Abstract
Interleukin (IL)-7 is known to up-regulate thymopoietic pathways of T-cell regeneration. Recent work also has shown it to potently enhance thymic-independent peripheral expansion and to restore immunocompetence in athymic T-cell–depleted hosts. We hypothesized that endogenous IL-7 could contribute to the restoration of T-cell homeostasis following T-cell depletion. To analyze this, we evaluated circulating IL-7 levels and lymphocyte subsets in multiple clinical cohorts with T-cell depletion of varying etiologies. In pediatric (n = 41) and adult (n = 51) human immunodeficiency virus–infected CD4-depleted patients, there were strong inverse correlations between IL-7 levels and CD4 counts (r = −0.77, P < .0001, andr = −0.68, P < .0001). Declines in IL-7 were temporally correlated with recovery of CD4 counts. Similar patterns were observed in CD4-depleted patients receiving cancer chemotherapy (r = −0.65, P = .009). Therefore, in 2 disparate clinical scenarios involving CD4 depletion, IL-7 levels dynamically respond to changes in CD4 T-cell number, making this cytokine uniquely suited as a candidate regulator of T-cell homeostasis. Furthermore, in patients with idiopathic CD4 lymphopenia, a much weaker relationship between IL-7 levels and peripheral blood CD4 counts was observed, suggesting that an impaired IL-7 response to CD4 depletion may contribute to the impaired lymphocyte homeostasis observed in this population. In light of the known effects of IL-7 on T-cell regeneration, we postulate that increased availability of IL-7 could play a critical role in restoring T-cell homeostasis following T-cell depletion.
Introduction
The size of the peripheral T-cell compartment remains tightly regulated throughout life, implying that homeostatic mechanisms function to carefully maintain peripheral T-cell numbers.1,2 The nature of such homeostatic mechanisms remains poorly understood. Because considerable reductions in thymic output are known to occur during the normal human lifespan,3-6 primary regulation of T-cell number is thought to occur peripherally.7-10 Currently held paradigms for peripheral mechanisms that contribute to T-cell homeostasis emphasize the critical role for major histocompatibility complex engagement in T-cell survival,11-14 and that antigen-driven peripheral expansion contributes significantly to modulating the size of the peripheral T-cell compartment, especially following T-cell depletion.15,16 Regulation of such events has been postulated to occur in the context of a vaguely defined entity termed the “lymphoid niche.”8 17 While the exact anatomic location and unique microenvironmental factors that exist within lymphoid niches are unknown, it is thought to be a site where adequate availability of antigen, major histocompatibility complex, and growth factors exist to allow competing T-cell populations to vie for survival and expansion signals. Growth factors that contribute to the regulation of the peripheral T-cell compartment within the putative lymphoid niche have not yet been defined.
Evidence compiled from clinical and experimental models of T-cell depletion provide some clues as to the factors that contribute to T-cell homeostasis. First, it has been observed that compensatory homeostatic responses to T-cell depletion are “blind” to CD4 and CD8 subsets18-20 because, in murine models, isolated depletion of CD4 or CD8 T-cell populations leads to subsequent expansion of the reciprocal subset.18 In the human immunodeficiency virus (HIV) infection, following bone marrow transplantation and following T-cell–depleting chemotherapy, declines in CD4 T cells are accompanied by concomitant rises in CD8 T cells, sometimes to supranormal levels.19,20 Second, large numbers of activated cells accumulate during periods of T-cell depletion.21-23 Because activated cells are susceptible to programmed cell death under normal circumstances, it is plausible that growth factors permissive to the survival of such cells may be active in this setting.
We have hypothesized that interleukin (IL)-7 may contribute to regulation of peripheral T-cell homeostasis for the following reasons. First, because IL-7 is produced predominantly by stromal tissues24,25 as well as by dendritic cells within the lymph node,26,27 high levels might exist within the lymphoid tissues and lymphocyte depletion would not diminish availability, as would occur with lymphocyte-produced growth factors. Second, the capacity of IL-7 to enhance the survival of mature T cells28-30 could allow the accumulation of activated cells, which is known to occur in T-cell–depleted hosts.16,23 Third, IL-7 expands both peripheral CD4 and CD8 T cells in T-cell–replete hosts,31 and we have observed that administration of IL-7 enhances the peripheral expansion of mature T cells after T-cell depletion in thymic-deficient hosts.49 Finally, IL-7 exerts profound effects on thymopoiesis,32-34 suggesting a potential role in the thymic rebound observed following T-cell depletion.4,35 36
Patients, materials, and methods
Patient groups
The pediatric HIV-infected cohort included 41 patients treated from 1995 to 1998 on approved protocols within the HIV and AIDS Malignancy Branch of the National Cancer Institute (NCI) after informed consent. Thirty-one protease inhibitor–naive patients were evaluated prior to enrollment on a ritonavir dose escalation study (NCI study 96-C-0003). Baseline serum was collected after a 2-week period during which time no antiretroviral agents were given. Samples from 10 additional pediatric patients were obtained after a 2-month period on stable dosages of antiretroviral agents prior to enrollment on an IL-2 clinical trial (NCI study 95-C-0183). Ages ranged from 6 months to 16 years. Centers for Disease Control (CDC) classification was as follows: 3 patients A2, 4 patients A3, 4 patients B1, 4 patients B2, 6 patients B3, 1 patient C1, 4 patient C2, 15 patients C3.37
Longitudinal analysis was performed on 18 HIV-infected pediatric patients treated on the ritonavir study. Briefly, patients received ritonavir monotherapy (dose escalation from 250 to 400 mg/m2 twice daily) for 12 weeks followed by combination therapy with ritonavir, didanosine (90 mg/m2 twice daily), and zidovudine (90 mg/m2 twice daily) for 96 weeks. Details of this trial have been reported elsewhere.38
Adult HIV-infected patients included 31 adult patients naive to protease inhibitors, with CD4 lymphocyte counts between 100 and 300 cells/μL, at initial screening enrolled on ACTG (AIDS Clinical Trials Group) protocol 315 after informed consent. Baseline plasma samples were collected after a 5-week “washout” period off all antiretroviral agents. A second adult cohort comprised 20 additional patients with CD4 lymphocyte counts below 100 cells/μL enrolled on clinical trials at the NCI. Patients enrolled on ACTG 315 were also followed longitudinally for 48 weeks on highly active antiretroviral therapy (HAART). Patients received ritonavir (600 mg/d begun day 0, 1200 mg/d begun day 7), lamivudine (300 mg/d begun day 10), and zidovudine (900 mg/d begun day 10). Details of the protocol have been reported previously.39
Following informed consent, patients were enrolled on one of 3 approved protocols within the Pediatric Oncology Branch of the NCI (86-C-169, 89-C-41, and 93-C-0125) for newly diagnosed sarcoma or non-Hodgkin's lymphoma. Lymphocyte depletion and immune reconstitution in these patients has been reported previously.4,40 41 Ages ranged from 5 to 23 years. Patients received 4 to 18 cycles of intensive multiagent chemotherapy with or without high-dose chemotherapy and stem cell rescue as described previously and, where appropriate, surgical resection and/or radiation therapy was administered. Lymphocyte subset analysis was performed at the time of maximal hematologic recovery from successive cycles of multiagent chemotherapy and following completion of therapy if the patient remained free of recurrent neoplastic disease.
Patients suspected of idiopathic CD4 lymphopenia were referred to the National Institutes of Health for evaluation. Serum samples were collected at initial screening after informed consent and enrollment on approved protocol. The case definition for this entity is lack of HIV infection, with CD4 count below 300 cells/μL in the absence of other coexisting disease that might be associated with CD4 lymphopenia.42 All patients referred for evaluation were included in the initial analysis of this group, including those subsequently found to not meet the case definition because CD4 lymphocyte count was above 300 cells/μL.
Normal IL-7 levels
Normal adult ranges for IL-7 were determined by analyzing samples obtained from 17 normal volunteers (age range 22.2-53.3 years, median 35.4 years). Simultaneous serum and plasma samples established the validity of the IL-7 assay using both sources. The normal values ranged from 0.3 to 8.4 pg/mL (median 2.7 pg/mL, mean 3.1 ± 2.5 pg/mL).
After obtaining informed consent, normal pediatric ranges for circulating IL-7 were determined from plasma obtained from HIV-noninfected children born to HIV-infected mothers. Data and specimens were kindly provided by the Women and Infants Transmission Study (WITS). Eleven patients were analyzed longitudinally at 6 months, 12 months, 18 months, 24 months, 36 months, and 48 months. Nineteen additional samples from HIV-negative children collected at presurgical screening at Children's National Medical Center (CNMC, Washington, DC) following informed consent were also analyzed (age range 2 months to 5.5 years). There was no correlation between IL-7 level and age in either group (r = −0.17, P = .49, andr = −0.19, P = .14, respectively), and there was no correlation between IL-7 level and CD4, CD8, or CD19 count at any of the ages analyzed in the WITS cohort. IL-7 levels were not significantly different between the 2 groups (medians, CNMC 12.7 pg/mL vs WITS 8.97 pg/mL, P = .09, Wilcoxon rank sum test). The range of IL-7 values for the combined cohort, using only the 6-month time point for the WITS group, was 5.6 to 15.9 pg/mL, with a median of 11.7 pg/mL (mean 10.7 ± 3.9 pg/mL).
Enzyme-linked immunosorbent assay
All samples were frozen at the time they were obtained and were previously unthawed. Prior to analysis, samples were thawed on ice and analyzed using a high-sensitivity colorimetric enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer's recommendations. A separate standard curve was prepared on each plate using serial dilutions. Samples exceeding the highest value on the standard curve were reanalyzed using serial dilutions. Plates were read with a Bio-Tek Instruments EL-340 Biokinetics reader (Winooski, VT) and analyzed using Delta Soft 3.0 software (Biometallics, Princeton, NJ). All samples were run in duplicate. At least 2 samples on each plate had been analyzed previously to determine interassay variability. Intraassay and interassay variability was less than 15%.
Immunologic and virologic assessments
Enumeration of lymphocyte subsets was performed using standard flow cytometric methods at central flow cytometry facilities according to National Committee for Clinical Laboratory Standards guidelines. B cells expressed CD19 or CD20. Naive CD4 lymphocytes were CD45RA+/CD45RO− and, where indicated, were also CD62L+. Memory CD4 subsets were CD45RA−/CD45RO+. HIV-1 RNA levels in the pediatric cohort were determined using standard polymerase chain reaction methods (Roche Amplicor Assay, Branchburg, NJ; lower limits of detection 200 copies/mL). For the ACTG group, plasma HIV-1 RNA levels were measured by nucleic acid sequence-based amplification (Organon Teknika, Durham, NC; lower limit of sensitivity adjusted to 100 copies/mL).
Statistical analysis
Association between IL-7 levels and other parameters was calculated using Spearman rank correlation. In the pediatric patients, multiple linear regression analysis was performed at each time point using IL-7 as the dependent variable, and absolute CD4 count,2 absolute CD8 count,2 absolute B-cell count,2 CD4:CD8 ratio,2 age, and CDC classification were considered as possible independent variables to include in the model. At each time point, we report the model that contained no more than 3 independent variables, resulted in the largest adjusted R2, and contained relatively few influential observations (as determined by Cook's D statistic43). The global correlation coefficient across all time points was derived using an adaptation of a method for combining dependent statistical tests using repeated measurements.44
Results
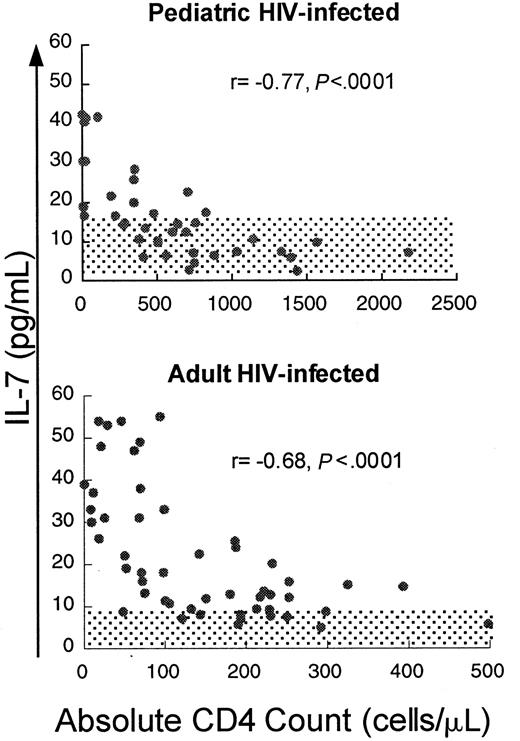
If IL-7 plays a role in the homeostatic regulation of T-cell number, T-cell depletion would be predicted to be associated with increased levels of IL-7. To test this, serum IL-7 levels were measured in HIV-infected pediatric patients (n = 41, median age 6.7 years) with varying degrees of CD4 T-cell depletion. We observed a strong inverse correlation between IL-7 and absolute CD4 count (Figure1) (r = −0.77,P < .0001) and lesser but significant correlations with CD3 count (r = −0.69, P < .0001) and CD8 count (r = −0.54, P = .0007) (Table1). IL-7 levels were only weakly correlated with B-cell counts (r = −0.33,P = .04). There was no correlation with viral load (r = 0.01, P = .96). Viral load analysis included only those samples obtained when patients were not on antiretroviral agents, because most patients receiving antiretroviral therapy had rapid, profound reductions in viral load, potentially confounding any correlation should it exist. No correlation existed between lymphocyte counts and other cytokines tested, including IL-2, IL-4, IL-6, IL-10, IL-15, and interferon-γ (data not shown).
Circulating IL-7 levels show a strong inverse correlation with CD4 counts in children and adults infected with HIV.
Scatter plot of CD4 count and circulating IL-7 level in adult and pediatric patients infected with HIV. Spearman correlation coefficient (r) and corresponding P values are indicated with each figure. Normal ranges for adults and children ± 2 SD are shaded.
Circulating IL-7 levels show a strong inverse correlation with CD4 counts in children and adults infected with HIV.
Scatter plot of CD4 count and circulating IL-7 level in adult and pediatric patients infected with HIV. Spearman correlation coefficient (r) and corresponding P values are indicated with each figure. Normal ranges for adults and children ± 2 SD are shaded.
Correlation between IL-7 and lymphocyte parameters in HIV-infected adults and children
| . | HIV-infected children (n = 41) . | HIV-infected adults (n = 51) . | ||||
|---|---|---|---|---|---|---|
| r . | P . | Median (range) . | r . | P . | Median (range) . | |
| CD3 (cells/μL) | −0.69 | < .0001 | 1552 (259-5813) | −0.43 | .002 | 845 (83-2775) |
| CD4 (cells/μL) | −0.77 | < .0001 | 504 (0-2558) | −0.68 | < .0001 | 121 (0-498) |
| CD8 (cells/μL) | −0.54 | .0007 | 1014 (42-4534) | −0.34 | .02 | 633 (72-2450) |
| CD4+CD45RA+(cells/μL) | −0.70 | .0004 | 375 (0-1171) | −0.69 | < .0001 | 47 (2-222)* |
| CD4+CD45RO+(cells/μL) | −0.78 | < .0001 | 188 (0-476) | 0.02 | .91 | 103 (14-307)* |
| CD4:CD8 | −0.57 | .0003 | 0.49 (0-1.88) | −0.60 | < .0001 | 0.16 (0-0.56) |
| B cell (cells/μL) | −0.33 | .04 | 753 (172-2789) | 0.03 | .87 | 372 (21-1029) |
| IL-7 (pg/mL) | 14.1 (2.5-64.0) | 18.0 (4.9-55.0) | ||||
| . | HIV-infected children (n = 41) . | HIV-infected adults (n = 51) . | ||||
|---|---|---|---|---|---|---|
| r . | P . | Median (range) . | r . | P . | Median (range) . | |
| CD3 (cells/μL) | −0.69 | < .0001 | 1552 (259-5813) | −0.43 | .002 | 845 (83-2775) |
| CD4 (cells/μL) | −0.77 | < .0001 | 504 (0-2558) | −0.68 | < .0001 | 121 (0-498) |
| CD8 (cells/μL) | −0.54 | .0007 | 1014 (42-4534) | −0.34 | .02 | 633 (72-2450) |
| CD4+CD45RA+(cells/μL) | −0.70 | .0004 | 375 (0-1171) | −0.69 | < .0001 | 47 (2-222)* |
| CD4+CD45RO+(cells/μL) | −0.78 | < .0001 | 188 (0-476) | 0.02 | .91 | 103 (14-307)* |
| CD4:CD8 | −0.57 | .0003 | 0.49 (0-1.88) | −0.60 | < .0001 | 0.16 (0-0.56) |
| B cell (cells/μL) | −0.33 | .04 | 753 (172-2789) | 0.03 | .87 | 372 (21-1029) |
| IL-7 (pg/mL) | 14.1 (2.5-64.0) | 18.0 (4.9-55.0) | ||||
n = 33. CD4+CD45RA+ cells in the adult HIV-infected group were also CD62L+.
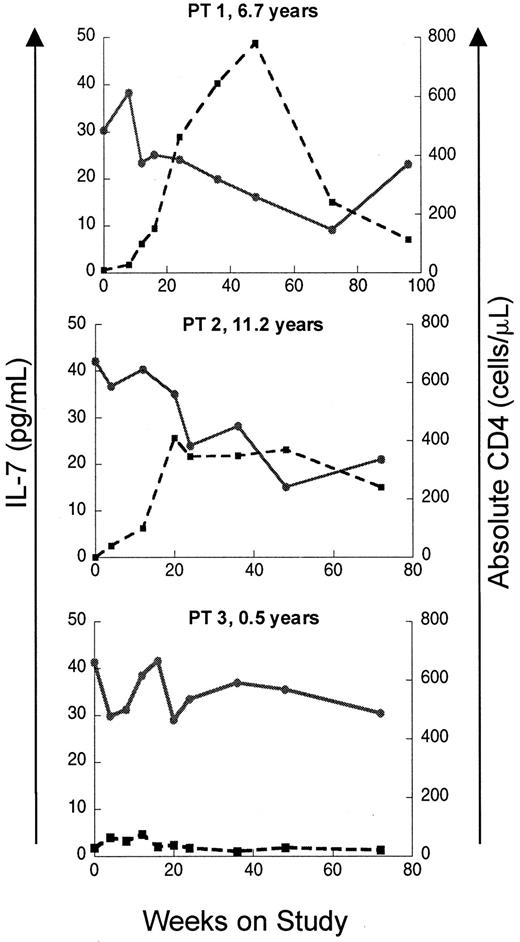
If elevated levels of IL-7 in patients with T-cell lymphopenia reflect a homeostatic response, recovery of CD4 counts should lead to a decline in IL-7 levels. To test this, IL–7 levels were analyzed longitudinally in a subset of the pediatric HIV-infected cohort enrolled on a protease inhibitor–based HAART. As seen in Figure2, patients with a good immunologic response to HAART showed temporally related declines in serum IL-7 levels, while patients with persistently low CD4 counts despite antiretroviral therapy maintained elevated serum IL-7 levels throughout the trial. Sequential multiple linear regression analyses were also performed on this cohort. Among the multiple independent variables considered (CD4 count, CD8 count, CD4:CD8 ratio, B-cell count, absolute lymphocyte count, age, and CDC classification), the most important variables in defining circulating IL-7 level were CD4 count, CD8 count, and CD4:CD8 ratio (Table 2). As a measure of the overall correlation between serum IL-7 levels and lymphocyte counts during the entire period of the study, global correlation coefficients were calculated for each parameter using data from all available time points. The strongest inverse correlation was between IL-7 and CD4 cells (global correlation coefficient −0.82,P < .0001) and total lymphocytes (−0.80,P < .0001). Weaker global correlation coefficients existed for CD8 cells (−0.62, P < .0001), CD4:CD8 (−0.54, P = .016), and absolute B cells (−0.65,P < .0001).
Recovery of CD4 counts following initiation of ritonavir-based antiretroviral therapy is associated with declines in circulating IL-7 levels in HIV-infected pediatric patients.
Plots of serum IL-7 level and absolute CD4 count versus time on study is shown for 3 patients. Patients 1 and 2 display an immunologic response to HAART with increases in CD4 count and concomitant declines in IL-7. Patient 3 had a poor response to therapy with persistently low CD4 counts and high IL-7 levels. Patient ages at enrollment are indicated. ● and solid line indicate IL-7; ■ and broken line, CD4.
Recovery of CD4 counts following initiation of ritonavir-based antiretroviral therapy is associated with declines in circulating IL-7 levels in HIV-infected pediatric patients.
Plots of serum IL-7 level and absolute CD4 count versus time on study is shown for 3 patients. Patients 1 and 2 display an immunologic response to HAART with increases in CD4 count and concomitant declines in IL-7. Patient 3 had a poor response to therapy with persistently low CD4 counts and high IL-7 levels. Patient ages at enrollment are indicated. ● and solid line indicate IL-7; ■ and broken line, CD4.
Multiple linear regression models for each time point during HAART in HIV-infected children
| Week . | Model . | No. . | Adjusted R2 . |
|---|---|---|---|
| 0 | IL-7= 42.1+0.016(CD4)−0.0147(CD8) −38(CD4:CD8) | 16 | 0.78 |
| 4 | IL-7= 27.9−0.021(CD4)+4.2E−6(CD4)2 | 15 | 0.66 |
| 8 | IL-7= 37.6−0.0088(CD8)−17.2(CD4:CD8) | 12 | 0.86 |
| 12 | IL-7= 41.4−0.0136(B cell)−8.6(CD4:CD8) | 13 | 0.63 |
| 16 | IL-7= 39.2−0.0094(CD8)−16.4(CD4:CD8) | 11 | 0.82 |
| 20 | IL-7= 37.2−0.0096(CD8)−12.3(CD4:CD8) | 13 | 0.66 |
| 24 | IL-7= 35.4−0.0078(CD8)−18.5(CD4:CD8) +4.0(CD4:CD8)2 | 14 | 0.84 |
| 36 | IL-7= 30.9−0.029(CD4)+7.8E−6(CD4)2 | 15 | 0.61 |
| 48 | IL-7= 32.5−0.0078(CD8)−13.3(CD4:CD8) | 12 | 0.84 |
| 72 | IL-7= 27.3−0.0075(CD8)−9.9(CD4:CD8) | 13 | 0.66 |
| 96 | IL-7= 27.1−0.033(CD4)+1.03E−5(CD4)2 | 10 | 0.70 |
| Week . | Model . | No. . | Adjusted R2 . |
|---|---|---|---|
| 0 | IL-7= 42.1+0.016(CD4)−0.0147(CD8) −38(CD4:CD8) | 16 | 0.78 |
| 4 | IL-7= 27.9−0.021(CD4)+4.2E−6(CD4)2 | 15 | 0.66 |
| 8 | IL-7= 37.6−0.0088(CD8)−17.2(CD4:CD8) | 12 | 0.86 |
| 12 | IL-7= 41.4−0.0136(B cell)−8.6(CD4:CD8) | 13 | 0.63 |
| 16 | IL-7= 39.2−0.0094(CD8)−16.4(CD4:CD8) | 11 | 0.82 |
| 20 | IL-7= 37.2−0.0096(CD8)−12.3(CD4:CD8) | 13 | 0.66 |
| 24 | IL-7= 35.4−0.0078(CD8)−18.5(CD4:CD8) +4.0(CD4:CD8)2 | 14 | 0.84 |
| 36 | IL-7= 30.9−0.029(CD4)+7.8E−6(CD4)2 | 15 | 0.61 |
| 48 | IL-7= 32.5−0.0078(CD8)−13.3(CD4:CD8) | 12 | 0.84 |
| 72 | IL-7= 27.3−0.0075(CD8)−9.9(CD4:CD8) | 13 | 0.66 |
| 96 | IL-7= 27.1−0.033(CD4)+1.03E−5(CD4)2 | 10 | 0.70 |
Given the evidence that a substantial amount of IL-7 is produced within the thymus,45 one potential source for the increased IL-7 observed in the HIV-infected pediatric cohort is thymic stroma. If the thymus was the principal site of production for the increased IL-7 seen in HIV-infected children with CD4 T-cell depletion, one would predict lower IL-7 levels in CD4-depleted HIV-infected adults as a result of reduced thymic mass in relation to total body mass.46 To address this, we measured IL-7 levels in a cohort of HIV-infected adult patients, including 20 adults with severe (< 100 cells/μL) and 31 patients with less severe CD4 depletion (100-300 cells/μL at initial screening). IL-7 levels in this cohort were significantly higher (median 15.8 pg/mL, mean 22.3 ± 14.6 pg/mL) than in normal adult volunteers (n = 17, median 2.7 pg/mL, mean 3.1 ± 2.5 pg/mL,P < .0001, Wilcoxon rank sum test) and were elevated to the same degree as the HIV-infected pediatric cohort (median 14.1 pg/mL, mean 17.2 ± 13.3 pg/mL, P = .15, Wilcoxon rank sum test). As observed in the HIV-infected pediatric cohort, there was a strong inverse correlation between IL-7 and CD4 cells (r = −0.68, P < .0001) and significant correlations with peripheral blood CD3 cells (r = −0.43,P = .002) and CD8 cells (r = −0.34,P = .02) but no correlation between IL-7 levels and B cells (r = 0.03, P = .87) (Table 1) or viral load (r = 0.28, P = .15). Thus, IL-7 levels were elevated to the same degree in adult patients with CD4 cell depletion due to HIV infection as in pediatric patients, providing evidence that a substantial amount of thymic mass was not required to generate high circulating IL-7 levels and suggesting an alternative source for IL-7 production.
Longitudinal data and extensive immunophenotyping for patients enrolled on a HAART clinical trial (ACTG 315, n = 31) allowed analysis of relationships between circulating IL-7 levels and lymphocyte subsets over time in this population with moderate CD4 cell depletion (median CD4 count at baseline, 213 cells/μL). When this population was analyzed independent of the more severely depleted HIV-infected adults for whom longitudinal data were not available, IL-7 levels were not correlated with the only moderately depleted CD4 counts (Table3). However, a strong inverse correlation was observed with the profoundly depleted CD4+RA+62L+ subset. Because recovery of this subset occurred during HAART, this inverse relationship progressively diminished and was lost by 24 weeks. Therefore, in HIV infection, an inverse correlation existed between CD4 counts and circulating IL-7 levels in the setting of profound CD4 cell depletion (CD4 count < 100 cells/μL), whereas this relationship is not observed in populations with moderate CD4 cell depletion or in healthy, non–CD4 cell–depleted individuals. Furthermore, these data suggest that isolated depletion of the CD4+45RA+62L+ subset is sufficient to maintain elevated circulating IL-7 levels but, again, the relationship is lost upon partial recovery of this subset and normalization of circulating IL-7 levels.
Correlation between IL-7 and lymphocyte or viral parameters in moderately CD4-depleted HIV-infected adults during HAART
| . | Baseline . | 4 weeks . | 12 weeks . | 24 weeks . | 48 weeks . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | |
| CD3 (cells/μL) | 0.19 | .92 | 911 | 0.13 | .47 | 1194 | 0.40 | .04 | 1321 | 0.50 | .03 | 1140 | 0.05 | .80 | 1148 |
| CD4 (cells/μL) | −0.23 | .21 | 213 | −0.22 | .24 | 298 | 0.11 | .58 | 334 | 0.46 | .06 | 336 | 0.13 | .49 | 363 |
| CD8 (cells/μL) | 0.06 | .74 | 735 | 0.18 | .34 | 824 | 0.42 | .03 | 1015 | 0.56 | .02 | 820 | 0.04 | .49 | 766 |
| CD4+CD45RA+62L+(cells/μL) | −0.67 | .0002 | 48 | −0.43 | .02 | 87 | −0.48 | .02 | 84 | −0.13 | .59 | 87 | 0.02 | .91 | 115 |
| CD4+CD45RO+RA−(cells/μL) | 0.07 | .71 | 114 | 0.27 | .15 | 173 | 0.22 | .27 | 176 | 0.55 | .02 | 189 | 0.13 | .52 | 179 |
| CD4:CD8 | −0.34 | .06 | 0.28 | −0.34 | .07 | 0.31 | −0.51 | .008 | 0.22 | −0.46 | .05 | 0.39 | −0.01 | .98 | 0.43 |
| HIV RNA log10 | 0.28 | .15 | 4.85 | −0.18 | .35 | 2.98 | −0.26 | .20 | 2.14 | −0.11 | .66 | 2.00 | 0.21 | .28 | 2.00 |
| . | Baseline . | 4 weeks . | 12 weeks . | 24 weeks . | 48 weeks . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | |
| CD3 (cells/μL) | 0.19 | .92 | 911 | 0.13 | .47 | 1194 | 0.40 | .04 | 1321 | 0.50 | .03 | 1140 | 0.05 | .80 | 1148 |
| CD4 (cells/μL) | −0.23 | .21 | 213 | −0.22 | .24 | 298 | 0.11 | .58 | 334 | 0.46 | .06 | 336 | 0.13 | .49 | 363 |
| CD8 (cells/μL) | 0.06 | .74 | 735 | 0.18 | .34 | 824 | 0.42 | .03 | 1015 | 0.56 | .02 | 820 | 0.04 | .49 | 766 |
| CD4+CD45RA+62L+(cells/μL) | −0.67 | .0002 | 48 | −0.43 | .02 | 87 | −0.48 | .02 | 84 | −0.13 | .59 | 87 | 0.02 | .91 | 115 |
| CD4+CD45RO+RA−(cells/μL) | 0.07 | .71 | 114 | 0.27 | .15 | 173 | 0.22 | .27 | 176 | 0.55 | .02 | 189 | 0.13 | .52 | 179 |
| CD4:CD8 | −0.34 | .06 | 0.28 | −0.34 | .07 | 0.31 | −0.51 | .008 | 0.22 | −0.46 | .05 | 0.39 | −0.01 | .98 | 0.43 |
| HIV RNA log10 | 0.28 | .15 | 4.85 | −0.18 | .35 | 2.98 | −0.26 | .20 | 2.14 | −0.11 | .66 | 2.00 | 0.21 | .28 | 2.00 |
No. ranged from 26 to 31.
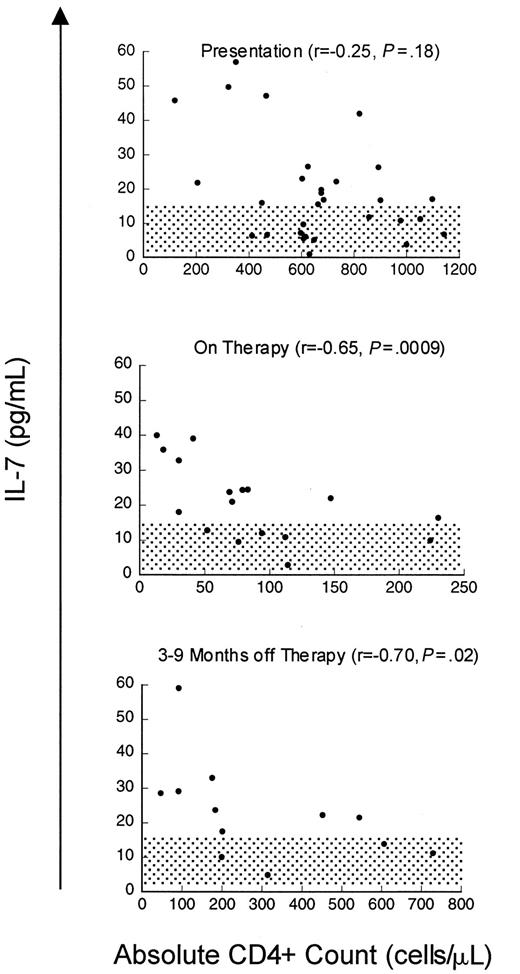
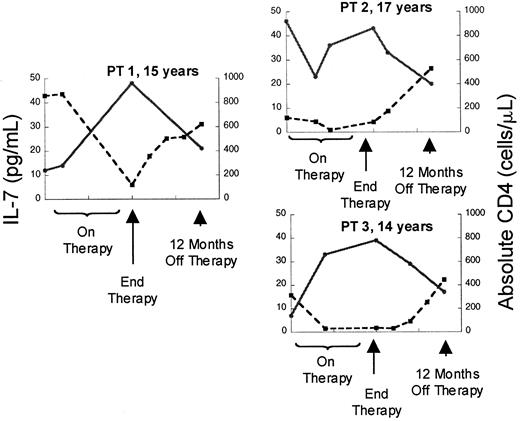
Although the relationship between CD4 cells or CD4 cell subsets and IL-7 levels in HIV is striking, the increases in serum IL-7 levels observed in patients with advanced HIV infection could be a disease-specific phenomenon related to direct or indirect effects of the virus rather than reflecting a causal relationship between CD4 cell depletion and IL-7 levels. Therefore, we also analyzed a cohort of patients with cancer treated with T-cell–depleting chemotherapeutic regimens to determine whether these findings could be extended to other states associated with T-cell deficiency. Prior to chemotherapy (n = 30, median CD4 count 639 cells/μL), there was no correlation between IL-7 levels and CD4 count, similar to non–T-cell–depleted, HIV-uninfected children. However, as seen in Figure3, depletion of CD4 cells with chemotherapy (treated with 3-10 cycles, median 76 cells/μL) resulted in a rise in serum IL-7 levels and a strong inverse correlation between IL-7 levels and CD4 counts (n = 17, r = −0.65,P = .009). During this time, the CD8 cells were also reduced (median 108 cells/μL) but no correlation was observed with this subset (r = 0.04, P = .82) (Table4). Following T-cell–depleting chemotherapy, as described previously, B cells and CD8 T cells typically recover within approximately 3 months, often increasing to supranormal levels,20 whereas CD4 T cells display variable recovery depending upon the age of the host.4 As seen in Figure 3, analysis of IL-7 levels during the period that spanned 6 to 9 months following chemotherapy (median CD4 count 200 cells/μL, median CD8 count 396 cells/μL, median CD20 count 200 cells/μL) showed that there remained a strong inverse correlation between circulating IL-7 and CD4 cell numbers (n = 12, r = −0.70,P = .02). There was no correlation between IL-7 and CD3, CD8, or CD20 cells during this time (Table 4). Further, in individual patients, recovery of CD4 counts following cessation of therapy was associated with declines in serum IL-7 levels (Figure4). Therefore, following chemotherapy for cancer, as in HIV infection, CD4 cell depletion leads to significant rises in circulating IL-7 levels. Because this cohort also sustained significant CD8 cell depletion, it is interesting that the relationship between CD4 T cells and IL-7 is stronger than that observed with CD8 T cells. These findings raise the possibility that CD4 cell populations alone are involved in regulation of circulating IL-7 levels.
Inverse correlation between CD4 counts and circulating IL-7 levels during chemotherapy-induced CD4 lymphopenia.
Scatter plot of serum IL-7 level and CD4 count in children and young adults with solid tumors at presentation (n = 30), during chemotherapy (n = 17), and 6 to 9 months after completing therapy (n = 12). Spearman correlation (r) and P value are shown with each graph. Normal IL-7 ranges for adults ± SD 2 are shaded.
Inverse correlation between CD4 counts and circulating IL-7 levels during chemotherapy-induced CD4 lymphopenia.
Scatter plot of serum IL-7 level and CD4 count in children and young adults with solid tumors at presentation (n = 30), during chemotherapy (n = 17), and 6 to 9 months after completing therapy (n = 12). Spearman correlation (r) and P value are shown with each graph. Normal IL-7 ranges for adults ± SD 2 are shaded.
Correlation between IL-7 and lymphocyte parameters in patients with cancer
| . | Baseline (n = 30) . | On therapy (n = 17) . | Off therapy (n = 12) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | |
| CD3 (cells/μL) | −0.14 | .44 | 1 080 | −0.24 | .34 | 272 | −0.32 | .29 | 812 |
| CD4 (cells/μL) | −0.25 | .18 | 639 | −0.65 | .009 | 76 | −0.70 | .02 | 200 |
| CD8 (cells/μL) | 0.04 | .82 | 348 | −0.14 | .57 | 108 | −0.06 | .84 | 396 |
| CD4+CD45RA+RO−(cells/μL) | −0.19 | .33 | 264 | −0.04 | .77 | 1.0 | −0.50 | .10 | 29 |
| CD4+CD45RO+RA−(cells/μL) | −0.23 | .23 | 269 | −0.60 | .02 | 70 | −0.76 | .02 | 127 |
| CD4:CD8 | −0.42 | .03 | 1.76 | −0.14 | .58 | 0.73 | −0.66 | .03 | 0.53 |
| B cells (cells/μL) | −0.21 | .40 | 174 | −0.46 | .05 | 4 | −0.26 | .39 | 200 |
| . | Baseline (n = 30) . | On therapy (n = 17) . | Off therapy (n = 12) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r . | P . | Median . | r . | P . | Median . | r . | P . | Median . | |
| CD3 (cells/μL) | −0.14 | .44 | 1 080 | −0.24 | .34 | 272 | −0.32 | .29 | 812 |
| CD4 (cells/μL) | −0.25 | .18 | 639 | −0.65 | .009 | 76 | −0.70 | .02 | 200 |
| CD8 (cells/μL) | 0.04 | .82 | 348 | −0.14 | .57 | 108 | −0.06 | .84 | 396 |
| CD4+CD45RA+RO−(cells/μL) | −0.19 | .33 | 264 | −0.04 | .77 | 1.0 | −0.50 | .10 | 29 |
| CD4+CD45RO+RA−(cells/μL) | −0.23 | .23 | 269 | −0.60 | .02 | 70 | −0.76 | .02 | 127 |
| CD4:CD8 | −0.42 | .03 | 1.76 | −0.14 | .58 | 0.73 | −0.66 | .03 | 0.53 |
| B cells (cells/μL) | −0.21 | .40 | 174 | −0.46 | .05 | 4 | −0.26 | .39 | 200 |
Depletion of CD4 counts by chemotherapy is associated with rises in circulating IL-7 followed by declines occurring concomitant with recovery of CD counts.
Plot of serum IL-7 level (l) and absolute CD4 count (n) versus time in 3 representative patients treated with dose-intensive chemotherapy for solid tumors. ● and solid line indicate IL-7; ■ and broken line, CD4.
Depletion of CD4 counts by chemotherapy is associated with rises in circulating IL-7 followed by declines occurring concomitant with recovery of CD counts.
Plot of serum IL-7 level (l) and absolute CD4 count (n) versus time in 3 representative patients treated with dose-intensive chemotherapy for solid tumors. ● and solid line indicate IL-7; ■ and broken line, CD4.
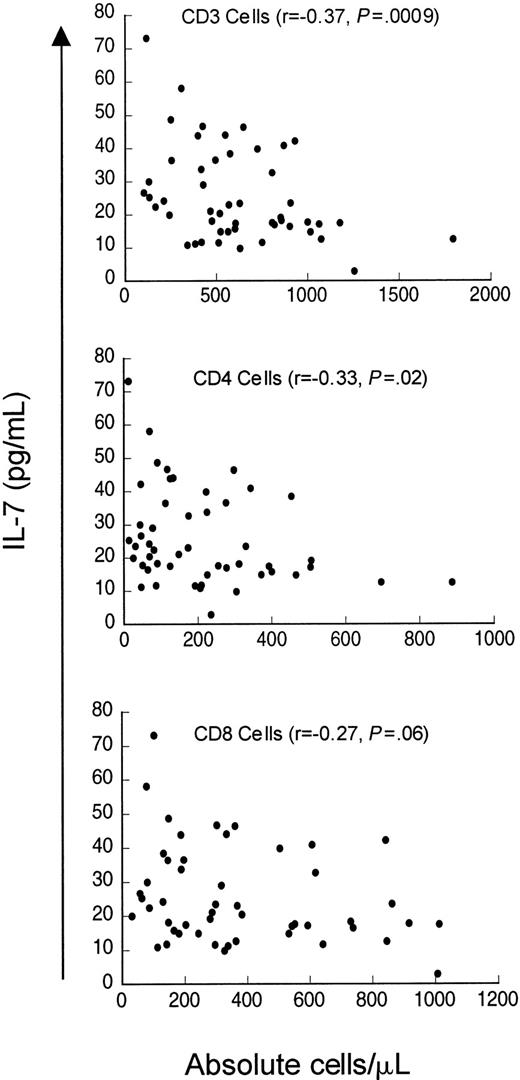
Finally, we analyzed serum IL-7 levels in a cohort of patients evaluated for idiopathic CD4 lymphopenia. When the entire cohort was analyzed (n = 50), including patients who did not meet the case definition at initial evaluation (median CD4 count 235 cells/μL, range 12-887 cells/μL), weak inverse correlations existed between serum IL-7 levels and CD4 counts (r = −0.33,P = .02) and CD3 counts (r = −0.37,P = .009) but not with CD8 counts (r = −0.27, P = .06) (Figure5). Interestingly, when only patients meeting the case definition (CD4 count < 300 cells/μL) were analyzed (n = 37), there was no correlation between serum IL-7 level and CD4 cells (r = −0.155, P = .35) or CD8 cells (r = −0.30, P = .07), but a weak inverse correlation between serum IL-7 level and CD3 count was observed (r = −0.33, P = .05). Therefore, in contrast to patients with CD4 depletion due to HIV infection or cancer chemotherapy, patients in this cohort of idiopathic CD4 lymphopenia did not show strong inverse relationships between serum IL-7 levels and peripheral blood CD4 counts. Interestingly, Figure 5 shows that there is a subset of patients in this cohort with unexpectedly low serum IL-7 levels despite extremely low CD4 counts (clustered in the lower left of the figure). This group partially explains the lack of correlation between CD4 counts and IL-7 levels in patients meeting the case definition and may suggest a potential factor contributing to the derangement in CD4 homeostasis in a subset of this heterogeneous group of patients.
Weak inverse correlation between circulating IL-7 and CD3 and CD4 counts but not with CD8 counts in patients evaluated for idiopathic CD4 lymphopenia.
Scatter plot of serum IL-7 and CD3+, CD4+, and CD8+ count in 51 patients evaluated for idiopathic CD4 lymphopenia. Spearman correlation (r) and P value are shown with each graph.
Weak inverse correlation between circulating IL-7 and CD3 and CD4 counts but not with CD8 counts in patients evaluated for idiopathic CD4 lymphopenia.
Scatter plot of serum IL-7 and CD3+, CD4+, and CD8+ count in 51 patients evaluated for idiopathic CD4 lymphopenia. Spearman correlation (r) and P value are shown with each graph.
Discussion
T-cell depletion is associated with increases in circulating IL-7 levels, suggesting that IL-7 may be involved in homeostatic regulation of T-cell numbers. These results confirm and expand on the study by Bolotin et al,47 which demonstrated increased IL-7 levels in pediatric patients undergoing bone marrow transplantation wherein the highest IL-7 levels were observed in patients with the most severe lymphocyte depletion. Additional mechanistic data from murine models indicate that IL-7 increases T-cell numbers, often to supranormal levels, both in T-cell–replete hosts31 and following bone marrow transplantation.48 IL-7 enhances both thymic-dependent pathways and thymic-independent peripheral expansion following T-cell depletion.49 Further, IL-7 therapy restores immune responses in both thymus-bearing48 and thymus-deficient hosts50 following T-cell depletion. Lastly, emerging data suggest that IL-7 can also enhance extrathymic pathways of T-cell differentiation (Kenneth Weinberg, written communication, 2000). Thus, the effects of IL-7 on multiple pathways of T-cell regeneration suggest a model wherein increased availability of IL-7 following CD4 depletion would be highly effective in restoring host immune competence.
The mechanism underlying the increases in circulating IL-7 levels observed in states of T-cell depletion are not clear and will require additional studies using animal systems. At least 2 scenarios are possible. In the first, decreased T-cell numbers result in diminished IL-7 receptor availability, leading to increased levels of free IL-7 with no change in overall IL-7 production, as has been suggested for thrombopoietin.51,52 Such a model requires no interaction between the IL-7–producing cells and peripheral T cells but could result in elevated levels of this cytokine in the microenvironment of the lymphoid tissues. Evidence against this model comes from analysis of the results presented here. If increased circulating IL-7 resulted solely from diminished target cell binding, this should occur in all clinical settings associated with CD4 depletion, including idiopathic CD4 lymphopenia, unless there is an increase in IL-7 receptor expression in this entity. Furthermore, while CD8 cells also express IL-7 receptor,53,54 preliminary results suggest that the level may be less than that seen on resting CD4 cells, which would provide a possible explanation for why expansions of this subset following cancer chemotherapy are not sufficient to lower circulating IL-7 levels in the absence of CD4 cell recovery. Finally, in patients recovering from CD4 depletion, there appears to be a delay before IL-7 levels return to normal, whereas regulation by binding to IL-7 receptor would be expected to occur rapidly. In the second model, changes in T-cell counts would lead to increased production of IL-7, as occurs with erythropoietin.55 This model invokes interaction between T-cell populations and IL-7–producing cells via a soluble mediator or through direct contact within the lymphoid microenvironment. The nature of such an interaction has not been defined but could involve local production of a factor such as transforming growth factor-β by T cells that can down-regulate stromal IL-7 secretion.56 However, naive CD4+cells appear to not express transforming growth factor-β,57 thus invoking another possible mediator for down-regulation of IL-7 secretion that occurs coincident with the recovery of this subset. Indeed, Napolitano et al have demonstrated increased IL-7 within the lymphoid tissues associated with follicular dendritic cells in the setting of HIV-induced CD4 depletion.58 Regardless of the mechanism, the levels of IL-7 we have observed in the circulation are likely to reflect more significant alterations of available IL-7 within the tissue microenvironment and would be compatible with IL-7 as a primary regulator of T-cell homeostasis.
Interestingly, in both HIV-infected groups, the inverse relationship is not linear with the slope, increasing substantially at CD4 counts below 200 cells/μL. In attempting to understand this, it is important to note that all examinations employed in this report are derived from the peripheral blood. As shown previously, peripheral blood lymphocyte counts correlate only roughly with tissue lymphocyte counts because lymphocyte numbers in nodal tissues are relatively well preserved despite relatively early depletion in the peripheral blood.59 60 Similarly, circulating IL-7 levels might only rise after increases have already occurred in the lymphoid tissue to a degree sufficient to allow “spillover” into the circulation. Therefore, the inflection point of this curve, while of interest, may not accurately reflect the absolute point at which an increased level of IL-7 is available to cells within the lymphoid tissue itself.
From these observations, a number of potential therapeutic implications emerge. Because replication of HIV is enhanced in activated CD4 cells61 and IL-7 can enhance the replication of HIV in in vitro systems,62 neutralizing of IL-7 in patients with increased circulating levels could potentially diminish viral replication. Additionally, lymphodepletion has been associated with the development of lymphoproliferative disorders and autoimmunity. In animal models, IL-7 can contribute to both scenarios,63-66potentially implicating chronic elevation of circulating IL-7 in such events. Finally, following control of viral replication with antiretroviral agents, and in the setting of chemotherapy-induced T-cell depletion, pharmacologic doses of IL-7 would be predicted to enhance immune reconstitution.
In summary, CD4 T-cell deletion in humans leads to elevated circulating levels of IL-7. In HIV and following cancer chemotherapy, significant inverse correlations exist between CD4+ and CD4+CD45RA+ T cells in patients with profound depletion of these subsets. Restoration of these subsets leads to normalization of circulating IL-7 levels and loss of the inverse relationship. Some patients with idiopathic CD4 lymphopenia have an aberrant relationship between IL-7 and CD4 count, suggesting a disruption of this system in a subset of these patients. We postulate that elevation of IL-7 within the lymphoid microenvironment in patients with CD4 depletion contributes to the increased peripheral expansion, accumulation of activated cells, and thymic rebound observed CD4-depleted hosts.
We thank Drs Claire Chougnet and Gene Shearer for providing the presurgical pediatric samples and Kathleen M. Wyvill for sample coordination and data analysis for the HIV-infected adults from the NCI. We also thank all members of the AIDS Clinical Trials Group and acknowledge WITS for providing samples for determination of IL-7 levels in HIV-uninfected children. WITS consists of 6 clinical centers (multiple sites located in Boston and Worcester, MA; Columbia-Presbyterian Medical Center, New York, NY; University of Illinois at Chicago; University of Puerto Rico; State University of New York at Brooklyn; and Baylor College of Medicine, Waco, TX), one coordinating center (Clinical Trials and Surveys Corporation), and is jointly funded by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, and National Institute on Drug Abuse. Finally, we thank Drs Ron Gress and Ron Germain for their careful review of the manuscript.
AIDS Clinical Trials Group studies were funded by the National Institutes of Health (AI-25879, AI-32770, AI-25915, AI-44748, AI-38855, AI-38858, R-0080, RR-00051, CA-46934).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
T. Fry, Bldg 10, Rm 13N240, MSC 1928, 10 Center Dr, Bethesda, MD 20892-1928; e-mail: tf60y@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal