Abstract
The Flt3 gene encodes a tyrosine kinase receptor that regulates proliferation and differentiation of hematopoietic stem cells. An internal tandem duplication of the Flt3 gene (Flt3/ITD) has been reported in acute myelogenous leukemia (AML) and may be associated with poor prognosis. We analyzed diagnostic bone marrow specimens from 91 pediatric patients with AML treated on Children's Cancer Group (CCG)-2891 for the presence of the Flt3/ITD and correlated its presence with clinical outcome. Fifteen of 91 samples (16.5%) were positive for the Flt3/ITD. Flt3/ITD-positive patients had a median diagnostic white count of 73 800 compared with 28 400 for the Flt3/ITD-negative patients (P = .05). The size of the duplication ranged from 21 to 174 base pairs (bp). Nucleotide sequencing of the abnormal polymerase chain reaction products demonstrated that all duplications involved exon 11 of theFlt3 gene and also preserved the reading frame. Lineage restriction analysis revealed that Flt3/ITD was not present in the lymphocytes, suggesting a lack of stem cell involvement for this mutation. None of the Flt3/ITD-positive patients had unfavorable cytogenetic markers, and there was no predominance of a particular FAB class. The remission induction rate was 40% in Flt3/ITD-positive patients compared with 74% in Flt3/ITD-negative ones (P = .005). The Kaplan-Meier estimates of event-free survival at 8 years for patients with and without Flt3/ITD were 7% and 44%, respectively (P = .002). Multivariate analysis demonstrated that presence of the Flt3/ITD was the single most significant, independent prognostic factor for poor outcome (P = .009) in pediatric AML.
Introduction
Flt3 is a member of the class III tyrosine kinase receptor family that includes the c-kit, c-fms, and PDGF receptors. The Flt3 receptor is preferentially expressed on hematopoietic stem cells and mediates stem cell differentiation and proliferation.1 Interaction of the Flt3 receptor with Flt3 ligand causes receptor dimerization, leading to the activation of the receptor tyrosine kinase and receptor autophosphorylation.2 The phosphorylated Flt3 transduces activation signals through association with various cytoplasmic proteins, including ras GTPase-activating protein, phospholipase C, and Src family tyrosine kinases.3Activation of the Flt3 receptor by ligand-dependent phosphorylation induces cellular proliferation via activation of cytoplasmic mediators. It has also been demonstrated that Flt3 receptor activation causes proliferation of AML cells in vitro, as it appears to both stimulate proliferation and inhibit apoptosis of the AML cells.4Thus, constitutive activation of the Flt3 pathway may lead to disease proliferation and may block the cellular apoptotic response to conventional chemotherapy.
An internal tandem duplication of the juxtamembrane (JM) domain-coding sequence of the Flt3 (Flt3/ITD) gene on chromosome 13 has been identified in a group of patients with AML.5 In the Flt3/ITD, a fragment of the JM domain-coding sequence (exons 11 and 12) is duplicated in direct head-to-tail orientation. The length of the ITD varies, but the duplicated sequence is always in-frame.5,6 In vitro studies have shown that mutant Flt3/ITD receptors are dimerized in a ligand-independent manner, leading to autophosphorylation of the receptor through constitutive activation of the tyrosine kinase moieties.7Such constitutive activation leads to autonomous, cytokine-independent growth in the mutant cells.8
Flt3 mutations have been implicated in the pathogenesis of AML. The Flt3/ITD has been associated with leukocytosis in acute promyelocytic leukemia.9 There is also evidence to suggest that Flt3/ITD is associated with leukemic transformation of myelodysplasia.10 Few studies have examined the prognostic significance of this mutation. Two adult studies demonstrated that the presence of the Flt3/ITD was associated with poor outcome.11,12 More recently, a small study of 56 pediatric AML cases showed that children with Flt3/ITD had significantly worse outcomes than children without this mutation.13 However, the prognostic significance of this mutation remains uncertain, as the patients studied were treated on numerous different treatment protocols. Also, patients who received bone marrow transplantation were censored from these studies, thus the impact of transplantation on outcome was not examined. In this study we determined the prevalence and prognostic significance of Flt3/ITD in a group of pediatric patients with AML who were treated on the same treatment protocol, and correlated its presence with clinical characteristics and outcome. We also assessed the stem cell involvement of this mutation by determining the presence of this mutation in lymphocytes and myeloblasts.
Patients, materials, and methods
Patients and treatments
Patients with newly diagnosed de novo AML registered on Children's Cancer Group (CCG) pediatric AML protocol CCG-2891 were included in this study. Patients with myelodysplastic syndrome (MDS), secondary AML, or Down syndrome were excluded. Ninety-one of the available cryopreserved marrow specimens were obtained from the CCG-AML reference laboratory. The study was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board and the CCG Biology Committee. The diagnosis of AML was made according to French-American-British (FAB) classification and was confirmed by the CCG-Central Review Committee.
Treatment protocol CCG-2891 has been described in detail elsewhere.14-16 In brief, CCG-2891 is a prospective randomized trial in which pediatric patients with AML were randomized at diagnosis to receive one of 2 induction regimens involving 4-day cycles of 5 chemotherapeutic agents (daunomycin, cytarabine, etoposide, 6-thioguanine, and dexamethasone). The second cycle was administered either after 10 days, despite low counts (intensive timing), or after 14 days, depending on the marrow status (standard timing). Induction consisted of a total of 4 courses in both groups. At the end of induction, patients who achieved remission and had a compatible matched-related donor went on to receive allogeneic bone marrow transplant (BMT). Patients without related donors were randomized to either nonmyeloablative chemotherapy or an autologous BMT.
Analysis of the Flt3/ITD
Genomic DNA was extracted from diagnostic marrow specimens using the Puregene protocol (Gentra Systems Inc, Minneapolis, MN). Exons 11 and 12 of the Flt3 gene were amplified by genomic polymerase chain reaction (PCR).11 PCR amplification was performed using primers 12R, 5′-CTTTCAGCATTTTGACGGCAACC-3′, and 11F, 5′-GCAATTTAGGTATGAAAGCCAGC-3′. The PCR mixture contained 500 ng DNA and 15 pmol of 12R and 11F primers. Standard concentrations of reaction mixtures were used as described previously.11 Denaturing, annealing, and extension steps were performed at 94°C for 30 seconds, 56°C for 1 minute, and 72°C for 2 minutes, respectively, for 35 cycles on a GeneAmp PCR system 2400 (Perkin Elmer, Emeryville, CA). There was an initial 3-minute denaturation step at 94°C and a final extension step at 72°C for 7 minutes. Products were resolved on 5% polyacrylamide gel, and high-molecular weight bands consistent with Flt3/ITD were cut out for direct nucleotide sequencing.
Direct nucleotide sequencing of the amplified fragments
Bands cut out from polyacrylamide gel were incubated overnight at room temperature in DEPC-H2O to extract double-stranded DNA (dsDNA) from the gel. The extracted dsDNA was directly sequenced using the Thermo Sequence Dye Terminator sequencing reaction.17
Flow cytometric isolation of lymphocytes
Lymphocytes were isolated by incubating diagnostic bone marrow samples with murine anti-CD3 and anti-CD20 antibodies, respectively, followed by FITC-conjugated goat antimurine antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) and flow cytometry as previously described.18 DNA was extracted from the sorted cells using the Puregene protocol (Gentra Systems Inc).
Statistical methods
The distributions of categorical and continuous variables were compared between Flt3/ITD-positive and Flt3/ITD-negative groups. Statistical comparisons between the study group and the CCG-2891 population were also made. Pearson chi-square statistic was used to test for differences in the distribution of categorical variables. Analysis of variance was used to test for differences in the means of distributions. The Wilcox and rank sum test was used to test for differences in the medians of continuous variables.
Nonparametric survival curves for censored data were computed by the Kaplan-Meier method.19 The significance of predictor variables was tested with the log-rank statistic. Significant predictors from univariate tests were studied with a Cox proportional hazards model.20 Variables were added in a step-wise manner, starting with variables known to be significant predictors from earlier studies. The likelihood-ratio statistic was used to test for the significance of model variables. Two-way interactions between predictor variables were also tested in this fashion.
Results
Patient characteristics—study population versus CCG-2891
We examined the clinical characteristics of our study population to determine whether they were representative of the CCG-2891 study population. Comparison of rate of induction remission, overall survival, and event-free survival showed no statistically significant difference between our population and the rest of the CCG-2891 AML patients (Table 1). Comparison of diagnostic white blood cell count (WBC) as a continuous variable showed that median WBC at diagnosis was higher in our study population (37 500/μL) than in the CCG-2891 population (20 400/μL) (P = .007). However, comparison of the diagnostic WBC by white count categories (less than 20 000, 20-100 000, and more than 100 000/μL) showed no statistically significant difference between the 2 groups (P = .25) (Table 2). Median Age at presentation was 10.5 years for the patients in the study group compared with 7.6 years for the rest of the CCG-2891 population (P = .05).
Patient characteristics—the study population versus the rest of the CCG-2891 population
| . | CCG-2891 (N = 797) . | Study population (N = 91) . | P value . |
|---|---|---|---|
| Median age (years) | 7.6 | 10.5 | .05 |
| Diagnostic WBC (median) | 20 400/mm3 | 37 500/mm3 | .007 |
| Remission induction (%) | 78% | 68% | .1 |
| Actuarial overall survival (%) at 8 years | 41% | 44% | .7 |
| Actuarial event-free survival (%) at 8 years | 34% | 36% | .9 |
| . | CCG-2891 (N = 797) . | Study population (N = 91) . | P value . |
|---|---|---|---|
| Median age (years) | 7.6 | 10.5 | .05 |
| Diagnostic WBC (median) | 20 400/mm3 | 37 500/mm3 | .007 |
| Remission induction (%) | 78% | 68% | .1 |
| Actuarial overall survival (%) at 8 years | 41% | 44% | .7 |
| Actuarial event-free survival (%) at 8 years | 34% | 36% | .9 |
WBC = white blood cell count.
Distribution of patients by WBC categories—the study population versus the rest of the CCG-2891 AML patients
| WBC categories . | CCG-2891 N (%) . | Study population N (%) . |
|---|---|---|
| <20 000/mm3 | 391 (49%) | 38 (42%) |
| 20-100 000/mm3 | 272 (34%) | 32 (35%) |
| >100 000/mm3 | 134 (17%) | 21 (23%) |
| Total | 797 | 91 |
| P = .25 | ||
| WBC categories . | CCG-2891 N (%) . | Study population N (%) . |
|---|---|---|
| <20 000/mm3 | 391 (49%) | 38 (42%) |
| 20-100 000/mm3 | 272 (34%) | 32 (35%) |
| >100 000/mm3 | 134 (17%) | 21 (23%) |
| Total | 797 | 91 |
| P = .25 | ||
P value calculated for trend across WBC categories by the method of Pearson chi-square statistics.
WBC = white blood cell count.
Genomic polymerase chain reaction and sequence analysis of theFlt3 gene
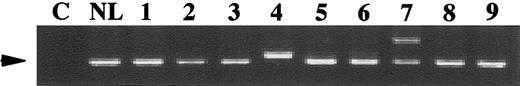
Genomic DNA was obtained from the diagnostic marrow specimens of 91 pediatric patients with AML and exons 11 and 12 of theFlt3 gene were amplified by PCR (Figure1). The Flt3/ITD amplification yields a higher-molecular weight product on polyacrylamide gel electrophoresis (Figure 1, lanes 4 and 7). Fifteen of 91 patients (16.5%) tested had the Flt3/ITD, all confirmed by sequence analysis. Fourteen patients demonstrated both a normal and an aberrant band. One patient (Figure 1, lane 4) had a single aberrant PCR product indicative of the Flt3/ITD. This patient was previously shown to have a loss of heterozygosity (LOH) of chromosome 13 in the region containing theFlt3 gene. The size of the duplicated region varied from 21- to 174-base pairs (bp). Exon 11 of the Flt3 gene was involved in every patient, and in 2 patients, it extended to exon 12. In 2 patients, insertions of 6- and 15-bp were noted between the duplicated regions. In all cases, duplications maintained the integrity of the reading frame.
Genomic PCR for the Flt3/ITD.
Genomic DNA prepared from diagnostic marrow specimens were PCR amplified and resolved on polyacrylamide gel. C, non-nucleic acid control; NL, normal marrow control; 1-9, samples from 9 different pediatric patients. High-molecular weight bands in lanes 4 and 7 represent the Flt3/ITD. Patient sample in lane 4 does not have a normal Flt3 band. This patient was previously shown to have a loss of heterozygosity (LOH) of chromosome 13 at the region of theFlt3 gene. Arrow points to the normal Flt3 gene product.
Genomic PCR for the Flt3/ITD.
Genomic DNA prepared from diagnostic marrow specimens were PCR amplified and resolved on polyacrylamide gel. C, non-nucleic acid control; NL, normal marrow control; 1-9, samples from 9 different pediatric patients. High-molecular weight bands in lanes 4 and 7 represent the Flt3/ITD. Patient sample in lane 4 does not have a normal Flt3 band. This patient was previously shown to have a loss of heterozygosity (LOH) of chromosome 13 at the region of theFlt3 gene. Arrow points to the normal Flt3 gene product.
Lineage restriction of Flt3/ITD
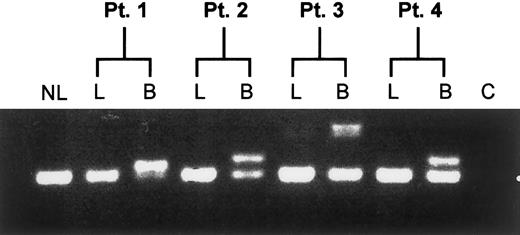
To determine whether the Flt3/ITD occurs at the level of the stem cell, lymphocytes from Flt3/ITD-positive patients were sorted from the diagnostic marrow specimens. DNA from the lymphocyte fractions was tested for the presence of the Flt3/ITD and compared with the blast population. Presence of the Flt3/ITD was restricted to the myeloblasts and was not detected in lymphocyte fractions tested (Figure2), demonstrating the lack of involvement of the stem cell compartment.
Lineage restriction analysis of the Flt3/ITD.
Diagnostic bone marrow specimens from Flt3/ITD-AML patients were flow sorted for lymphocyte and blast fractions and assayed for the presence of the Flt3/ITD. Result of the assay from 4 representative patients is shown. NL, normal bone marrow control; C, non-nucleic acid control; L, lymphocyte fraction; B, myeloblast fraction.
Lineage restriction analysis of the Flt3/ITD.
Diagnostic bone marrow specimens from Flt3/ITD-AML patients were flow sorted for lymphocyte and blast fractions and assayed for the presence of the Flt3/ITD. Result of the assay from 4 representative patients is shown. NL, normal bone marrow control; C, non-nucleic acid control; L, lymphocyte fraction; B, myeloblast fraction.
Clinical characteristics of the study population
Clinical characteristics of patients with and without the Flt3/ITD were compared (Table 3). There was no significant difference in the age of patients (P = .46), although it was noted that, of the 15 patients younger than 3 years in the study, none had the Flt3/ITD mutation. Patients with the Flt3/ITD had a diagnostic WBC of 73 800/μL compared with 28 400/μL for the patients without the mutation (P = .05).
Characterization of the 91 study patients with and without the Flt3/ITD
| Characteristics N = 91 . | Flt3/ITD-negative N = 76 (83.5%) . | Flt3/ITD-positive N = 15 (16.5%) . | P value . |
|---|---|---|---|
| Median age (range) | 10.9 (0.2-17.9) | 8.3 (3.1-18.7) | .46 |
| Median WBC × 103/mm3 (range) | 28.4 (0.5-480) | 73.8 (4.9-453) | .05 |
| Remission rate (%) | 74% | 40% | .005 |
| Actuarial overall survival (%) at 8 years | 50% | 13% | .02 |
| Actuarial event-free survival (%) at 8 years | 44% | 7% | .002 |
| Characteristics N = 91 . | Flt3/ITD-negative N = 76 (83.5%) . | Flt3/ITD-positive N = 15 (16.5%) . | P value . |
|---|---|---|---|
| Median age (range) | 10.9 (0.2-17.9) | 8.3 (3.1-18.7) | .46 |
| Median WBC × 103/mm3 (range) | 28.4 (0.5-480) | 73.8 (4.9-453) | .05 |
| Remission rate (%) | 74% | 40% | .005 |
| Actuarial overall survival (%) at 8 years | 50% | 13% | .02 |
| Actuarial event-free survival (%) at 8 years | 44% | 7% | .002 |
Flt3/ITD = Flt3 internal tandem duplication; WBC = white blood cell count.
Characteristics of patients with Flt3/ITD
The parameters of age, WBC at diagnosis, induction regimen, FAB classification, cytogenetic abnormalities, clinical outcome, and the size of the duplication for the 15 patients with the Flt3/ITD are shown in Table 4. On average, this group of patients had a substantially higher WBC, although there were 4 patients who had WBC less than 20 000/μL. There was not a predominance of a particular FAB class in Flt3/ITD-positive patients, though FAB classes M5, M6, or M7 were not represented. None of the 15 patients with Flt3/ITD had unfavorable cytogenetic markers. One patient had an 11q23 translocation, which in CCG-2891, it was not found to be of prognostic significance. There were 4 patients (27%) with t(15;17), inv 16, or t(8;21) translocation traditionally considered as favorable cytogenetic markers. This compares with a prevalence of 29% (22 patients) in patients without the Flt3/ITD.
Characteristics of patients with the Flt3/ITD
| Registration no. . | Age (y) . | WBC (×103) . | Induction regimen . | Remission . | Relapse ? . | Alive ? . | FAB class . | Karyotype . | Postinduction treatment . | Duplication size (bp) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 48846 | 17.3 | 73.8 | STD | Yes | No | Yes | M2 | NL | Chemo | 30 |
| 59237 | 11.7 | 436 | INT | No | Yes | Yes | M4 | NL | Allo × 2 | 69 |
| 59719 | 3.9 | 240.2 | INT | No | Yes | Yes | M2 | ND | Allo | 21 |
| 47604 | 3.1 | 43.7 | STD | No | Yes | No | M2 | ND | Allo | 33 |
| 51207 | 6.3 | 52.6 | INT | Yes | Yes | No | M4 | NL | Chemo | 27 |
| 51181 | 9.5 | 345.8 | STD | Yes | Yes | No | M4 | Inv 16 | Chemo | 48 |
| 53588 | 14.7 | 4.9 | INT | No | Yes | No | M3 | t (15;17) | Auto | 39 |
| 52416 | 3.6 | 9.4 | INT | Yes | Yes | No | M3 | t (15;17) | Auto | 45 |
| 53177 | 15.5 | 9.4 | STD | No | Yes | No | M2 | NL | Allo | 24 |
| 56586 | 17.8 | 453.1 | STD | Yes | Yes | No | M2 | del 14 (q21;q24) | Auto | 24 |
| 47053 | 12 | 410 | STD | No | NLM | No | M1 | Trisomy 8 | Chemo | 39 |
| 49447 | 8.1 | 42.7 | STD | Yes | NLM | No | M1 | del 13 (q12;q22) | Allo | 174 |
| 52454 | 7.8 | 246 | STD | No | NLM | No | M4 | NL | Chemo | 54 + 64-150 |
| 57258 | 8.3 | 165 | INT | No | NLM | No | M0 | 11q23 | None | 54 + 154-150 |
| 58340 | 18.7 | 7.9 | INT | Death | NLM | No | M2 | t (8;21) | Not applicable | 48 |
| Registration no. . | Age (y) . | WBC (×103) . | Induction regimen . | Remission . | Relapse ? . | Alive ? . | FAB class . | Karyotype . | Postinduction treatment . | Duplication size (bp) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 48846 | 17.3 | 73.8 | STD | Yes | No | Yes | M2 | NL | Chemo | 30 |
| 59237 | 11.7 | 436 | INT | No | Yes | Yes | M4 | NL | Allo × 2 | 69 |
| 59719 | 3.9 | 240.2 | INT | No | Yes | Yes | M2 | ND | Allo | 21 |
| 47604 | 3.1 | 43.7 | STD | No | Yes | No | M2 | ND | Allo | 33 |
| 51207 | 6.3 | 52.6 | INT | Yes | Yes | No | M4 | NL | Chemo | 27 |
| 51181 | 9.5 | 345.8 | STD | Yes | Yes | No | M4 | Inv 16 | Chemo | 48 |
| 53588 | 14.7 | 4.9 | INT | No | Yes | No | M3 | t (15;17) | Auto | 39 |
| 52416 | 3.6 | 9.4 | INT | Yes | Yes | No | M3 | t (15;17) | Auto | 45 |
| 53177 | 15.5 | 9.4 | STD | No | Yes | No | M2 | NL | Allo | 24 |
| 56586 | 17.8 | 453.1 | STD | Yes | Yes | No | M2 | del 14 (q21;q24) | Auto | 24 |
| 47053 | 12 | 410 | STD | No | NLM | No | M1 | Trisomy 8 | Chemo | 39 |
| 49447 | 8.1 | 42.7 | STD | Yes | NLM | No | M1 | del 13 (q12;q22) | Allo | 174 |
| 52454 | 7.8 | 246 | STD | No | NLM | No | M4 | NL | Chemo | 54 + 64-150 |
| 57258 | 8.3 | 165 | INT | No | NLM | No | M0 | 11q23 | None | 54 + 154-150 |
| 58340 | 18.7 | 7.9 | INT | Death | NLM | No | M2 | t (8;21) | Not applicable | 48 |
Insertions of 6 and 15 base pairs upstream of the duplicated region.
WBC = white blood cell count; STD = standard timing; INT = intensive timing; NLM = nonleukemic mortality; NL = normal; ND = not determined; Allo = allogeneic bone marrow transplant; Auto = autologous bone marrow transplant; Chemo = chemotherapy.
Clinical outcome and evaluation of prognostic factors
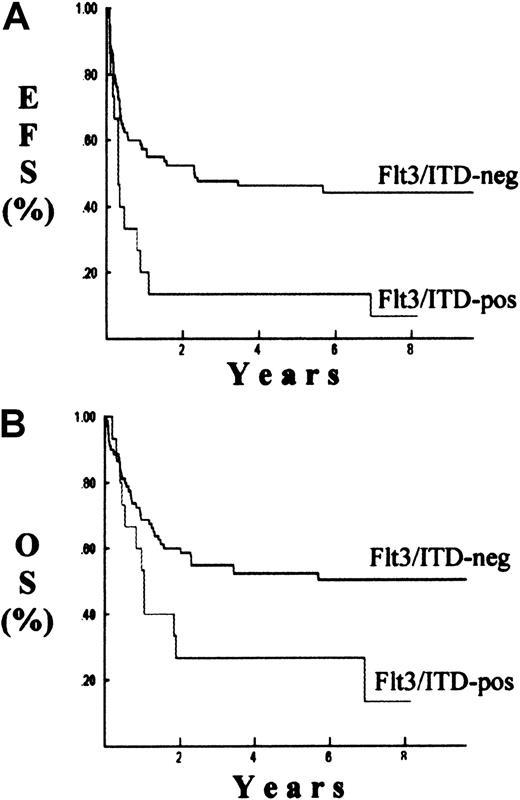
Clinical outcome was determined for patients with and without the Flt3/ITD. The remission induction rate was 40% for patients with the Flt3/ITD compared with 74% for patients without this mutation (P = .005) (Table 3). The Kaplan-Meier estimate for event-free survival for patients with and without the Flt3/ITD was 7% and 44%, respectively (P = .002) (Figure3). Overall survival for patients with the Flt3/ITD was 13% compared with 50% for patients without the Flt3/ITD (P = .02).
Survival probabilities.
Probabilities of event-free survival (EFS), (A); or overall survival (OS), (B). Results are Kaplan-Meier estimate for patients with (Flt3/ITD-pos) and without (Flt3/ITD-neg) the Flt3/ITD mutation.
Survival probabilities.
Probabilities of event-free survival (EFS), (A); or overall survival (OS), (B). Results are Kaplan-Meier estimate for patients with (Flt3/ITD-pos) and without (Flt3/ITD-neg) the Flt3/ITD mutation.
Prognostic factors were evaluated for overall and event-free survival. We used univariate analysis to assess the prognostic significance of the Flt3/ITD, induction regimen, diagnostic WBC, cytogenetic class, and age. From the factors analyzed, only the presence of the Flt3/ITD was a significant prognostic marker for event-free and overall survival (Table5). The hazard ratio for death and poor outcome (death, induction failure, or relapse) in patients with the Flt3/ITD compared with mutation-free patients was 2.1 (P = .03) and 2.6 (P = .002), respectively. Multivariate analysis of the Flt3/ITD, induction regimen, and diagnostic WBC showed that Flt3 mutation was the only independent prognostic factor for overall and event-free survival (P = .04 and .009, respectively) (Table6).
Univariate analysis of prognostic factors for event-free survival and overall survival
| . | Event-free survival . | Overall survival . | ||
|---|---|---|---|---|
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Flt3/ITD positive (compared with negative) | 2.6 | .002 | 2.1 | .03 |
| Standard induction regimen (compared with intensive) | 1.5 | .2 | 1.1 | .7 |
| WBC > 100 000/mm3 (compared with <100 000) | 1.2 | .6 | 1.0 | 1.0 |
| . | Event-free survival . | Overall survival . | ||
|---|---|---|---|---|
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Flt3/ITD positive (compared with negative) | 2.6 | .002 | 2.1 | .03 |
| Standard induction regimen (compared with intensive) | 1.5 | .2 | 1.1 | .7 |
| WBC > 100 000/mm3 (compared with <100 000) | 1.2 | .6 | 1.0 | 1.0 |
WBC = white blood cell count.
Multivariate analysis of prognostic factors for event-free survival and overall survival
| . | Event-free survival . | Overall survival . | ||
|---|---|---|---|---|
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Flt3/ITD positive (compared with negative) | 2.4 | .009 | 2.1 | .04 |
| Standard induction regimen (compared with intensive) | 1.2 | .5 | 1 | 1.0 |
| WBC > 100 000/mm3 (compared with <100 000) | 1.2 | .5 | 1 | .9 |
| . | Event-free survival . | Overall survival . | ||
|---|---|---|---|---|
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Flt3/ITD positive (compared with negative) | 2.4 | .009 | 2.1 | .04 |
| Standard induction regimen (compared with intensive) | 1.2 | .5 | 1 | 1.0 |
| WBC > 100 000/mm3 (compared with <100 000) | 1.2 | .5 | 1 | .9 |
WBC = white blood cell count.
We furthermore compared the Flt3/ITD distribution and outcome for these patients stratified by WBC at diagnosis. Four of the 38 patients (10%) with low WBC (WBC less than 20 000/μL) were positive for the Flt3/ITD compared with 11 of 53 patients (21%) with high WBC (more than 20 000/μL). Kaplan-Meier estimates for event-free survival at 8 years for patients with low WBC was 41% in Flt3/ITD-negative patients, and 0% for patients with the Flt3/ITD (P = .07). Kaplan-Meier estimate for event-free survival at 8 years for patients with high WBC was 46% for Flt3/ITD-negative patients, and 9% for patients with the Flt3/ITD (P = .009).
We finally compared the clinical outcome of patients with and without Flt3/ITD on the basis of induction regimen. Of the study population, 76 patients were Flt3/ITD-negative, of which 53 received intensive and 23 received standard timing induction therapy. Event-free survival at 8 years for patients who received intensive timing therapy was 49% compared with 34% for patients who received standard timing induction regimen (P = .23). Of the 15 Flt3/ITD-positive patients, 7 received intensive timing and 8 received standard timing induction therapy. In the Flt3/ITD-positive patients, event-free survival at 8 years for patients who received intensive timing was 0% (0 of 7) compared with 12.5% (1 of 8) for the patients who received standard timing regimen (P = .37). Thus, patients with Flt3/ITD have a poor outcome, regardless of diagnostic WBC or induction regimen used.
Discussion
We examined the prevalence and prognostic significance of the Flt3/ITD in pediatric patients with AML who were treated on a single treatment protocol. We demonstrated that a significant proportion (16.5%) of pediatric patients with AML who were treated on CCG-2891 had the Flt3/ITD mutation and that this mutation was a powerful, independent risk factor for poor outcome, regardless of diagnostic WBC or induction regimen used. Furthermore, we have shown that the Flt3/ITD is not a stem cell mutation and is restricted to the myeloid lineage.
Previous studies showing association of this mutation with poor outcome in pediatric, adult, and elderly AML were limited by the fact that patients were not treated uniformly, making it difficult to assess the prognostic significance of this mutation. In our study, all patients were treated on the same treatment protocol (CCG-2891), and the population was a representative sample of the CCG-2891 population. Although median WBC and age at diagnosis for patients in the study were slightly higher than the median for the rest of the CCG-2891 patient population, this is most likely due to the fact that the marrow specimens available through the reference laboratory are enriched for older patient specimens and the ones with higher WBCs. Very young patients and patients with very low WBCs at diagnosis may have very few or no specimens available at the reference laboratory. This problem is inherent to the use of archived specimens and could be overcome by conducting a prospective study.
Evaluation of the characteristics of the patients with the Flt3/ITD demonstrated the association of this mutation with leukocytosis. The Flt3/ITD has been shown to cause constitutive activation of the receptor tyrosine kinase,7 leading to autonomous, cytokine-independent cellular proliferation.8,12 Thus, the Flt3/ITD may be a causative factor for leukocytosis in patients who harbor this mutation. The mechanism by which this mutation leads to tyrosine kinase activation is unknown. Evaluation of the region of duplication has defined a general region of involvement within the JM domain coding region (exons 11 and 12). However, within this region, there is no common area of duplication shared by all patients. Deletions and point mutations in the JM coding domain of c-kit tyrosine kinase receptor gene have been reported that lead to constitutive activation of the c-kit receptor tyrosine kinase.21 22 In this case, it is the disruption of the JM domain of the receptor that allows ligand-independent receptor dimerization and receptor kinase activation. Thus, one may speculate that it is not a specific duplication that leads to the Flt3 receptor activation, but rather a conformational change of the JM domain of the Flt3 receptor, caused by the ITD, that leads to constitutive receptor dimerization and receptor tyrosine-kinase activation.
Our study highlights the role of the Flt3/ITD as a predictor for poor outcome in pediatric AML. We have demonstrated that within a uniformly treated patient population, the Flt3/ITD is a powerful, independent prognostic factor, regardless of diagnostic WBCs, induction regimen, or cytogenetic markers. For example, 4 of the 15 study patients with the Flt3/ITD had favorable cytogenetics (inv 16, t[15;17] or t[8;21]), yet all died. This contrasts the 22 of 76 Flt3/ITD-negative patients with favorable cytogenetic markers who had an event-free survival of 64% at 8 years (14 of 22). Patients with the Flt3/ITD appear to be refractory to primary induction therapy, and for those who achieve remission, there is a high rate of relapse and death.
Given the aggressive nature of the Flt3/ITD AML and the extremely poor outcome for these patients on current therapy, it may be appropriate to consider alternative therapies. Some patients with Flt3/ITD have had favorable outcome with allogeneic BMT. Of the 3 patients with Flt3/ITD who are alive in our series, 2 received unrelated bone marrow transplants after failing induction therapy. In a prior pediatric study, the lone survivor with Flt3/ITD had received an allogeneic bone marrow transplant.13 Thus, allogeneic stem cell transplantation as a postinduction therapy may improve outcome in this population. Patients without matched-related donors may benefit from unrelated stem cell transplants. For the significant number of patients with Flt3/ITD refractory to the primary induction therapy, or those with relapsed disease, more novel therapies such as tyrosine kinase inhibitors may prove useful. Constitutive activation of the Flt3 receptor tyrosine kinase may contribute to the pathogenesis of AML in the patients with Flt3/ITD. In chronic myelogenous leukemia (CML) in which constitutive activation of the c-abl kinase is responsible for CML leukemogenesis, inhibitors of tyrosine kinase have been successfully used for targeted therapy.23Understanding the mechanism of action for Flt3/ITD and its role in leukemogenesis may eventually lead to the development of novel, targeted therapies for AML patients with Flt3/ITD.
Supported by NIH grants T32 CA09351, CA 18029, and ACS U10 CA13539.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Soheil Meshinchi, Fred Hutchinson Cancer Research Center, Clinical Research Division, D4-100, 1100 Fairview Ave N, Seattle, WA 98103; email: smeshinc@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal