Abstract
The polymorphism responsible for the PlA2 alloantigen on the β3-component of β3-containing integrins is reported to be a risk factor for coronary thrombosis. This study examined the effect of PlA2 on the function of β3-integrins using platelets from subjects homozygous and heterozygous for PlA1 and PlA2. There was overlap in the distribution of the dissociation constant (Kd) and maximum fibrinogen binding (Bmax) values for fibrinogen binding to αIIbβ3 on platelets from PlA1 and PlA2 homozygotes and PlA1/PlA2 heterozygotes. However, whereas there was no statistical difference in these values for the PlA1homozygotes and PlA2 heterozygotes, the Kd for the PlA2 homozygotes was significantly lower than that for the PlA1/PlA2 heterozygotes, but was not statistically different from that for the PlA1 homozygotes. No differences were detected in ADP sensitivity between platelets from PlA1 homozygotes and PlA1/PlA2heterozygotes, in the IC50 for RGDS inhibition of fibrinogen binding to αIIbβ3, in the αvβ3-mediated adhesion of platelets to osteopontin and vitronectin, and in the phorbol ester-stimulated adhesion to fibrinogen of B lymphocytes expressing αIIbβ3 containing either the PlA1 or the PlA2 polymorphism. Finally, no differential effects of PlA2 on turbidometric platelet aggregation, platelet secretion, or platelet thrombus formation were found as measured in the PFA-100. Because no differences were detected in the ability of β3-integrins to interact with ligands based on the presence or absence of the PlA2 polymorphism, the results suggest that factors unrelated to β3-integrin function may account for the reported association of the PlA2 allele with coronary thrombosis.
Introduction
Platelet aggregation results from platelet cross-linking by fibrinogen or von Willebrand factor (or both) bound to the platelet-specific integrin αIIbβ3(GPIIb-IIIa).1 Because of critical roles played by platelets and αIIbβ3 in the pathogenesis of arterial thrombosis,2 it is noteworthy that a case-control study of patients with myocardial infarction and unstable angina suggested that the presence of at least one allele for the β3 alloantigen PlA2 was associated with an odds ratio for a coronary event of 2.8; the odds ratio increased to 6.2 for patients less than 60 years old.3 Because nearly 20% of non-Asian populations are heterozygous for PlA2,4 its presence could put a substantial portion of these populations at increased risk for coronary thrombosis.
PlA2 results from a L→P (single-letter amino acid code) polymorphism at amino acid 33 in the extracellular portion of β3.5 Although amino acid 33 is located at a substantial distance in the linear β3 sequence from its putative ligand binding region, β3 is a highly folded molecule and it is conceivable that amino acid 33 is in proximity to the ligand binding region of the mature protein.6 If PlA2 affects platelet function, it likely does so by altering the interaction of αIIbβ3 with ligands such as fibrinogen, von Willebrand factor, or fibronectin.6,7 β3-Integrin is also a component of the platelet vitronectin (VN) receptor (αvβ3). Although platelets contain only a small amount of αvβ3, it is capable of supporting platelet adhesion to substrates such as osteopontin (OPN) and VN.8 Thus, PlA2 could also affect platelet αvβ3 function. To provide a biochemical basis for the suggestion that PlA2 is a risk factor for coronary thrombosis, we measured αIIbβ3 and αvβ3 function on platelets expressing PlA1 or PlA2 or both. Because the number of subjects homozygous for PlA2 is limited, many of our measurements were made using platelets of individuals who expressed one PlA2 allele. Nevertheless, we found that the presence of at least one PlA2 allele did not alter the function of either platelet αIIbβ3 and αvβ3 and conclude that factors unrelated to the function of these integrins likely account for the reported association of PlA2 with coronary thrombosis.
Materials and methods
Identification of subjects whose platelets express PlA2
DNA was purified from the lymphocytes of 100 healthy volunteers. The polymerase chain reaction (PCR) was then used to amplify a 266-bp fragment from exon 2 of the β3 gene that contains the polymorphism responsible for the PlAphenotype.5 PCR was performed according to the manufacturer's instructions in a 25-μL reaction volume containing 6.5 pM of each primer, 1.25 U Amplitaq (Applied Biosystems, Foster City, CA), and 150 ng genomic DNA. The antisense primer was 5′-TCTCTCCCCATGGCAAAGAGT-3′ and the sense primer was 5′-TTCTGATTGCTGGACTTCT-3′. Following heating at 94°C for 7 minutes, 30 PCR cycles were performed using the following conditions: denaturation, 94°C for 10 seconds; annealing, 62°C for 30 seconds; extension, 69°C for 90 seconds. This was followed by an extension for 7 minutes at 70°C. Allele-specific hybridization was performed at 40°C using the probes 5′-CCTGCCTCTGGGCTCAC-3′ and 5′-CCTGCCTCCGGGCTCAC-3′. Genotyping was validated by sequencing the affected region of the β3 gene in 5 PlA1/ PlA1 homozygotes and 5 PlA1/PlA2 heterozygotes.
Fibrinogen binding to ADP-stimulated platelets
Citrate-anticoagulated platelet-rich plasma (PRP) was obtained from 21 healthy normotensive PlA1/ PlA1homozygotes and PlA1/PlA2 heterozygotes whose ages were evenly distributed between 29 and 62. Seventeen subjects were women and 8 were African American. The subjects did not smoke tobacco products and were not taking aspirin or other medications known to affect platelet function. PRP was also obtained by apheresis at the Musser Blood Center of the Penn Jersey Region of the American Red Cross from 5 known white male PlA2 homozygotes whose ages ranged from 40 and 56 and was transported to the University of Pennsylvania for study. Platelets were isolated from PRP by gel filtration on Sepharose 2B (Pharmacia, Piscataway, NJ) as previously described.7 Fibrinogen binding to ADP-stimulated platelets was measured using 125I-labeled human fibrinogen.7 Briefly, 0.5-mL aliquots of ≈5 × 107 gel-filtered platelets (GFP) were mixed with125I-fibrinogen, 0.5 mM CaCl2, and 10 μM ADP. Following a 5-minute incubation at 37°C without stirring, the platelets were sedimented through silicone oil in an Eppendorf centrifuge (Brinkman Instruments, Westbury, NY). The tips of the centrifuge tubes containing the pelleted platelets were amputated and counted for 125I. Nonspecific binding was determined by including a 15-fold excess of unlabeled fibrinogen in the assay. The effect of 2 concentrations of the tetrapeptide RGDS (Sigma) on fibrinogen binding was determined by adding the peptide to the platelet suspensions prior to the addition of ADP.9
The dissociation constant (Kd) of αIIbβ3 for fibrinogen and maximum fibrinogen binding (Bmax) were obtained by Scatchard analysis.7 The inhibitory constant (Ki) for RGDS was derived by plotting the slopes of double reciprocal plots of fibrinogen binding obtained in the presence or absence of RGDS against the RGDS concentration.10
Expression of β3 in Epstein-Barr virus–transformed B lymphocytes
Overlap PCR was used to convert the codon for β3L33 to P.11 pREP vectors containing αIIb and β3 complementary DNAs (cDNAs) were introduced into 7.5 × 106 GM1500 B lymphocytes by electroporation (250 V and 960 μF). Stable cotransfectants were selected using G418 and hygromycin. The αIIbβ3 on the lymphocyte surface was quantified by flow cytometry using the monoclonal antibody (mAb) A2A9.12 The PlA allotype of each cell line was verified using polyclonal antisera that specifically recognize PlA1 or PlA2.13 Phorbol myristate acetate (PMA)–stimulated adhesion of35S-methionine-labeled lymphocytes to fibrinogen was measured as previously described.11 Adherence data were normalized for the level of αIIbβ3expression by dividing the percent adherence by the mean fluorescence intensity (MFI) determined by flow cytometry.11
Assays of platelet function
Platelet-dependent hemostasis was assessed using the PFA-100 Platelet Function Analyzer (Dade-Behring, Deerfield, IL).14 Turbidometric platelet aggregation was measured using a Bio-Data PAP-4 aggregometer.15 Thrombin receptor-activating peptide (TRAP)–stimulated P-selectin expression was measured by flow cytometry using the ADIAflo Platelet Gp kit (American Diagnostica, Greenwich, CT) according to the manufacturer's instructions. Briefly, TRAP-stimulated platelets were incubated sequentially with the mAb CD62P (Biocytex, Marseilles, France) and fluorescein isothiocyanate (FITC)–labeled antimouse IgG, followed by flow cytometry. The measured platelet fluorescence was then compared to that obtained using 4 populations of 2 μm latex beads, each population coated with a known quantity of mouse IgG. The amount of P-selectin expressed per platelet was calculated from plots of bead fluorescence versus the amount of antibody bound per bead. The effect of the αIIbβ3 antagonist RWJ 53308 on TRAP-stimulated platelet aggregation was tested after determining whether PlA allotype affected RWJ 53308 binding. RWJ 53308 is a peptidomimetic whose structure is based on the K-Q-A-G-D sequence present at the carboxyl terminus of the fibrinogen γ chain.16 Platelets in citrated whole blood were stained with 2 anti-αIIbβ3 mAbs: Mab1, whose binding was not affected by RWJ 53308, and Mab2, whose binding to αIIbβ3 is inhibited by RWJ 53308. Flow cytometry measurements were converted to occupied αIIbβ3 complexes using microbeads coated with known amounts of FITC.
Platelet adhesion to OPN and VN
Platelet adhesion to OPN and VN was measured as previously described.8 Aliquots of GFP (100 μL) were added to the wells of microtiter plates coated with 5 μg/mL recombinant OPN or human VN (Sigma). Platelets were stimulated with 10 μM ADP. After a 30-minute incubation at 37°C, the number of adherent platelets was determined using a colorimetric assay.8
Statistical analysis
Power calculations were based on the variability of our fibrinogen binding assay.7 Sample size was estimated as 10 per genotype to detect a log difference in Kd and Ki with 90% power. Statistical significance of differences was determined using a 2-tailed t test for unpaired samples.
Results
Effect of PlA genotype on fibrinogen binding to αinfIIbβ3
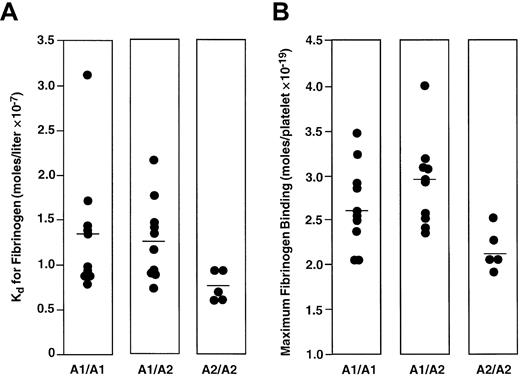
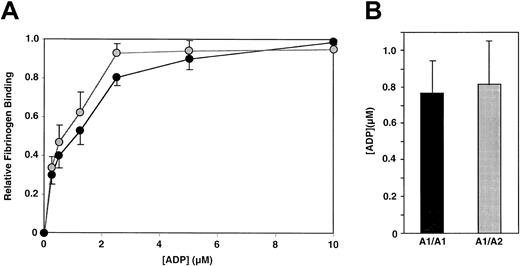
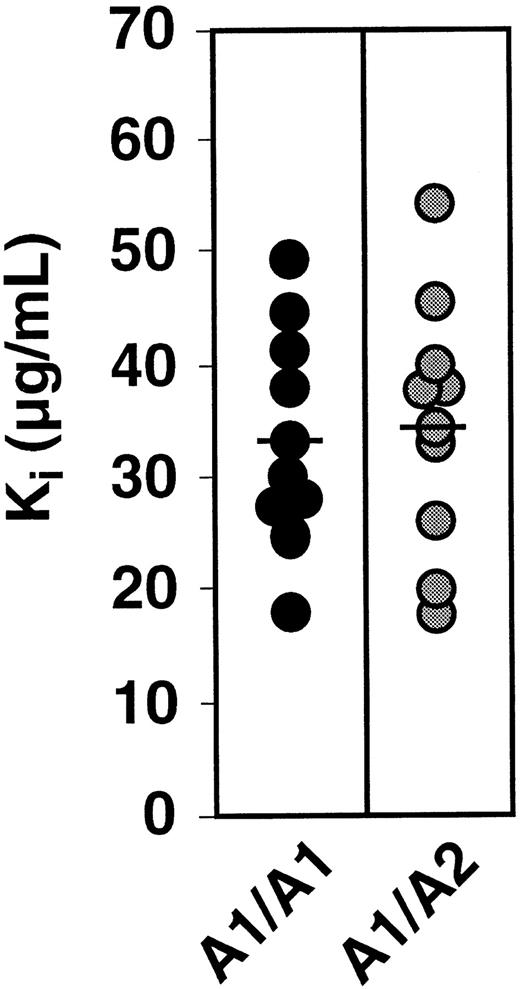
To study the effect of PlA genotype on the affinity of αIIbβ3 for fibrinogen, we determined the PlA genotype of 100 healthy subjects. The frequency of the PlA2 allele among the 100 subjects was 16%, consistent with the frequency of the PlA2 allele in non-Asian populations.4 No PlA2 homozygotes were identified. We then measured both the Kd and Bmax of fibrinogen binding to ADP-stimulated platelets of 10 subjects homozygous for PlA1 and 11 subjects heterozygous for PlA1/ PlA2. We also measured the Kd and Bmax of fibrinogen binding to the platelets of 5 subjects homozygous for PlA2 obtained from the Musser Blood Center of the Penn Jersey Region of the American Red Cross. As shown in Figure 1A, there was overlap in the distribution of Kd values among the 3 groups of subjects. The means of these distributions were 1.36 ± 0.22 × 10−7 M, 1.28 ± 0.13 × 10−7 M, and 0.73 ± 0.08 × 10−7 M for the PlA1homozygotes, PlA1/PlA2 heterozygotes, and PlA2 homozygotes, respectively. Although the means for the PlA1 homozygotes and PlA1/PlA2heterozygotes were not statistically different (P = .74), the mean for the PlA2 homozygotes was significantly lower than that for the PlA1/PlA2 heterozygotes (P = .02), but not for the PlA1 homozygotes (P = .07). The distribution of values for Bmaxis shown in Figure 1B. The means of these distributions ranged from 2.65 ± 0.15 × 10−19 mole/platelet for PlA1 homozygotes, 2.90 ± 0.14 × 10−19mole/platelet for PlA1/PlA2 heterozygotes, and 2.17 ± 0.11 × 10−19 mole/platelet for PlA2 homozygotes. Again, there was no significant difference between PlA1 homozygotes and PlA1/PlA2 heterozygotes (P = .24), whereas the mean Bmax of the PlA2 homozygotes was significantly less than that of the other 2 groups (P = .048 and .006, respectively). The data shown in Figure 1 indicate that the presence of a single PlA2 allele did not alter the ability of αIIbβ3 to interact with fibrinogen when the platelets were maximally stimulated by ADP. To address the possibility that a difference might be apparent at lower agonist concentrations, we measured fibrinogen binding to platelets of individuals homozygous for PlA1 and heterozygous for PlA1/PlA2 as a function of ADP concentration. As shown in Figure 2A, there was no difference in fibrinogen binding to the platelets of these individuals as the concentration of ADP was varied from 0.25 μM to 10 μM. Moreover, the median effective concentration (EC50) values for ADP-stimulated fibrinogen binding (0.77 ± 0.18 μM and 0.82 ± 0.24 μM, respectively) were not statistically different (Figure 2B). RGD-containing peptides competitively inhibit fibrinogen binding to αIIbβ3.17 To determine if the inhibitory effect of the tetrapeptide RGDS is affected by the presence of PlA2, we measured the Ki for RGDS using platelets from 10 PlA1/PlA1 and 10 PlA1/PlA2 subjects. As shown in Figure3, the distribution of Kivalues for the 20 subjects overlapped completely, nor was there a significant differences in the means of these distributions (33.9 ± 3.0 μg/mL and 35.0 ± 3.6 μg/mL, respectively,P = .82).
Dissociation constant and maximum fibrinogen binding.
Distribution of the Kd (A) and Bmax (B) of fibrinogen binding to ADP-stimulated platelets from PlA1/PlA1, PlA1/PlA2, and PlA2/PlA2 subjects. The horizontal lines indicate the mean of each distribution.
Dissociation constant and maximum fibrinogen binding.
Distribution of the Kd (A) and Bmax (B) of fibrinogen binding to ADP-stimulated platelets from PlA1/PlA1, PlA1/PlA2, and PlA2/PlA2 subjects. The horizontal lines indicate the mean of each distribution.
Effect of ADP concentration on fibrinogen binding to platelets from 3 PlA1/PlA1 and 3 PlA1/PlA2 subjects.
(A) Mean and SEM of fibrinogen binding at various ADP concentrations. ●, A1/A1; , A1/A2. (B) Mean and SEM of the EC50 for ADP.
, A1/A2. (B) Mean and SEM of the EC50 for ADP.
Effect of ADP concentration on fibrinogen binding to platelets from 3 PlA1/PlA1 and 3 PlA1/PlA2 subjects.
(A) Mean and SEM of fibrinogen binding at various ADP concentrations. ●, A1/A1; , A1/A2. (B) Mean and SEM of the EC50 for ADP.
, A1/A2. (B) Mean and SEM of the EC50 for ADP.
Distribution of the Ki for RGDS inhibition of fibrinogen binding to ADP-stimulated platelets.
Fibrinogen binding to the platelets of PlA1 homozygotes and PlA1/PlA2 heterozygotes was measured in presence of RGDS at 25 and 75 μg/mL. The horizontal lines indicate the mean of each distribution.
Distribution of the Ki for RGDS inhibition of fibrinogen binding to ADP-stimulated platelets.
Fibrinogen binding to the platelets of PlA1 homozygotes and PlA1/PlA2 heterozygotes was measured in presence of RGDS at 25 and 75 μg/mL. The horizontal lines indicate the mean of each distribution.
Effect of the PlA polymorphism on αIIbβ3 function in B lymphocytes
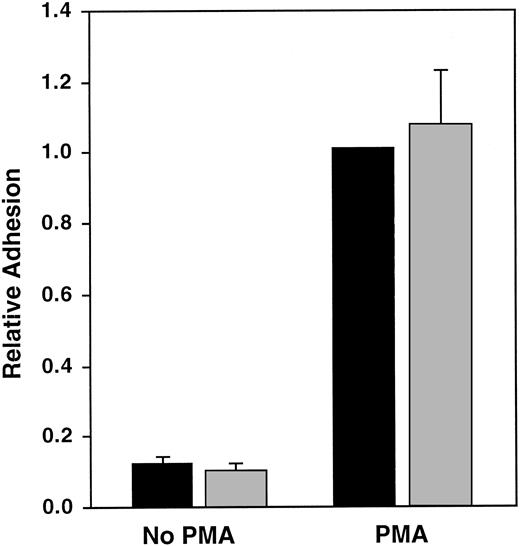
It is possible that sequence differences in β3linked to PlA, but not PlA itself, could affect αIIbβ3 function. To address this possibility, we cotransfected the B-lymphocyte line GM1500 with an αIIb cDNA and a β3 cDNA encoding either PlA1 or PlA2 and measured unstimulated and PMA-stimulated lymphocyte adhesion to immobilized fibrinogen.11 As shown in Figure4, there was no significant difference in either the unstimulated or the PMA-stimulated adhesion of lymphocytes expressing αIIbβ3 containing either the PlA1 or PlA2 alloantigen (P = .45 and P = .65, respectively).
Adhesion of unstimulated and PMA-stimulated GM1500 B cells expressing PlA1 or PlA2 to immobilized fibrinogen.
Data were normalized for αIIbβ3 expression and were expressed relative to PMA-stimulated cells expressing PlA1. The data shown are the mean and SEM of 7 experiments. ▪, A1; ░, A2.
Adhesion of unstimulated and PMA-stimulated GM1500 B cells expressing PlA1 or PlA2 to immobilized fibrinogen.
Data were normalized for αIIbβ3 expression and were expressed relative to PMA-stimulated cells expressing PlA1. The data shown are the mean and SEM of 7 experiments. ▪, A1; ░, A2.
Effect of PlA on platelet aggregation and secretion
Postreceptor events, including platelet secretion, are thought to stabilize platelet aggregates.18 To determine whether macroscopic platelet aggregation is affected by the presence of a PlA2 allele, we compared the aggregation of PlA1/PlA1, PlA1/PlA2, and PlA2/PlA2 platelets induced by TRAP in a conventional platelet aggregometer. We found no significant differences in either the extent (Figure 5A) or rate (data not shown) of platelet aggregation as the TRAP concentration was increased from 0.4 to 1.0 μM. At low concentrations, even strong agonists like TRAP require αIIbβ3-mediated platelet aggregation to induce platelet secretion. To determine whether this is affected by PlA2, we measured P-selectin expression as an indication of platelet secretion on TRAP-stimulated platelets (Figure 5B). We found baseline P-selectin expression was slightly increased on unstimulated PlA2/PlA2 platelets. Nonetheless, when P-selectin expression was corrected for this difference in baseline expression, there were no differences in TRAP-stimulated P-selectin expression between PlA1/PlA1, PlA1/PlA2, and PlA2/PlA2 platelets. We next compared the rate of platelet thrombus formation in whole blood as a function of PlA allotype using the PFA-100 instrument.14As seen in Figure 6, there were no detectable differences in the time required to make an occlusive thrombus among the 3 groups of subjects. We also studied the effect of PlA allotype on the ability of the low-molecular-weight αIIbβ3 antagonist RWJ 53308 to inhibit macroscopic platelet aggregation.16 We first determined whether there were differences in binding of the compound to platelets according to their PlA allotype. As seen in Figure7A, RWJ 53308 bound equally well to platelets regardless of PlA allotype. We then measured TRAP-stimulated platelet aggregation in the presence of increasing concentrations of RWJ 53308. As seen in Figure 7B, there were no significant differences in the ability of RWJ 53308 to inhibit the aggregation of PlA1/PlA1, PlA1/PlA2, and PlA2/PlA2 platelets.
Effect of PlA2 on TRAP-stimulated platelet adhesion and P-selectin expression.
(A) Extent of TRAP-stimulated aggregation of platelets from 10 PlA1/PlA1 (●), 9 PlA1/PlA2 (○), and 9 PlA2/PlA2( ) subjects. The data shown are the mean and SEM. (B) P-selectin expression on TRAP-stimulated platelets as measured by flow cytometry using the monoclonal antibody CD62P. The data shown are the mean and SEM.
) subjects. The data shown are the mean and SEM. (B) P-selectin expression on TRAP-stimulated platelets as measured by flow cytometry using the monoclonal antibody CD62P. The data shown are the mean and SEM.
Effect of PlA2 on TRAP-stimulated platelet adhesion and P-selectin expression.
(A) Extent of TRAP-stimulated aggregation of platelets from 10 PlA1/PlA1 (●), 9 PlA1/PlA2 (○), and 9 PlA2/PlA2( ) subjects. The data shown are the mean and SEM. (B) P-selectin expression on TRAP-stimulated platelets as measured by flow cytometry using the monoclonal antibody CD62P. The data shown are the mean and SEM.
) subjects. The data shown are the mean and SEM. (B) P-selectin expression on TRAP-stimulated platelets as measured by flow cytometry using the monoclonal antibody CD62P. The data shown are the mean and SEM.
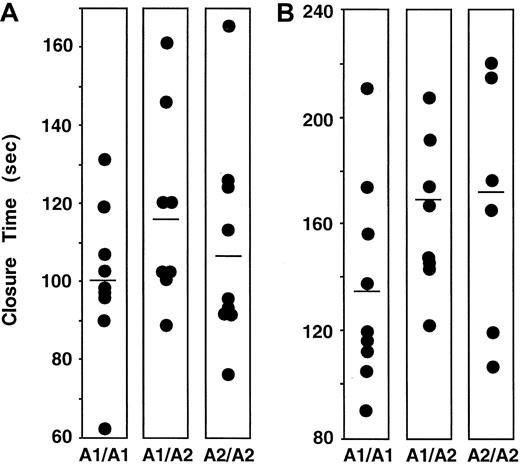
Effect of PlA2 on platelet thrombus formation in the PFA-100.
The data shown are the distribution of times required to occlude the aperture of the PFA-100 using citrate-anticoagulated whole blood from PlA1/PlA1, PlA1/PlA2, and PlA2/PlA2 subjects. The horizontal lines indicate mean values. (A) Collagen-ADP cartridge. (B) Collagen-epinephrine cartridge.
Effect of PlA2 on platelet thrombus formation in the PFA-100.
The data shown are the distribution of times required to occlude the aperture of the PFA-100 using citrate-anticoagulated whole blood from PlA1/PlA1, PlA1/PlA2, and PlA2/PlA2 subjects. The horizontal lines indicate mean values. (A) Collagen-ADP cartridge. (B) Collagen-epinephrine cartridge.
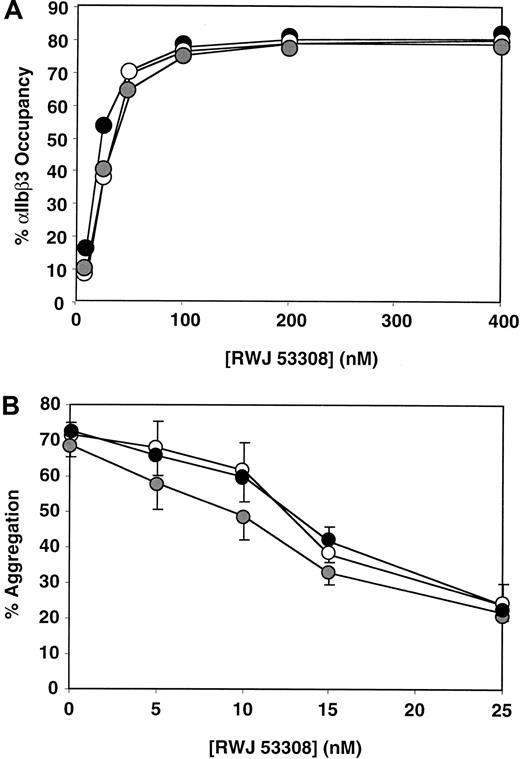
Inhibition of platelet aggregation by RWJ 53308.
(A) Percent occupancy of αIIbβ3 by RWJ 53308 on platelets from 10 PlA1/PlA1 (●), 9 PlA1/PlA2 (○), and 9 PlA2/PlA2( ) subjects. The data shown are mean values. (B) Effect of RWJ 53308 on platelet aggregation stimulated by 1 μM TRAP. The data shown are the mean and SEM.
) subjects. The data shown are mean values. (B) Effect of RWJ 53308 on platelet aggregation stimulated by 1 μM TRAP. The data shown are the mean and SEM.
Inhibition of platelet aggregation by RWJ 53308.
(A) Percent occupancy of αIIbβ3 by RWJ 53308 on platelets from 10 PlA1/PlA1 (●), 9 PlA1/PlA2 (○), and 9 PlA2/PlA2( ) subjects. The data shown are mean values. (B) Effect of RWJ 53308 on platelet aggregation stimulated by 1 μM TRAP. The data shown are the mean and SEM.
) subjects. The data shown are mean values. (B) Effect of RWJ 53308 on platelet aggregation stimulated by 1 μM TRAP. The data shown are the mean and SEM.
Effect of PlA2 on platelet adhesion to OPN and VN
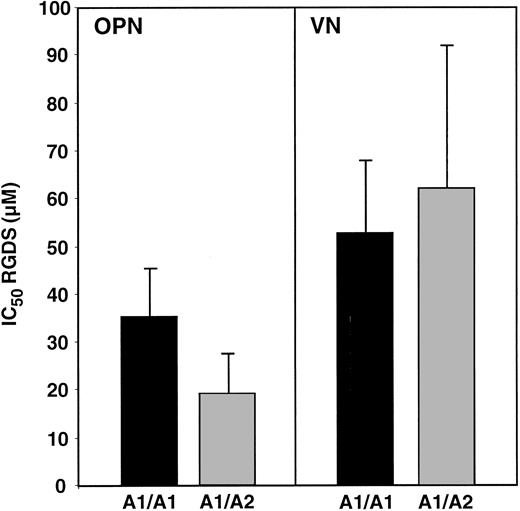
The integrin αvβ3 mediates platelet adhesion to OPN- and VN-coated surfaces.8 To determine if the presence of a PlA2 allele affects platelet adherence to OPN and VN, we compared platelet avidity for each substrate by determining the 50% inhibitory concentration (IC50) for RGDS, reasoning that there is a direct relationship between the IC50 and the avidity of platelet adhesion. There were no significant differences in the IC50 for RGDS inhibition of platelet adhesion to OPN (35.0 ± 10.5 μM versus 19.1 ± 8.5 μ, P = .3) or VN (52.5 ± 15.6 μM and 62.1 ± 29.8 μM, P = .79) as a function of PlA allotype (Figure 8). These data suggest that platelet αvβ3, as well as αIIbβ3, is not affected by the presence of a PlA2 allele.
Effect of PlA2 on platelet adhesion to OPN and VN.
ADP-stimulated platelets from 3 PlA1 homozygotes and 3 PlA1/PlA2 heterozygotes were added to the wells of microtiter plates coated with OPN or VN. The avidity of platelet adhesion was measured by determining the IC50 for the tetrapeptide RGDS. The data presented are the mean and SEM of 3 experiments.
Effect of PlA2 on platelet adhesion to OPN and VN.
ADP-stimulated platelets from 3 PlA1 homozygotes and 3 PlA1/PlA2 heterozygotes were added to the wells of microtiter plates coated with OPN or VN. The avidity of platelet adhesion was measured by determining the IC50 for the tetrapeptide RGDS. The data presented are the mean and SEM of 3 experiments.
Discussion
The formation of a coronary thrombus in vivo is a complex process that is influenced by a number of factors, including the nature of the vascular wall pathology, the ambient shear rate, and platelet reactivity. Therefore, it is not surprising that the contribution of a single factor, such as the polymorphism responsible for the PlA2 alloantigen on platelet αIIbβ3, to the risk for coronary artery thrombosis is uncertain. Moreover, it is noteworthy that of 37 epidemiologic studies addressing this question, only 15 implicated PlA2 in various aspects of arterial thrombosis.3,19-54 We reasoned that if the presence of PlA2 directly affects coronary thrombus formation, it should be possible to detect an effect of PlA2 on β3-integrin function. Although our studies were limited by the small number of subjects who were homozygous for PlA2, heterozygous subjects were available in the expected numbers, and the presence of a single PlA2 allele in heterozygous individuals has been reported to confer significant thrombotic risk in all of the positive studies3,41-52,54but one.53
Initially, we measured parameters of αIIbβ3function using radiolabeled fibrinogen. We found no differences in the affinity of fibrinogen binding or in maximal fibrinogen binding using platelets from 10 subjects homozygous for PlA1 and 11 subjects heterozygous for PlA1/PlA2. Although it is conceivable that differences might have become apparent if more subjects were studied, the extent of the overlap of these values within the groups suggests that this would be unlikely. On the other hand, the values for Kd and Bmax of platelets from subjects homozygous for PlA2 were clustered tightly at the lower end of the distribution ranges of the other 2 groups, raising the possibility that the slightly increased affinity of these platelets for fibrinogen could enhance their ability to form thrombi. However, the Kd for fibrinogen binding of each allotype is well below the plasma concentration of fibrinogen,7 a disparity likely to be magnified within a thrombus because agonist-stimulated platelets secrete fibrinogen. Thus, under conditions of substantial fibrinogen excess, small differences in affinity are likely to have a negligible effect on fibrinogen binding and would not be expected to account for the reported effects of PlA2.10
A more likely possibility is that the presence of PlA2enhances the ability of agonists to induce ligand binding to αIIbβ3 and a number of functional studies comparing PlA1 and PlA2 platelets have been reported. However, like the epidemiologic studies cited above, these reports have been contradictory. Thus, although Feng and colleagues55 reported that threshold concentrations for epinephrine-stimulated platelet aggregation were significantly lower when platelets expressed one or 2 copies of PlA2 and Michelson and coworkers concluded that PlA2-positive platelets displayed a lower threshold for activation,56Lasne and associates measured the threshold for biphasic aggregation induced by TRAP and ADP and found that platelets expressing PlA2 were actually less sensitive to either agonist.57 Similarly, Cooke and coworkers reported that the platelets of PlA1/PlA2 heterozygotes were more sensitive to the inhibitory effects of aspirin,58whereas Andrioli and colleagues reported that PlA2platelets were hyporesponsive to thromboxane A2.39 In addition, Goodall and coworkers found increased fibrinogen binding to PlA2 platelets stimulated by ADP, but not when the platelets were stimulated by thrombin.59 On the other hand, Meiklejohn and colleagues reported that there was no difference in fibrinogen binding to ADP-stimulated PlA1 and PlA2platelets.60 We found that the presence of a single PlA2 allele had no effect when fibrinogen binding was measured as a function of ADP concentration. Moreover, we did not detect a difference in the TRAP-stimulated aggregation of homozygous or heterozygous PlA2 platelets compared to homozygous PlA1 platelets when platelet aggregation was measured as a function of TRAP concentration, nor did we detect differences in PMA-stimulated adhesion of B cells expressing either PlA1or PlA2 to fibrinogen-coated surfaces. Similarly, Wang and Newman did not detect functional differences between platelets expressing the Pena and Penb alloantigens that result from an R143Q polymorphism located in the RGD-binding domain of β3.61 Thus, our measurements argue against the possibility that the presence of the PlA2 polymorphism facilitates the agonist-induced conversion of αIIbβ3 from a resting to an activated ligand-binding state.
αIIbβ3 function is associated with a number of “postreceptor” events including platelet aggregation, platelet secretion when platelets are stimulated by weak agonists such as ADP and epinephrine, clot retraction, and phosphorylation of focal adhesion kinase. It is worth noting that each of these events firstrequires ligand binding to αIIbβ3. Thus, each is either absent or perturbed in platelets from patients with Glanzmann thrombasthenia, a hereditary bleeding disorder due to the absence of sufficient numbers of functional αIIbβ3 complexes. Vijayan and associates addressed the influence of PlA2 on postreceptor events initiated through αIIbβ3 by expressing αIIbβ3 heterologously in Chinese hamster ovary (CHO) and 293 cells.62 Although they found no difference in soluble fibrinogen binding to transfected CHO cells expressing either PlA1 or PlA2, they observed increased adhesion of both CHO and 293 cells expressing PlA2 to immobilized fibrinogen. We performed similar experiments using transfected PMA-stimulated human B lymphocytes, but detected no significant difference in the adhesion of cells expressing either PlA1 or PlA2. We also measured agonist-stimulated platelet aggregation using both conventional aggregometry and the PFA-100 instrument and P-selectin expression as a measure of platelet secretion in patients who expressed 0, 1, or 2 PlA2 alleles. Again, we detected no differences. However, like Michelson and coworkers,56 we did find a slight increase in basal P-selectin expression on unstimulated platelets homozygous for PlA2. In our case, this was likely due to a slight degree of platelet activation during apheresis at the Red Cross or during the transport of blood from the Red Cross to the University of Pennsylvania because there was no difference in basal P-selectin on PlA1/PlA1 and PlA1/PlA2platelets obtained at the University of Pennsylvania.
β3-integrin is a component of the VN receptor αvβ3. We have observed that despite the small number of αvβ3 complexes present on platelets, this integrin can mediate platelet adhesion to surfaces coated with OPN or VN.8 Thus, it is conceivable that perturbation of αvβ3 function could be responsible for the association of the PlA2 with myocardial infarction. Nonetheless, we found that the presence of PlA2did not effect the avidity of αvβ3-mediated adhesion of platelets to either OPN or VN, suggesting that the presence of a PlA2 allele does not modify the function of this integrin.
In summary, we have examined the effect of the PlA2polymorphism on β3-integrin function in human platelets, focusing on agonist-stimulated ligand binding because this is central to the function of these integrins. Because we did not detect substantial differences in the ability of β3-integrins to interact with ligands based on this polymorphism, our results suggest that factors unrelated to β3-integrin function may account for the reported association of the PlA2 allele with coronary thrombosis.
Supported in part by grants HL40387, HL51258, and MO1RR00040 from the National Institutes of Health and by funds from SmithKline Beecham Pharmaceuticals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joel S. Bennett, Rm 914, BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: bennetts@mail.med.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal