Abstract
Systemic vasculitis is an uncommon manifestation of X-linked lymphoproliferative disease (XLP), a disorder in which there is a selective immune deficiency to Epstein-Barr virus (EBV). The molecular basis for XLP has recently been ascribed to mutations within SLAM-associated protein (SAP), an SH2 domain–containing protein expressed primarily in T cells. The authors describe a patient who died as a result of chronic systemic vasculitis and fulfilled clinical criteria for the diagnosis of XLP. Sequencing of this patient'sSAP gene uncovered a novel point mutation affecting the SH2 domain. The patient presented with virus-associated hemophagocytic syndrome (VAHS) and later had chorioretinitis, bronchiectasis, and hypogammaglobulinemia develop. He further developed mononeuritis and fatal respiratory failure. Evidence of widespread small and medium vessel vasculitis was noted at autopsy with involvement of retinal, cerebral, and coronary arteries as well as the segmental vessels of the kidneys, testes, and pancreas. Immunohistochemical analysis using antibodies to CD20, CD45RO, and CD8 revealed that the vessel wall infiltrates consisted primarily of CD8+ T cells, implying a cytotoxic T-lymphocyte response to antigen. EBV DNA was detected by polymerase chain reaction (PCR) in arterial wall tissue microdissected from infiltrated vessels further suggesting that the CD8+ T cells were targeting EBV antigens within the endothelium. The authors propose that functional inactivation of the SAP protein can impair the immunologic response to EBV, resulting in systemic vasculitis.
Introduction
X-linked lymphoproliferative syndrome (XLP) or Duncan disease is a fascinating disease that presents as an Epstein-Barr virus (EBV)-specific immune defect. The original case, described by David Purtilo in 19751 involved an 8-year-old boy who died of fulminant hepatitis and bone marrow failure 1 month after the onset of acute infectious mononucleosis (IM). A few years later, it was noted that 2 brothers had also died of illnesses resembling fulminant IM, and it was learned that 3 maternally related male cousins had also died as a result of complications of an EBV infection. Purtilo correctly deduced that a novel X-linked disease was present in this family — a disease that resulted in substantial mortality and morbidity after primary exposure to EBV. By 1978, an XLP registry was established to facilitate the study of the disease, and 3 main clinical presentations were ascribed2: (I) fulminant infectious mononucleosis and death, (ii) dysgammaglobulinemia with elevated levels of IgM and IgA and decreased levels of IgG, and (iii) extranodal lymphomas of either B- or T-cell origin. Other less common expressions of the disease have been reported, including aplastic anemia, virus-associated hemophagocytosis (VAHS), and pulmonary vasculitis. Many XLP kindred have been studied extensively in an effort to characterize the nature of the immune defect, leading to a specific susceptibility to EBV. Interest in these cases continues as EBV infection is ubiquitous and has been implicated in the genesis of both lymphoid and mesenchymal tumors. It has been hoped that a better understanding of patients with XLP and their immunologic defects may shed light on the unique nature of the relationship between EBV and human infection.
The molecular basis for XLP has recently been ascribed to mutations affecting the SLAM-associated protein (SAP).3-5 SAP is a Src-homology 2 (SH2) domain-containing protein. Expression of SAP protein has been detected in both murine thymus and human peripheral blood lymphocytes.3 SAP RNA has been detected primarily in T cells, T-cell lines, and in both T- and B-cell neoplasms.5 Expression of RNA has been detected in lymphoid germinal centers and natural killer (NK) cells by some4 but not others.3 The SAP gene contains 4 exons and encodes a protein of 128 amino acids with a single SH2 domain. This SH2 domain has been shown to interact with the costimulatory surface molecule termed signaling lymphocyte activation molecule (SLAM)/CDw150 in lymphocytes3 and the costimulatory molecule 2B4 present in NK cells and some T cells.6 The binding of SAP to 2B4 or SLAM has been proposed to regulate cell signaling through these molecules. How the described mutations in SAP produce an XLP phenotype is still unclear.
We describe a patient who died as a result of chronic systemic vasculitis and fulfilled clinical criteria for the diagnosis of XLP. Sequencing of this patient's SAP gene revealed a novel mutation affecting the SH2 domain. Immunohistochemical analysis using antibodies to CD45RO and CD8 revealed that the vessel infiltrates consisted primarily of T cells. Lymphoid vasculitis has rarely been described in association with XLP. Patients with features of pulmonary lymphomatoid granulomatosis, pulmonary Wegener disease, and necrotizing vasculitis with aneurysmal dilatation have been reported.2,3,7 To our knowledge, this is the third reported case of necrotizing lymphoid vasculitis associated with XLP and the first to document chorioretinitis as a manifestation of this vasculitic syndrome. Large-vessel arteritis and Kawasaki-like arteritis have been associated with chronic EBV infection, and γ-herpesvirus 68,8-10 a virus related to EBV, has been shown to cause large-vessel arteritis in mice.11 We propose that functional inactivation of the SAP protein can impair the immunologic response to EBV, resulting in systemic vasculitis.
Materials and methods
Autopsy and patient samples
Postmortem examinations of both the index patient and his maternal first cousin were performed at British Columbia's Children's Hospital. Tissues were processed and stained both with hematoxylin and eosin (H&E) and Martius-scarlet-blue (MSB) for histologic examination. A pedigree of the proband and his family was constructed, and peripheral blood samples were obtained from relatives after obtaining informed consent (UBC Clinical Research Ethics Board).
SAP cloning and sequencing
Paraffin sections of autopsy tissue were obtained from the tissue archive for amplification of the SAP gene and DNA sequencing. Paraffin sections of liver were dewaxed with xylene, dehydrated with increasing concentrations of alcohol, and the DNA was isolated by phenol-chloroform extraction. SAP exons were amplified by polymerase chain reaction (PCR) using methods previously described.4 Briefly, the primers were: exon 1 forward GTT GAG CTA AGT TAT TCC TG, exon 1 reverse TGA GGC GAA AGT GTG TTC CA, exon 2 forward CAA TGA CAC CAT ATA CGT GT, exon 2 reverse GCT TTC TTA ATG ATC CAT GA, exon 3 forward CAA GTT ACA CAA ATG TTA, exon 3 reverse CTT GGA CTC ATA ACT CTG, exon 4 forward TCA TTG TGA GTT TTA TGC AT, and exon 4 reverse GCT CAC CGA ACT GTA TTA. All 4 exons were amplified at 95°C × 5 minutes, followed by 30 cycles of 95°C × 40 seconds, 55°C × 40 seconds, 72°C × 40 seconds, and a final extension of 72°C × 10 minutes. The PCR product was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) and sequenced by the dideoxynucleotide method. Restriction enzyme analysis was performed by digesting PCR-amplified exon 2 products with BfaI (New England Biolabs, Beverly, MA).
Immunohistochemistry
Selected paraffin sections of tissue containing vasculitic lesions were adhered to silane slides and incubated with antibodies to CD20 (L26, DAKO, Carpinteria, CA), CD45R0 (UCHL1, Signet Laboratories Inc, Dedham, MA), or CD8 (DAKO), followed by biotinylated secondary antibody, Ultra Streptavidin-HRP (Signet Laboratories Inc) and substrate.
Laser capture microdissection and Epstein-Barr virus detection
Paraffin sections of 10 μM were stained with H&E by conventional methods and dehydrated through a graded ethanol series to xylene, then allowed to air dry. Dry sections were subjected to laser capture microdissection (LCM) using a Pix Cell LCM instrument (Arcturus Engineering Inc, Mountain View, CA) following the manufacturer's protocols. Vascular endothelium, free of obvious lymphocytic infiltration, was selected for microdissection using a beam size of 30 μm. Cells were fixed to the polymer film on CapShur LCM caps (Arcturus Engineering Inc) and placed tightly into 0.5 mL microcentrifuge tubes for subsequent DNA isolation and PCR analysis using the “hand's free” LCM cap loading station (Arcturus Engineering Inc). Individual cells from formalin-fixed, paraffin-embedded sections of an EBV-infected cell line were dissected as a positive control. Single cells prepared by this method were lysed overnight in 50 μL digestion buffer (0.04% proteinase K, 10 mM Tris-HCL pH 8.0, 1 mM EDTA, and 1% Tween-20), and the resulting solution was used directly as template for PCR in a final reaction volume of 50 μL containing 10 μL DNA template, 20 nM each of the EBV forward primer (GCC AGG AGT CCA CAC AAA TGT A) and the EBV reverse primer (CCA GGG CCT TCA CTT CGG TC), which target the BamHIW repeat region of the EBV genome,12 and standard concentrations of Taq buffer (Perkin Elmer, CA) and dNTPs. The PCR parameters were 40 seconds at 94°C, 40 seconds at 60°C, and 40 seconds at 72°C for 35 cycles. Control DNA consisted of 5 ng DNA extracted from the EBV-transformed cell line. DNA was resolved in a 2.5% agarose gel containing ethidium bromide.
Results
Case presentation
A 13-month-old First Nations boy was first admitted to British Columbia's Children's Hospital in March of 1986 with a 1-week history of fever, icterus, lethargy, anorexia, and dark urine. Hepatosplenomegaly was noted. Laboratory workup documented anemia, thrombocytopenia, and an absolute lymphocytosis with many atypical lymphocytes. He had a coagulopathy with hypofibrinogenemia. Biochemical studies showed hepatitis with elevated liver enzymes. A bone-marrow aspirate and biopsy specimen showed myeloid and erythroid hypoplasia associated with histiocytosis and hemophagocytosis. Serologic studies for EBV (VCA), cytomegalovirus (CMV), herpes simplex virus (HSV), hepatitis A virus (HAV), and hepatitis B virus (HBV) were negative. Immunologic studies revealed both an absolute and relative CD8+ T-cell pleocytosis (71%, n = 20 to 30) and an inverted CD4+/CD8+ T-cell ratio (0.64, n = 1 to 2). IgG subclass analysis revealed decreased levels of IgG1 to IgG4. Family history further revealed that a maternal male cousin had died at the age of 18 months, with a presumptive diagnosis of virus-associated hemophagocytic syndrome. The patient received supportive therapy and was discharged 2 weeks after admission. The discharge diagnosis was virus-associated hemophagocytic syndrome. The patient was readmitted at age 8 with choroiditis and chronic lung disease. Chest x-ray films and computed tomographic (CT) scans confirmed bronchiectasis. Mild global developmental delay was noted. CT and magnetic resonance (MR) imaging of the brain was normal, but cerebrospinal fluid protein was elevated at 1.56 g/L. Cerebrospinal fluid (CSF) cytology was negative as were all bacterial, fungal, and viral cultures. CSF analysis showed 2 white blood cells, 3 red blood cells, and CSF antibody studies were negative for EBV, HSV, CMV, toxoplasmosis, syphilis and Borrelia burgdorferi. Serologic assays, performed in the clinical virology laboratory at this time, indicated positive IgG responses to CMV, HSV, varicella-zoster virus (VZV), and EBV (VCA). Epstein-Barr virus–associated nuclear antigen (EBNA) studies were not performed. The choroiditis resulted in gradual blindness. By age 10 he had worsening bronchiectasis and underwent a resection of the left lower lobe and lingula. This showed both bronchial and vascular inflammation. He was again noted to have depressed levels of total IgG (4.75 g/L, n = 8 to 18). Monthly treatment with intravenous immunoglobulin was begun. He was readmitted to British Columbia's Children's Hospital at age 12 with recurrent bronchiectasis. A history of left leg weakness was obtained. MR imaging showed left pectineus muscle atrophy consistent with denervation. Electromyography confirmed femoral nerve neuropathy. There were no other overt clinical findings of vasculitis. Antineutrophil cytoplasmic antibody was negative by immunofluorescence and enzyme-linked immunosorbent assay (ELISA). Antinuclear antibodies were undetectable. Von Willebrand factor–related antigen levels were normal. He had respiratory failure develop, requiring intubation and ventilation. Echocardiography revealed coronary artery aneurysmal dilatation. He died of intractable respiratory failure 1 month after final admission.
An autopsy revealed widespread microscopic necrotizing arteritis that resembled polyarteritis nodosa (Figure1). The lesions were focal, asynchronous, and associated with aneurysmal dilatation and, in acute areas, were associated with fibrinoid necrosis. There was medium and small vessel arteritis affecting the cerebral hemispheres, pons, and dura. Microscopic examination of the heart showed irregular atrophy of the media of the coronary arterial tree with intimal plaque formation and irregular destruction of the internal elastic lamina. Segmental involvement of the vessels of the kidneys, testes, and pancreas was noted. There was evidence of acute arteritis of the left femoral nerve vasa nervorum and old chorioretinitis. The final autopsy diagnosis was vasculitis compatible with polyarteritis nodosa.
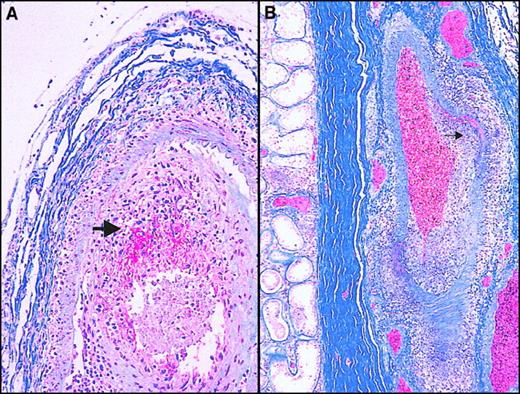
Histopathologic findings obtained at postmortem.
(A) Choroidal vessel stained with Martius-scarlet-blue (MSB) shows necrotizing vasculitis with a mononuclear cellular infiltrate and fibrin deposition (red). (B) Hilar testicular vessel stained with MSB shows necrotizing arteritis, segmental/asymmetric accumulation of mononuclear cells in the vessel wall (large arrowhead). Note the focal nodose dilatation characteristic of polyarteritis nodosa (small arrowhead).
Histopathologic findings obtained at postmortem.
(A) Choroidal vessel stained with Martius-scarlet-blue (MSB) shows necrotizing vasculitis with a mononuclear cellular infiltrate and fibrin deposition (red). (B) Hilar testicular vessel stained with MSB shows necrotizing arteritis, segmental/asymmetric accumulation of mononuclear cells in the vessel wall (large arrowhead). Note the focal nodose dilatation characteristic of polyarteritis nodosa (small arrowhead).
A novel SLAM-associated protein mutation
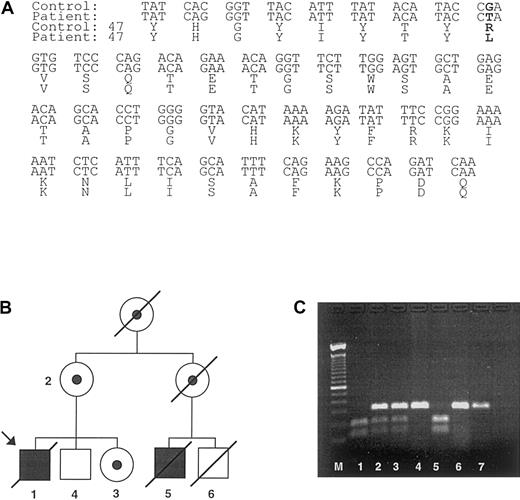
The combination of prior hemophagocytosis and IgG hypogammaglobulinemia, along with a history of a maternal male relative dying of hemophagocytosis, led us to speculate that the patient presented herein had XLP. To confirm a diagnosis of XLP, we cloned and sequenced the SAP gene from our patient (Figure2A). This revealed the presence of a single base substitution in exon 2, resulting in an arginine (R) to leucine (L) transposition within the SH2 domain (Arg55Leu). Genotyping of the proband's extended family using a mutation-induced restriction fragment polymorphism confirmed maternal segregation of the mutation (Figure 2B,C).
SAP mutation and identification of patients.
(A) The gene mutation present in the index case consisted of a G to T mutation in exon 2 of SAP that leads to an arginine to leucine transposition at Arg55 (bold letters). (B) A pedigree was constructed based on the index case. The symbols used are proband (arrow), males (box), females (circle), deceased (line through), affected male (black fill), female carriers (dotted fill), and unaffected subjects (no fill). The SAP mutation introduces a restriction endonuclease recognition site into exon 2 of the SAP gene. DNA isolated from (1) index case and (5) affected male cousin; (2) mothers of index case; (3) sister of index case; (4) brother of index case; (6) unaffected male cousin and (7) unrelated healthy control were subjected to PCR amplification with primers specific for SAPexon 2, and the amplified product was digested with BfaI and visualized on an agarose gel (C). The numbers on the pedigree match the numbers on the agarose gel. Affected male patients are identified by 2 bands of the digested products, whereas carriers have a mixture of the undigested and digested exon 2. The unaffected relative (6) died of a condition unrelated to XLP.
SAP mutation and identification of patients.
(A) The gene mutation present in the index case consisted of a G to T mutation in exon 2 of SAP that leads to an arginine to leucine transposition at Arg55 (bold letters). (B) A pedigree was constructed based on the index case. The symbols used are proband (arrow), males (box), females (circle), deceased (line through), affected male (black fill), female carriers (dotted fill), and unaffected subjects (no fill). The SAP mutation introduces a restriction endonuclease recognition site into exon 2 of the SAP gene. DNA isolated from (1) index case and (5) affected male cousin; (2) mothers of index case; (3) sister of index case; (4) brother of index case; (6) unaffected male cousin and (7) unrelated healthy control were subjected to PCR amplification with primers specific for SAPexon 2, and the amplified product was digested with BfaI and visualized on an agarose gel (C). The numbers on the pedigree match the numbers on the agarose gel. Affected male patients are identified by 2 bands of the digested products, whereas carriers have a mixture of the undigested and digested exon 2. The unaffected relative (6) died of a condition unrelated to XLP.
Immunohistochemistry and polymerase chain reaction analysis of vasculitic lesions
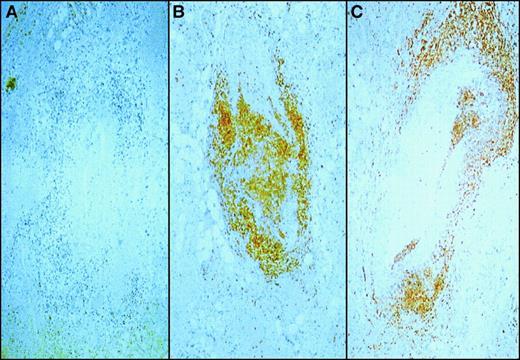
To determine the nature of the cells invading the arterial endothelium, we immunophenotyped the mononuclear infiltrate in the affected vessels of our patient. Fixed sections of testes were stained with markers for B cells (CD20, Figure3A), T cells (CD45RO, Figure 3B), and CTL (CD8, Figure 3B). Histochemical staining revealed that the infiltrate did not comprise B cells but was largely composed of T cells expressing the CD8+ coreceptor. Immunostaining for CD4 antigen was negative (data not shown), and no other antigen markers were tested.
Immunophenotype of vessel wall infiltrate.
Sections of testes containing vessels infiltrated with mononuclear cells were stained with monoclonal antibodies to CD20 (panel A), a B-cell marker; CD45R0 (panel B), a pan T-cell marker and CD8 (panel C), a cytotoxic T lymphocyte marker. The primary antibodies were then labeled with horseradish peroxidase–conjugated antibody and reacted with color substrate. Note the absence of CD20 (panel A) staining and the large proportion of mononuclear cells staining brown indicating the presence of CD45R0 and CD8 cells.
Immunophenotype of vessel wall infiltrate.
Sections of testes containing vessels infiltrated with mononuclear cells were stained with monoclonal antibodies to CD20 (panel A), a B-cell marker; CD45R0 (panel B), a pan T-cell marker and CD8 (panel C), a cytotoxic T lymphocyte marker. The primary antibodies were then labeled with horseradish peroxidase–conjugated antibody and reacted with color substrate. Note the absence of CD20 (panel A) staining and the large proportion of mononuclear cells staining brown indicating the presence of CD45R0 and CD8 cells.
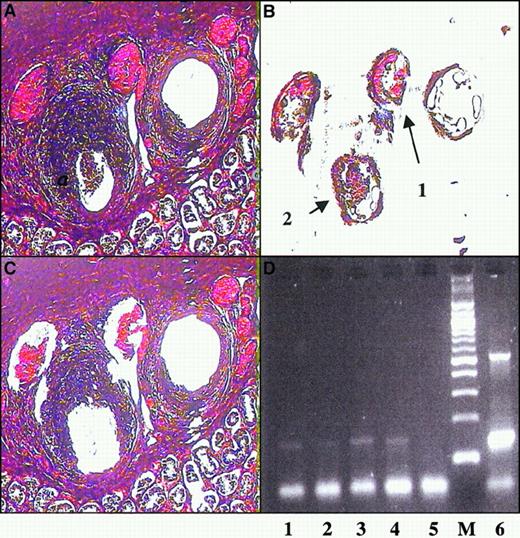
Because the vessel wall infiltrate comprised primarily CD8+T cells and because CTL are known to be important determinants of immunity to EBV,13 we sought to determine whether the EBV antigen was present in vascular tissue. In situ hybridization for the EBV genome was negative (data not shown), suggesting either the absence of viral DNA or the presence of minute amounts undetectable by hybridization techniques. Therefore, we chose to search for viral DNA by PCR. Although the vascular infiltrate was composed primarily of T cells and the latent EBV genome resides mainly within B lymphocytes,14 we used LCM15 to separate vessel wall from infiltrate to discount the possibility of significant B-cell contamination (Figure 4A-C). We were able to detect the presence of the EBV genome in both the vascular tissue and the testes by PCR (Figure 4D), suggesting a widespread distribution of EBV, including a presence in vascular tissue.
PCR amplification of vessel wall tissue.
Sections containing affected testicular arteries were microdissected to remove vessel wall cells. H&E stain of section before dissection (A) and after dissection (C) show that portions of the vessel were removed. DNA was prepared from the removed sections labeled 1 and 2 (B) and subjected to EBV-specific amplification by PCR (D). The amplified products were visualized by gel electrophoresis and ethidium bromide staining. Lanes 1 and 2, vessel wall dissections as labeled in panel B; lane 3, amplification of an EBV-positive lymphoblastoid cell line; lane 4, seminiferous tubule; lane 5, negative control; and lane 7, 1 ng EBV-positive genome.
PCR amplification of vessel wall tissue.
Sections containing affected testicular arteries were microdissected to remove vessel wall cells. H&E stain of section before dissection (A) and after dissection (C) show that portions of the vessel were removed. DNA was prepared from the removed sections labeled 1 and 2 (B) and subjected to EBV-specific amplification by PCR (D). The amplified products were visualized by gel electrophoresis and ethidium bromide staining. Lanes 1 and 2, vessel wall dissections as labeled in panel B; lane 3, amplification of an EBV-positive lymphoblastoid cell line; lane 4, seminiferous tubule; lane 5, negative control; and lane 7, 1 ng EBV-positive genome.
Discussion
Several mutations of SAP, including stop codons, truncations, and missense mutations all of which affect the SH2 domain, have been described in patients with XLP.3-5,16 The phosphotyrosine binding pocket of the SH2 domain contains 3 positively charged arginine residues (Arg13, Arg 32, and Arg55). Such positively charged residues have been shown to mediate the binding of a ligand in a homologous SH2-containing protein, Abl.17 In addition, the crystal structure of SAP complexed to SLAM peptides confirms that Arg 55 participates in binding of SAP to SLAM.18 Our patient is the first to have a described missense mutation affecting Arg55, and we propose that the transposition of a positively charged residue (arginine) with a neutral residue (leucine) interferes with SAP function. The described mutation in SAP both confirms the diagnosis of XLP in our patient and provides a possible mechanistic explanation for his immunodeficiency.
Polyarteritis, either macroscopic or microscopic, is a rare disease in childhood.19,20 Polyarteritis nodosa has been associated with HBV, human immunodeficiency virus, CMV, parvovirus B19, human T-lymphotropic virus type I, and hepatitis C virus.21 Chronic EBV infection has been associated with large-vessel arteritis and Kawasaki-like arteritis,9,10and the EBV genome has been detected in the diseased aortic tissue by PCR.10,22 In an animal model of vasculitis, infection of mice with murine γ-herpesvirus 68 caused a severe large-vessel vasculitis with detectable viral antigen in the arteritic lesions and in vascular smooth muscle.11 Arteritis and chronic viral infection occurred in interferon-γ (IFN-γ) null or IFN-γ receptor null mice demonstrating a role for IFN-γ in regulating chronic γ-herpesvirus infection. Arteritis also occurred in infected weanling but not adult healthy mice. This demonstrates that γ-herpesvirus 68, a virus related to EBV, has a tropism for smooth muscle, can induce arteritis, and that IFN-γ is essential to control vascular infection or resultant inflammation. EBV likewise may have tropism for smooth muscle, as the EBV genome is detected within smooth muscle tumors.23 As we have shown the presence of EBV DNA in the affected vascular structures of our patient, the murine model described may parallel the pathophysiology of our case.
Lymphoid vasculitis has rarely been reported in association with XLP. A single case report describes an 8-year-old boy with XLP who died of a fatal generalized lymphoid vasculitis.7 The vascular infiltrate in this patient was not immunophenotyped, but in another patient with a similar presentation, both infiltrating B cells and T cells were noted.24 Interestingly, a single case of bilateral retinal necrosis has been described in a child with XLP who died of aplastic anemia. The EBV genome was detected in affected tissue by PCR.25 Presence or absence of vasculitis elsewhere was not commented upon. Five patients have been described with lymphomatoid granulomatosis2 and at least one with Wegener's granulomatosis.3 This case thus highlights a rare complication of XLP and further suggests that chorioretinitis in patients with XLP may be a clue to concomitant systemic vasculitis.
Signaling through SLAM/CDw150 in T cells results in T-cell activation, and costimulation through SLAM induces significant IFN-γ production.26 Likewise, 2B4 has been shown to be an activating molecule in murine NK and T cells.27,28Mutations in SAP such as the mutation we describe may interfere with this signaling in either T cells or NK cells. Indeed, a number of reports, all originating from Purtilo's group, have also documented defective NK and CTL activity in patients with XLP.29-32 Similarly, male patients in our kindred have defective NK cell function.33 An important function of NK cells is to rapidly secrete IFN-γ in response to viral infection and in addition to cytotoxicity defects, NK cells from patients with XLP have been shown to have deficient IFN-γ production.34 We propose that SAP mutations result in a functional deficit, allowing productive infection of vascular structures by EBV. These structures may then become the target of prolonged cytotoxic T-cell assault culminating in clinical vasculitis.
Acknowledgments
We thank Ms Liz Rowlands for assistance in collecting family data and Mr Richard Mah and Dr Pirouz Daftarian for technical expertise.
Supported by the British Columbia Health Research Foundation and British Columbia's Children's Hospital New Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rusung Tan, Department of Pathology & Laboratory Medicine, British Columbia's Children's Hospital, 4480 Oak St, Rm 2G5, Vancouver, British Columbia V6H 3V4, Canada; email:roo@interchange.ubc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal