Abstract
The clinical activity of rituximab, a chimeric monoclonal antibody which binds to the CD20 antigen, was evaluated as a single first-line therapy for patients with follicular non-Hodgkin lymphoma (NHL). Fifty patients with follicular CD20+ NHL and a low tumor burden were analyzed for clinical and molecular responses. They received 4 weekly infusions of rituximab at a dose of 375 mg/m2. The response rate a month after treatment (day 50) was 36 of 49 (73%), with 10 patients in complete remission, 3 patients in complete remission/unconfirmed, and 23 patients in partial remission. Ten patients had stable disease, and the disease progressed in 3 patients. One of 13 (8%) patients in complete remission, 9 of 23 (39%) patients in partial remission, and 5 of 10 (50%) patients with stable disease exhibited disease progression during the first year. Within the study population, 32 patients were initially informative for polymerase chain reaction (PCR) data on bcl-2–JHrearrangement. On day 50, 17 of 30 patients (57%) were negative for bcl-2–JH rearrangement in peripheral blood, and 9 of 29 (31%) were negative in bone marrow; a significant association was observed between molecular and clinical responses (P < .0001). At month 12, 16 of 26 patients (62%) were PCR negative in peripheral blood. These results indicate that early molecular responses can be sustained for up to 12 months and that this response is highly correlated with progression-free survival. Rituximab has a high clinical activity and a low toxicity and induces a high complete molecular response rate in patients with follicular lymphoma and a low tumor burden.
Introduction
The optimal treatment of advanced-stage follicular non-Hodgkin lymphoma (NHL) is yet to be determined. In patients with a low tumor burden and without adverse prognostic factors, retrospective analysis1 and prospective studies2 3 have shown that postponing treatment until progression has no negative influence on survival. Nevertheless, almost all patients will experience disease progression and ultimately die of their disease. Thus, new treatment approaches for this subgroup of patients are needed and must meet several requirements—high efficacy, low morbidity, minimal influence on the sensitivity of lymphoma cells to subsequent treatments, and, especially, no induction of chemoresistance.
Recent studies have been devoted to antibody-mediated therapy. Treatments with unmodified monoclonal antibodies (mAbs) have included patient-specific mAbs,4,5 anti-CD20,6anti-CD19 and CD22 Abs coupled with immunotoxins,7-9 and anti-CD20 mAbs radiolabeled with iodohippurate sodium I131or yttrium Y90 in low doses10,11 and in high doses with stem cell support.12,13 The chimeric human–mouse anti-CD20 mAb rituximab is a human IgG1κ antibody, with mouse variable regions isolated from a murine anti-CD20 mAb, IDEC-2B8.14 In vitro studies showed that this chimeric antibody is able to lyse CD20+ cells using human-complement or antibody-dependent cell-mediated cytotoxicity (ADCC)14and that it induces apoptosis in the presence of either goat antimouse IgG or Fc receptor-expressing cells.15
Several trials16-18 have shown that rituximab as single therapy has a significant clinical activity in pretreated patients with follicular lymphoma.16-18 Recently, results of a large phase II trial were reported.19 One hundred sixty-six patients with relapsed low-grade or follicular lymphoma were given 4 weekly doses of 375 mg/m2 rituximab as outpatients; 48% of patients responded, and the median time to progression was 13 months among responders. Adverse events were mostly moderate and were limited to infusion-related events, especially during the first infusion. Overall, severe reactions to rituximab are rare and are observed mainly in patients with bulky tumors,20 leukemic involvement,21 or both.
However, the clinical activity of rituximab alone as a first-line therapy in patients with low-grade NHL has been evaluated only in a limited number of patients with various histologic subtypes of low-grade NHL.22 23 This report summarizes results of a multi-institutional trial of a 4-dose course of rituximab in previously untreated patients with follicular lymphoma with a low tumor burden. The objectives of the study were: (1) to assess clinical activity; (2) to monitor molecular responses in blood and bone marrow and to correlate clinical and molecular responses; and (3) to assess toxicity in patients without bulky tumor.
Patients, materials, and methods
Patients
Patients were eligible for inclusion in this study if they had follicular CD20+ NHL according to the REAL classification,24 whatever the cell type. All slides of pathologic specimens were reviewed by one hemato-pathologist. Patients were required to have histologic diagnosis of NHL established less than 5 months before treatment with rituximab and no prior therapy, including steroids or radiation treatment. They were 18 to 75 years of age. Disease stage had to be II to IV according to Ann-Arbor classification, and the presence of at least one measurable site was required. Bone marrow involvement was not considered measurable. The study protocol was approved by the institutional ethics committee, and informed written consent was obtained from each patient before therapy.
Patients were required to have a low tumor burden according to the GELF criteria3—that is, no nodal or extranodal involvement of more than 7 cm, no B symptoms, no significant splenomegaly, no pleural effusion, no complications such as ascites or organ compression, and normal serum lactate dehydrogenase and β2-microglobulin levels. Patients had to have good performance status (0 or 1 on the World Health Organization scale) and adequate hematologic, renal, and hepatic functions. They were excluded if they had human immunodeficiency virus, hepatitis B surface antigen, hepatitis B core antigen, hepatitis C virus antibodies, or if they were pregnant.
Therapy
Rituximab (Mabthera; Produits Roche, Neuilly, France) was given at a dose of 375 mg/m2 by intravenous infusion for a total of 4 dosages (days 1, 8, 15, 22) on an outpatient basis. The drug was reconstituted in normal saline to a concentration of 1 mg/mL. Rituximab was administered intravenously at an initial rate of 50 mg/h. If no toxicity was observed during the first hour, the infusion rate was increased to 50 mg/h every 30 minutes, to a maximum rate of 300 mg/h. The infusion was interrupted if the patient contracted hypotension, edema, fever, rigors, dyspnea, or any other serious adverse effect. If that occurred, infusion was resumed at half the previous rate after resolution of the event. Oral premedication with acetaminophen and diphenhydramine hydrochloride was recommended. No steroids were given.
Clinical monitoring
To assess all sites of disease involvement, baseline evaluation included clinical documentation, radiography of the chest, computed tomography (CT) of the chest, abdomen, and pelvis, and unilateral bone marrow biopsy. Laboratory testing included routine hematology, serum chemistries, serum immunoglobulin levels, lactate dehydrogenase, and β2-microglobulin assays in blood and urinalysis. Monitoring included hematology and serum chemistry evaluations before each treatment and full tumor restaging 28 days after the end of treatment, 1 month after that, every 3 months for 1 year, and then every 6 months for 2 years.
Molecular analysis of bcl-2–JHgene rearrangement
All samples were centralized in a single laboratory, and DNA was extracted using standard procedures and the usual precautions to avoid cross-contamination. For each assay at diagnosis, 1 μg DNA was amplified using MBR- (TATGGTGGTTTGACCTTTAG) ormcr- (CGTGCTGGTACCACTCCTG′) specific oligonucleotides, together with JH consensus (ACCTGAGGAGACGGTGACCAGGGT) oligonucleotide and AmpliTaq Gold polymerase (Perkin Elmer). Each reaction included 10 minutes at 95°C, then 50 cycles—each comprising 3 steps at 94°C for 30", 56°C (MBR) or 58°C (mcr) for 30", and 72°C for 30"—followed by 9′ at 72°C. Amplified products were visualized on agar gel stained with ethidium bromide; under these conditions, the sensitivity of detection did not exceed 10−3 For follow-up samples, nested polymerase chain reaction (PCR) analysis was performed using MBR(CAGCCTTGAAACATTGATGG) or mcr (CGTGCTGGTACCACTCCTG) withJH- (ACCTGAGGAGACGGTGACC) specific primers for the first round (30 cycles for MBR, 25 cycles for mcr), then a reamplification of 4% of the reaction product with internal MBR (CTATGGTGGTTTGACCTTTAGAG) or mcr (GGACCTTCCTTGGTGTGTTG) and JH (ACCAGGGTCCCTTGGCCCCAG) oligonucleotides (30 cycles each) under the same conditions as above, except that each PCR step lasted 1 minute. The sensitivity of this assay was routinely greater than or equal to 10−4. All samples at the time of diagnosis and during follow-up were tested twice, and all negative results were controlled with another PCR assay using tumor necrosis factor gene primers25 to ensure DNA integrity.
Endpoints
The primary efficacy endpoint was the objective response rate—that is, the proportion of patients achieving either complete remission (CR) or partial response (PR) according to the criteria recently reported by Cheson et al.26 Complete response required the resolution of all symptoms and the disappearance of all detectable clinical and radiologic lesions (greatest transverse diameter, less than 1.5 cm), including bone marrow cleansing, for at least 28 days. Complete remission/unconfirmed (CRu) was defined by either a residual lymph node mass larger than 1.5 cm at the greatest transverse diameter, with the sum of the products of perpendicular diameters regressing by more than 75%, or an indeterminate bone marrow result (increased number or size of aggregates without atypical cytologic or architectural features). PR required a decrease greater than or equal to 50% of the sum of the products of perpendicular diameters of the 6 largest dominant nodes or nodal masses without any evidence of disease progression for at least 28 days. Progressive disease was defined as any occurrence of a new lesion during or at the end of therapy or a 50% increase in the size of any previously identified lesion. Stable disease was defined as no change of the target lesions or a change not corresponding to PR or progressive disease. An independent panel of radiologists reviewed all CT scans of all the included patients.
Clinical response was evaluated on days 50 and 78, and maximal response at either of these stagings (if confirmed on day 180 and if the maximal response was observed on day 78) was taken into account for this report. Comparison of clinical response by individual prognostic variables was performed using logistic regression.
All patients were evaluated for progression at 12 months according to the Cheson et al26 criteria. Patients in CR or CRu with bone marrow (BM) cleansing on day 50 and BM showing again some involvement at 12 months were considered to have progressive disease. Patients in PR with negative BM biopsy findings on day 50 and minimal or moderate involvement at 12 months were not considered in progression because the latter could be related to technical aspects (sample size). An independent panel of lymphoma specialists reviewed all responses. Comparison of clinical responses at 12 months with the day 50 results of PCR analysis was performed using the Fisher exact test.
The secondary endpoints were PCR outcome and progression-free survival. Progression-free survival was calculated according to the method of Kaplan and Meier27 and was measured from the start of treatment until progression/relapse or death. Comparison of the survival functions by results of PCR analysis on day 50 was performed using the log-rank test.
Results
Patient characteristics
Fifty patients were enrolled in 16 centers from October 1997 to August 1998. One patient was excluded after histologic review. Thus, 49 patients were evaluable for response and 50 for tolerance.
There were 26 men and 24 women. The median age was 52 years (range, 32 to 75 years). Twenty-two patients had grade I, 24 had grade II, and 3 had grade III follicular NHL. Four patients had Ann Arbor stage II, 11 had Ann Arbor stage III, and 35 had Ann Arbor stage IV disease. Sixty-six percent of patients had bone marrow involvement, and 82% of the patients had at least 2 measurable sites (Table1).
Patient characteristics (n = 50)
| Characteristics . | No. patients . |
|---|---|
| Sex | |
| Male | 26 |
| Female | 2 |
| Histology (grade) | |
| I | 22 |
| II | 24 |
| III | 3 |
| Other | 1 |
| Stage | |
| II-III | 15 |
| IV | 35 |
| Marrow | |
| Negative | 17 |
| Positive | 33 |
| No. extranodal involvements | |
| 0-1 | 41 |
| 2 or more | 9 |
| Peripheral blood bcl-2 | |
| Negative | 17 |
| Positive | 31 |
| Characteristics . | No. patients . |
|---|---|
| Sex | |
| Male | 26 |
| Female | 2 |
| Histology (grade) | |
| I | 22 |
| II | 24 |
| III | 3 |
| Other | 1 |
| Stage | |
| II-III | 15 |
| IV | 35 |
| Marrow | |
| Negative | 17 |
| Positive | 33 |
| No. extranodal involvements | |
| 0-1 | 41 |
| 2 or more | 9 |
| Peripheral blood bcl-2 | |
| Negative | 17 |
| Positive | 31 |
Clinical response rate
The response rate (RR) was 73% (36 of 49) with 10 patients (20%) in CR, 3 patients (6%) in CRu, and 23 patients (47%) in PR. Ten patients (20%) had stable disease, whereas 3 patients (6%) experienced disease progression during treatment. Lung carcinoma was diagnosed in one patient 10 months after treatment, but it appeared to have existed at the time of NHL diagnosis. The lung radiologic abnormality was considered lymphoma involvement. Hodgkin disease developed in another patient 11 months after treatment.
Sex, stage, bone marrow involvement, number of extranodal sites, and detectable bcl-2 gene rearrangement by PCR in peripheral blood at diagnosis were analyzed to define individual prognostic variables for clinical response. No factor was found of prognostic value.
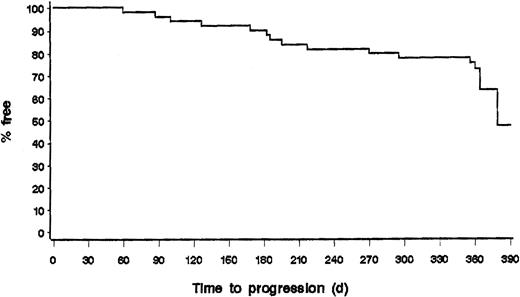
Of the 36 patients who were responders on day 78, 10 patients progressed during the first year of follow-up (1 CR and 9 PR); 5 of the 10 patients with stable disease were also progressing at 12 months (Figures 1,2). Two progressions to Hodgkin lymphoma and diffuse large-cell lymphoma were observed. Of note, we observed that 7 of 23 patients in PR on day 78 were in CR or CRu at 12 months and that 3 of 10 patients with stable disease on day 78 were in PR at 12 months. Thus, if we consider the response rate at any staging during the first year of follow-up after rituximab treatment, 20 of 49 patients (41%) reached CR/CRu, and 19 of 49 patients (39%) reached PR, for an overall response rate of 39 of 49 patients (80%).
Progression-free survival of all patients evaluable for response (n = 49).
Results of the PCR analysis of bcl-2–JHrearrangement
Of the 49 patients with follicular lymphoma included in the study, 48 were evaluated using PCR analysis before treatment. The bcl-2–JH rearrangement was detected in peripheral blood in 32 of 48 patients tested, in 29 of 45 of those patients tested in bone marrow, and in 11 of 17 patients tested in lymph node. All patients who were positive in peripheral blood were also positive in lymph node or bone marrow, and this 32-patient (67%) total was therefore considered informative for further follow-up, 28 with a rearrangement in theMBR region and 4 in the mcr region.
On day 50, after the start of rituximab treatment, 30 patients were evaluated in peripheral blood and 17 of them (57%) were negative for bcl-2–JH rearrangement (Table2). Among the latter, 9 were also PCR negative in bone marrow, whereas 7 remained PCR positive in bone marrow (one patient did not have bone marrow evaluation). Therefore, 9 of 30 (30%) of the evaluable patients had a clearance of the molecular markers in both blood and bone marrow. When molecular response was compared with clinical response assessed on days 50 and 78, a significant association (P < .0001) was observed between molecular and clinical responses: 10 of the 17 patients who were PCR negative in peripheral blood were in CR/CRu, whereas none of the 13 patients PCR positive in peripheral blood had CR (Table 2).
Results of the bcl-2–JH PCR analysis on day 50 and clinical outcome (n = 30)
| . | Patient number . | CR/CRu . | PR . | SD/Prog . | Progression at 12 months* . |
|---|---|---|---|---|---|
| Peripheral blood PCR negative | 17 | 10‡ | 4 | 3 | 1 |
| With BM PCR negative | 9 | 5 | 3 | 1 | 0 |
| With BM PCR positive | 7 | 4 | 1 | 2 | 1 |
| With BM untested | 1 | 1 | 0 | 0 | 0 |
| Peripheral blood PCR positive† | 13 | 0 | 9 | 4 | 8 |
| . | Patient number . | CR/CRu . | PR . | SD/Prog . | Progression at 12 months* . |
|---|---|---|---|---|---|
| Peripheral blood PCR negative | 17 | 10‡ | 4 | 3 | 1 |
| With BM PCR negative | 9 | 5 | 3 | 1 | 0 |
| With BM PCR positive | 7 | 4 | 1 | 2 | 1 |
| With BM untested | 1 | 1 | 0 | 0 | 0 |
| Peripheral blood PCR positive† | 13 | 0 | 9 | 4 | 8 |
Only 30 of 32 patients with bcl-2–JH rearrangement at diagnosis were evaluated on day 50.
2-tailed Fisher exact test for comparison of PCR analysis on day 50 and clinical outcome at 12 months (P < .0001).
All patients who were PCR positive in peripheral blood were also PCR positive in bone marrow, except 2 untested in bone marrow.
2-tailed Fisher exact test for comparison of PCR analysis on day 50 and clinical outcome on day 78 (P < .0001).
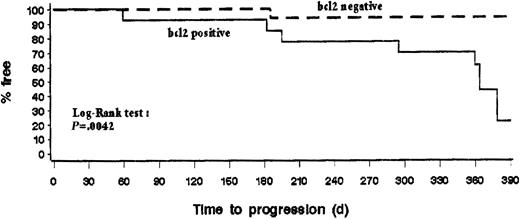
The data from follow-up samples obtained at 6 months in peripheral blood and at 12 months in peripheral blood and bone marrow showed that the overall proportions of patients who were PCR negative in peripheral blood were comparable at the 3 time points—17 of 30 (57%), 18 of 26 (69%), and 16 of 26 (62%), respectively—indicating that the PCR response was maintained over that period. The kinetics and duration of the molecular response were assessed by comparing the results obtained from patients on day 50 and at month 12 for whom serial samples were obtained and analyzed. Interestingly, 6 of 6 evaluable patients who were PCR negative in peripheral blood and bone marrow on day 50 remained PCR negative in both sites at 12 months. Of 7 patients with discordant results in peripheral blood and bone marrow, 4 became PCR negative in both sites, whereas 3 reverted to PCR positivity. Finally, 2 of 11 patients who were PCR positive in peripheral blood and bone marrow on day 50 became PCR negative at 12 months, whereas the 9 others remained PCR positive in blood, marrow (2 with negative PB), or both (Table 3). Interestingly, only 1 of 17 patients (6%) with negative PCR results in peripheral blood on day 50 had disease progression during the first year compared to 8 of 13 patients (62%) with PCR positive results (Table 2). Overall, these results indicate that early molecular responses in blood and bone marrow after rituximab treatment can be sustained for up to 12 months and that some patients may have delayed molecular response. Moreover, progression-free survival curves showed that early molecular response is associated with a lower rate of disease progression during the first year of follow-up (P < .005) (Figure 3).
Kinetics and duration of the molecular response from day 50 until 12 months
| PCR results on day 50 . | PCR results at month 12 . |
|---|---|
| 9 pts with PB negative and BM negative | 1 pt with PB missing and BM missing |
| 1 pt with PB missing and BM negative | |
| 1 pt with PB negative and BM missing | |
| 6 pts with PB negative and BM negative | |
| 7 pts with PB negative and BM positive | 2 pts with PB positive and BM positive |
| 1 pt with PB negative and BM positive | |
| 4 pts with PB negative and BM negative | |
| 1 pt with PB negative and BM missing | 1 pt with PB positive and BM positive |
| 11 pts PB positive and BM positive | 2 pts with PB missing and BM missing |
| 5 pts with PB positive and BM positive | |
| 1 pt with PB negative and BM missing | |
| 1 pt with PB negative and BM positive | |
| 2 pts with PB negative and BM negative | |
| 2 pts with PB positive and BM missing | 2 pts with PB positive and BM positive |
| PCR results on day 50 . | PCR results at month 12 . |
|---|---|
| 9 pts with PB negative and BM negative | 1 pt with PB missing and BM missing |
| 1 pt with PB missing and BM negative | |
| 1 pt with PB negative and BM missing | |
| 6 pts with PB negative and BM negative | |
| 7 pts with PB negative and BM positive | 2 pts with PB positive and BM positive |
| 1 pt with PB negative and BM positive | |
| 4 pts with PB negative and BM negative | |
| 1 pt with PB negative and BM missing | 1 pt with PB positive and BM positive |
| 11 pts PB positive and BM positive | 2 pts with PB missing and BM missing |
| 5 pts with PB positive and BM positive | |
| 1 pt with PB negative and BM missing | |
| 1 pt with PB negative and BM positive | |
| 2 pts with PB negative and BM negative | |
| 2 pts with PB positive and BM missing | 2 pts with PB positive and BM positive |
Progression-free survival according to the results of bcl-2 PCR analysis in blood performed 1 month after treatment of 30 patients with initial bcl-2–JH rearrangement.
Progression-free survival according to the results of bcl-2 PCR analysis in blood performed 1 month after treatment of 30 patients with initial bcl-2–JH rearrangement.
Adverse events
All patients received the 4 weekly infusions at full dose. The most common adverse events thought to be related to rituximab infusion are listed in Table 4. The most frequent events were grade 1-2 fever, headache, asthenia, pain, rash, laryngitis, rhinitis, paresthesia, hypotension, and nausea. Two cases of grade 3-4 hypotension and hypertension resolved after management according to the protocol procedures. No hematologic toxicity was observed, and only one minor infection developed.
Incidence of adverse events per patient (n = 50)
| Event . | NCI grade . | |
|---|---|---|
| 1-2 . | 3 . | |
| General | ||
| Fever | 21 | |
| Headache | 22 | |
| Pain | 15 | |
| Asthenia | 11 | |
| Pruritus | 9 | |
| Hypotension | 7 | 1 |
| Arthralgia | 6 | |
| Rash | 6 | |
| Anxiety | 6 | |
| Paresthesia | 5 | |
| Rigors | 6 | |
| Dizziness | 2 | |
| Hypertension | 1 | 1 |
| Digestive | ||
| Nausea | 6 | |
| Vomiting | 2 | |
| Diarrhea | 3 | |
| Constipation | 3 | |
| Respiratory | ||
| Laryngitis | 18 | |
| Rhinitis | 11 | |
| Coughing | 4 | |
| Pneumonitis | 1 | |
| Event . | NCI grade . | |
|---|---|---|
| 1-2 . | 3 . | |
| General | ||
| Fever | 21 | |
| Headache | 22 | |
| Pain | 15 | |
| Asthenia | 11 | |
| Pruritus | 9 | |
| Hypotension | 7 | 1 |
| Arthralgia | 6 | |
| Rash | 6 | |
| Anxiety | 6 | |
| Paresthesia | 5 | |
| Rigors | 6 | |
| Dizziness | 2 | |
| Hypertension | 1 | 1 |
| Digestive | ||
| Nausea | 6 | |
| Vomiting | 2 | |
| Diarrhea | 3 | |
| Constipation | 3 | |
| Respiratory | ||
| Laryngitis | 18 | |
| Rhinitis | 11 | |
| Coughing | 4 | |
| Pneumonitis | 1 | |
Discussion
We report a trial evaluating the clinical efficacy and safety of single-agent rituximab therapy as first-line therapy of follicular NHL with low tumor burden. In addition, patients were evaluated for molecular response in blood and bone marrow. Previous experience with 4 weekly courses of rituximab as single first-line therapy has been reported in refractory or relapsing low-grade lymphoma.16-19 A pivotal multicenter trial19was performed in 166 patients with recurrent or refractory low-grade, mostly follicular, B-cell lymphoma. The overall response rate was 48% (6% CR, 42% PR), confirming the phase I-II data. The efficacy of rituximab had also been shown in an 8-week infusion program28 and as retreatment.29
We have treated 49 patients with follicular NHL and a low tumor burden; the response rate was 73%—CR, 20%; CRu, 6%; and PR, 47%. This rate is similar to the response rate to other agents—such as an alkylating agent or interferon alfa—in a similar subgroup of patients with follicular NHL.3 We confirmed also that a delayed response could be observed after rituximab therapy. Ten patients had clinical responses between day 78 and month 12, so that the overall response rate was 79%. In the current study, no clinical or biologic parameters were found to be associated with the clinical response after rituximab treatment. This could be related to the low number of patients, but it is likely to reflect the homogeneity of the population, which comprised only patients with follicular lymphoma with a low tumor burden. Additional studies are warranted to identify patients who may not respond to rituximab.
The possibility of clearing the residual disease evaluated by PCR technique appears particularly interesting. On day 50, of 30 patients initially positive for bcl-2–JH rearrangement in peripheral blood, 17 were negative after treatment and 9 were also negative in bone marrow. Two other patients had a delayed response in peripheral blood. Serial PCR analysis after treatment showed that PCR response persisted over time. At 12 months, 12 of 26 patients remained PCR negative in blood and bone marrow. Conversely, a few patients (3 of 17) who became PCR negative in blood after treatment did not reach clinical response.
These results appear comparable to those reported in patients with progressive or relapsed disease. McLaughlin et al19 and, more recently, Gupta et al30 indicated reversion to negative status in blood and bone marrow of relapsed patients after rituximab therapy. A clear association was observed between PCR negativity and clinical response.
The significance of molecular remission in follicular lymphoma remains unclear. Recently, Lopez-Guillermo et al31 tested, during and after treatment, the peripheral blood and, when possible, the bone marrow of 194 patients exhibiting either MBR or mcr bcl-2 rearrangement at diagnosis. Patients who achieved molecular response during the first year of treatment had a significantly longer failure-free survival than those who did not (4-year progression-free survival, 76% versus 38%). Similar prognostic value of bcl-2 negativity has been reported after autologous bone marrow transplantation. Gribben et al32have indicated that after autologous bone marrow transplantation, patients with negative blood bcl-2 PCR findings have significantly lower relapse rates and better progression-free survival rates than patients who remain bcl-2 positive. On the other hand, some patients with localized follicular NHL remain PCR positive during several years without clinical relapse.33
In our group of patients, PCR negativity also appeared to be associated with a good prognosis because only one patient with PCR negativity has had a relapse during the first year (whereas 8 of 13 patients who were PCR positive after treatment have had relapses). Nevertheless, the clinical significance of molecular remission after rituximab therapy remains unknown. It may reflect either a true molecular response in all disease sites or a predominant effect of rituximab on blood and bone marrow involvements. Of note, we observed that most patients remained PCR negative after the usual delay of the reappearance of normal B-lymphocytes in blood and bone marrow that occurs 6 to 9 months after treatment with rituximab.19
Importantly, these results were obtained without the toxicity of conventional chemotherapy. The adverse events consisted of grade 1 or 2 symptoms such as fever, headache, nausea, hypotension, rhinitis, angioedema, rash, pain, or pruritus. Only 2 cases of grade 3 toxicity, manifested as hypotension or hypertension, were observed during the first infusion. As described in previous trials, the number and the severity of adverse events decreased with subsequent infusions. The small number of adverse events could be explained by the low tumor burden of the patients included in our study.20 We did not observe any appearance of severe infusion-related symptom complex recently described.21
Two other studies of rituximab used as first-line treatment of low-grade NHL have been reported recently. Hainsworth et al22 reported a 52% response rate among 25 patients with follicular lymphoma. Gutheil et al23 observed 8 responses among 14 patients with follicular lymphoma. Molecular results were not reported in any of these studies.
In conclusion, these results demonstrate that rituximab has high clinical activity in previously untreated patients with follicular NHL. Response duration has yet to be determined. In addition, rituximab as a single agent induces a molecular response rate that has not been reported with any other cytotoxic agent. These responses have to be confirmed with larger phase III trials in which rituximab will be compared with other therapeutic approaches. Many additional issues about this agent remain to be explored: combination with chemotherapy,34 with growth factors such as G-CSF35 or GM-CSF which could increase ADCC, or with an immunotherapy such as interferon alfa.36 Whether repeated treatments with rituximab of responders after recovery of CD20+ cells may delay clinical relapse remains to be investigated. This is being tested in another trial.22
Acknowledgments
We thank Carole Charlot and Marie-Claude Chamard for expert technical assistance. We also thank J. N. Munck, F. Morschauser, B. Grosbois, and A. Delmer for clinical reviews; Y. Menu and A. Scherrer for radiologic reviews; and J. M. Goehrs (Medical Department, Produits Roche).
Supported by Produits Roche, Neuilly-sur-Seine, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ph. Solal-Celigny, Centre Jean Bernard, 9 rue Beauverger, 72000 Le Mans, France; e-mail: coutard@cybercable.tm.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal