 20 (9.9-38) | 16 (9.9-33) | 2.2 (0-5.6) | 6 |
| Pli (1 mg) | 7.1 (0.44-31) | 6.4 (0.44-26) | 0.34 (0-5.7) | 7 |
| P | .032 | .015 | .28 |
Immunohistochemical staining
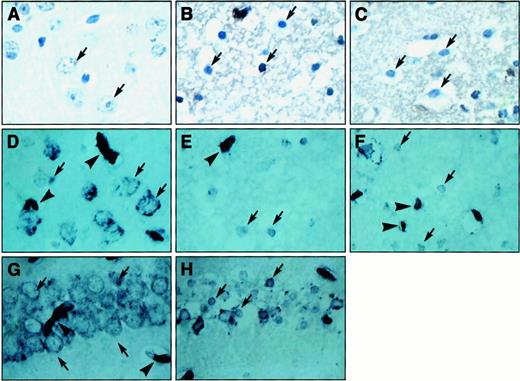
Light microscopic analysis of brain sections of the contralateral cerebral cortex (Figure 2) stained for hematoxylin revealed normal neurons (Figure 2A) surrounded by laminin immunoreactivity (Figure 2D) both in saline- and in Pli-treated mice. In the FII zone, neuronal atrophy was observed (Figure 2B,C), and immunoreactivity of laminin was slightly but not significantly reduced and condensed around neurons (Figure 2E,F). No difference was observed between saline-treated animals (Figure 2B,E) and Pli-treated animals (Figure 2C,F). Laminin immunoreactivity of blood vessels in the infarct area was very similar among saline-treated mice (Figure 2E) and Pli-treated mice (Figure 2F) and comparable to that of the contralateral area (Figure 2D). In the CA1 region in the hippocampus, a similar change in laminin staining was observed by injection of kainic acid (Figure 2G) relative to the contralateral side (Figure 2H). Fibrin(ogen) immunoreactivity (brown staining) was not observed in the contralateral area (Figure 2A) but was comparably present in the infarct area of saline-treated animals (Figure 2B) and Pli-treated animals (Figure 2C).
Histologic analysis of laminin and fibrin(ogen) staining in BALB/c mice.
(A-C) Sections stained for fibrin(ogen) (brown) followed by hematoxylin (dark blue); (A) contralateral area of cerebral cortex with normal neuronal morphology (arrow) and diffuse fibrin(ogen) staining, which were comparable in the saline-treated and Pli-treated groups; (B,C) area of FII in saline-treated (B) and Pli-treated (C) mice. Neuronal morphology (arrows) and fibrin(ogen) staining are similarly affected in the saline-treated and Pli-treated groups. (D,H) Sections stained for laminin (dark blue); (D) contralateral area of cerebral cortex with strong immunoreactivity in basal lamina of vessels (arrowheads) and with immunoreactivity surrounding neurons (arrows), which were comparable in the saline-treated and Pli-treated groups; (E,F) area of FII in saline-treated (E) and Pli-treated (F) mice. The immunoreactivity of neurons (arrows) and vessels (arrowheads) in the infarct area was comparably reduced in both groups. Likewise, the immunoreactivity in neurons was reduced in the CA1 region of the hippocampus following injection of 1.5 nM kainic acid (H, arrows), relative to the contralateral side (G, arrows).
Histologic analysis of laminin and fibrin(ogen) staining in BALB/c mice.
(A-C) Sections stained for fibrin(ogen) (brown) followed by hematoxylin (dark blue); (A) contralateral area of cerebral cortex with normal neuronal morphology (arrow) and diffuse fibrin(ogen) staining, which were comparable in the saline-treated and Pli-treated groups; (B,C) area of FII in saline-treated (B) and Pli-treated (C) mice. Neuronal morphology (arrows) and fibrin(ogen) staining are similarly affected in the saline-treated and Pli-treated groups. (D,H) Sections stained for laminin (dark blue); (D) contralateral area of cerebral cortex with strong immunoreactivity in basal lamina of vessels (arrowheads) and with immunoreactivity surrounding neurons (arrows), which were comparable in the saline-treated and Pli-treated groups; (E,F) area of FII in saline-treated (E) and Pli-treated (F) mice. The immunoreactivity of neurons (arrows) and vessels (arrowheads) in the infarct area was comparably reduced in both groups. Likewise, the immunoreactivity in neurons was reduced in the CA1 region of the hippocampus following injection of 1.5 nM kainic acid (H, arrows), relative to the contralateral side (G, arrows).
Discussion
Thrombolytic therapy for ischemic stroke is based on the premise that timely recanalization of the occluded cerebral artery may salvage “the ischemic penumbra,”20 the hypoperfused but potentially viable zone adjacent to the central ischemic area, limit infarct size, and improve functional recovery and survival. Early thrombolysis with alteplase indeed restored reperfusion, salvaged jeopardized brain tissue, and limited cerebral infarct size in experimental animals21 and reduced morbidity and mortality in patients.1,2 Studies in experimental animal models,3,4 however, indicate that at least in cases with persistent occlusion there may be deleterious side effects of the thrombolytic agent, causing infarct expansion. To the extent that extrapolation of these findings to thrombolytic therapy of ischemic stroke is warranted, this would suggest that the recently observed beneficial clinical outcome with alteplase1,2 versus the detrimental outcome with streptokinase22-24 might relate to the higher efficacy for arterial recanalization of alteplase. Because it appears to be impossible to distinguish a priori between patients who will or who will not achieve cerebral arterial recanalization with alteplase, the development of specific conjunctive strategies to counteract the harmful effects of thrombolytic agents on persisting focal cerebral ischemia appears to be warranted. In view of the interactive effects on neuronal degeneration between oxidative stress, excitotoxin induction,25 and with thrombolytic agents, one could speculate that oxygen radical scavengers, glutamate antagonists,26 or both might beneficially affect the clinical outcome of thrombolytic therapy for ischemic stroke.
Alternatively, gene deficiency of plasminogen increased FII volume, and a positive correlation was found between infarct size and α2-AP genotype, with heterozygotes displaying infarct sizes between those of the wild-type and homozygous genotypes.6 Furthermore, bolus injection of human α2-AP in α2-AP−/− mice caused a dose-related infarct expansion, and subsequent injection of Fab fragments from affino-specific polyclonal rabbit antihuman α2-AP antibodies significantly reduced FII. These observations suggested that thrombolytic agents exacerbate cerebral infarction via other mechanisms27 than plasmin generation and that in vivo depletion of α2-AP might reduce FII.
In the present study, the hypothesis that reduction of α2-AP, either by infusion of Pli or mPli or by immunoneutralization with Fab-4H9—the Fab fragment of a murine monoclonal antibody neutralizing murine α2-AP—might affect FII in the absence of arterial recanalization was confirmed in mice with MCA ligation. The effect of Pli on FII could not be ascribed to changes in hemodynamic parameters such as blood pH, Pco2, Po2, Hb concentration, or arterial blood pressure. MCA ligation was found to cause FII of significantly different sizes in different genetic backgrounds, which is in agreement with earlier observations.6 28 The effects of plasmin on FII were, however, observed in all genetic backgrounds tested (BALB/c, SV129, and C57Bl/6). For convenience, all subsequent experiments in the present study were performed in BALB/c mice, which developed the largest FII (average 27 μL in the control groups), although the relative reduction with plasmin of FII (20%) was smaller than in the other genetic backgrounds. Both mPli and Fab-4H9, but not inactivated Pli, reduced FII, confirming that the effect was caused by depletion of α2-AP.
A mouse of 30 g has an estimated total body pool of α2-AP of 100 μg (based on a 1.0 mL plasma pool, a 0.5 mL extravascular space, and a concentration of 1 μM α2-AP with Mr70 000). In view of the high selectivity and rapid kinetics of inhibition of Pli by α2-AP,29 30infused Pli is expected to react nearly exclusively and stoichiometrically with its physiologic inhibitor. An intravenous dose of 0.07 mg Pli thus should reduce but not deplete the circulating α2-AP pool, as confirmed by the residual α2-AP levels of 44% ± 4% and residual fibrinogen levels of 60%. This was associated with a reduction of FII with approximately 20%. Somewhat surprisingly, similar infarct size reductions were obtained when a larger dose (0.2 mg) was used, which resulted in depletion of both α2-AP and fibrinogen. In all experimental groups, α2-AP and fibrinogen levels had returned to normal within 24 hours, when infarct size was determined. Finally, no clear difference in efficacy was observed when plasmin was given 15 minutes before or after MCA ligation.
FII may induce cerebral edema, which in turn may exacerbate ischemic damage. Determination of the relative contribution of necrosis and edema to infarct size, however, indicated that plasmin caused alterations of necrosis and not of edema.
The hypothesis that FII reduction was the result of α2-AP depletion and not of the proteolytic activity of injected plasmin was confirmed by the experiments with Fab-4H9, the Fab fragment of a murine monoclonal antibody neutralizing murine α2-AP. The autologous antibody was elicited in α2-AP–deficient mice. A similar 20% reduction of FII was observed in BALB/c mice either with 1 mg Fab-4H9, which reduces the α2-AP level to around 40%, given before MCA ligation or with 1.7 mg Fab-4H9 given after MCA ligation. Pli inactivated with aprotinin did not affect FII, because it did not alter α2-AP levels. To exclude species specificity of α2-AP reduction on FII, the effect of Pli was confirmed using an adapted MCA occlusion model in the hamster. In the present study, a 30-minute instead of a 10-minute occlusion period of the right CCA was used to obtain larger lesions.5 This resulted in the death of one of the control animals but none of the hamsters receiving plasmin.
Cerebral infarct expansion mediated by tissue-type plasminogen activator is thought to occur via degradation of laminin that surrounds neurons, resulting in enhanced excitotoxin-mediated neuronal cell death.4 In the present study, laminin immunoreactivity around neurons in the infarct area seemed to be somewhat reduced at 24 hours, but to a similar extent in saline- and Pli-treated animals, indicating that the observed effect could not be ascribed to alterations in laminin turnover. It is possible that reduction of α2-AP might enhance endogenous fibrinolytic degradation of fibrin in vessels of the penumbra after ischemic exposure. Platelets and polynuclear leukocytes are recruited in microvessels after ischemia followed by reperfusion,31,32 resulting in impaired microcirculation.32 Whether reduction of α2-AP protects against failure of the microcirculation remains to be established. If that were the case, thrombolytic agents would have a similar protective affect, which might, however, be overridden by toxic effects.
The concentration of α2-AP in human plasma is 1 μM,33 corresponding to a total body pool in human beings of approximately 400 mg. An equivalent dose of Pli is approximately 400 mg and that of Fab fragment approximately 200 mg, which would be high but not excessive for a single bolus administration. In this context, the observation that miniplasmin, a derivative of intact plasmin that lacks 4 of the 5 kringles, has a molecular weight that is less than half of that of native plasmin is encouraging. Such truncated variants might be more amenable to production by recombinant DNA technology. Furthermore, the observation in gene-inactivated mice that infarct size is proportional to the α2-AP level5 suggests that, provided the present observations can be extrapolated to human beings, even a partial reduction of the plasma level might have a beneficial effect. In view of the excessive morbidity associated with ischemic stroke, further exploration of this potential avenue to reduction of FII would seem to be warranted. Pharmacodynamic studies to delineate the time window and the kinetics of α2-AP depletion on the reduction of FII, both in terms of anatomic and functional end points, as well as mechanistic studies aiming to delineate the role of factors such as leukocytes, blood-brain barrier alterations, secondary microthrombosis, matrix metalloproteinases, and apoptosis appear to be indicated.
Supported in part by a sponsored research agreement between the Center for Molecular and Vascular Biology and Thromb-X, NV, a spin-off company of the University of Leuven in which D.C. has an equity interest.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
D. Collen, Center for Transgene Technology and Gene Therapy, Flanders Interuniversity Institute for Biotechnology, University of Leuven, Campus Gasthuisberg O&N, Herestraat 49, B-3000 Leuven, Belgium; e-mail: desire.collen@med.kuleuven.ac.be