Abstract
Tumoral lymphocytes from patients with B-chronic lymphocytic leukemia (B-CLL) are long-lived cells in vivo, but they die rapidly by apoptosis in vitro. Here, it is reported that endothelial cells (ECs) inhibit the apoptosis of B-CLL cells, as determined by 4 different flow cytometric methods, and that this antiapoptotic effect is mediated mainly by soluble factor(s), as can be deduced from the following findings. First, EC-conditioned medium (ECCM) inhibited the apoptotic rate in B-CLL to approximately 50% of control. Second, the antiapoptotic effect mediated by EC/B-CLL cell contact was more apparent than real; using a fluorescence-based phagocytosis assay, it was demonstrated that this effect was due to the phagocytic capacity of ECs, which internalized apoptotic cells. Third, the protective effect of ECCM was associated neither with proliferation nor differentiation signals. Fourth, the survival factor was a dimeric form of IL-6 because anti–IL-6 antibodies completely neutralized the antiapoptotic effect mediated not only by the crude ECCM but also by the 45- to 55-kd active fractions obtained after gel filtration, which contained high levels of IL-6. These IL-6 dimers (IL-6D) were noncovalently associated. Sixth, human recombinant IL-6D(hrIL-6D) inhibited B-CLL apoptosis, whereas hrIL-6 monomers (hrIL-6M) did not. Binding and functional competition experiments showed not only that monomers and dimers had similar affinity for the IL-6R, but also that hrIL-6Minhibited the antiapoptotic activity of hrIL-6D. These data suggest that IL-6D derived from ECs promote the survival of B-CLL cells.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by sustained lymphocytosis in the peripheral blood (PB) of monoclonal CD5+ B-lymphocytes. These cells are frozen in the G0/G1 phase of the cell cycle and are long-lived in vivo.1,2 Indeed, it has been suggested that the prolonged lifespan is the main factor for the accumulation of the tumoral cells.1 However, in culture, PB B-CLL cells undergo rapid and spontaneous apoptosis,3 suggesting that programmed cell death in cultured B-CLL cells may be the result of the absence of several humoral factors, cellular factors, or both present in vivo. Thus, the prevention of apoptosis in B-CLL cells by several cytokines, such as interferon (IFN)-α,4,6IL-4,5 IL-13,7 and IL-88 have been studied. These studies have always examined the effect of recombinant cytokines added exogenously to B-CLL cultures. As a consequence, it is not yet clear in most cases whether these cytokines are produced by the tumor cells themselves or by accessory cells.
There is growing evidence that all cells are constantly supported in vivo by survival factors produced by other cells.9Therefore, regulation of the size of the B-CLL tumoral population is likely to depend directly on signals external to the cell that determine whether the cell-death program is engaged. As such, the factors that can modulate B-CLL survival include not only cytokines but also cell-cell contacts, extracellular matrix (ECM) components, or both. In this sense, 2 recent reports10 11 suggest that cell-cell interactions mediated by bone marrow stromal cells prevent B-CLL apoptosis, though the adhesion molecules mediating this effect have not been characterized.
It is well known that B-CLL lymphocytes are recirculating cells that probably interact with vascular endothelial cells (ECs), the cell population that covers the inner surfaces of blood vessels.12 However, ECs are absent or rarely present in venous blood extraction. Because B-CLL cells undergo apoptosis within hours of removal from the body, in contrast to their prolonged survival in vivo, we hypothesized that ECs may affect the viability of circulating B-CLL cells. Our results indicate that IL-6 dimers (IL-6D) contained in the conditioned medium produced by ECs (ECCM), but not by close cell-cell interactions, are potent factors for preventing B-CLL cells from entering apoptosis in culture.
Patients, materials, and methods
Patients
Thirty-five untreated patients with B-CLL were given diagnoses according to standard clinical, morphologic, and immunologic criteria. PB contained 7.9 × 109 to 198 × 109leukocytes per liter, of which 57% to 93% were lymphocytes. Rai staging distribution13 was 13 patients with stage 0, 11 patients with stage I, 10 patients with stage II, and 1 patient with stage III-IV disease.
Antibodies and cytokines
Purified fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled mouse monoclonal antibodies (mAbs) were used in this study. Control IgG1, CD3, CD14, CD20, CD23, and CD38 were purchased from Becton Dickinson (San Jose, CA); CD2, CD5, CD19, and CD5 were obtained from Coulter (Hialeah, FL); hrIL-6 derived fromEscherichia coli was purchased from Prepotech (Rocky Hill, NJ); hrIL-6 derived from Chinese hamster ovary (CHO) cells, neutralizing rabbit polyclonal antibodies against IL-6, granulocyte macrophage colony-stimulating factor (GM-CSF), IL-4, IL-8, and IL-13, and preimmune rabbit serum were obtained from Genzyme (Boston, MA).
Cell purification
Peripheral blood mononuclear cells (PBMCs) from patients or healthy subjects were obtained by density gradient centrifugation of heparinized blood on Ficoll-Paque (Pharmacia, Uppsala, Sweden). Non-B cells were removed using magnetic beads (Dynabeads; Dynal, Oslo, Norway) coated with antibodies CD2 and CD14. B-CLL preparations contained 97% ± 1% CD19+ cells, as determined by flow cytometry (FACScan; Becton Dickinson). Total tonsillar B cells were prepared as previously described.14
EC cultures and preparation of ECCM
Human umbilical vein endothelial cells (HUVECs) were obtained through a previously described method15 and used at the second or third passage. Human umbilical cord-derived ECV-304 cells (American Type Culture Collection, Rockville, MD) were plated at a cell density of 0.1 × 106/mL and cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS) (complete media; CM). The cells were passaged every 3 to 4 days by trypsinization.
After cultivation for different time intervals, the conditioned medium from HUVECs or ECV-304 cells was collected and filtered (0.22 μm), and the supernatant was frozen at −20°C until use. Maximal antiapoptotic activity in the ECCM was observed when HUVECs or ECV-304 cells were grown to near confluence (7 and 3 days, respectively). ECCM and cytokines were added at the onset of the culture.
Cell cultures
Purified tumoral B-lymphocytes and normal PBMCs and tonsillar B cells were cultured at a final concentration of 0.5 × 106 cells/mL in a 24-well plate (Costar, Cambridge, MA). In parallel experiments, tumoral B-lymphocytes (0.3 × 106 cells/well) were cocultured either in direct contact with ECs or physically separated from ECs using a transwell insert (Costar) with a 0.4-μm microporous membrane that permits the passage of soluble factors but prevents direct B-CLL–endothelium contact.
Adhesion blocking assay and preparation of ECM
The following blocking mAbs were incubated with B-CLL cells for 30 minutes at 4°C before the cell-cell contact assay: TS2/16 (anti-CD29), TS1/18 (anti-CD18), BU75 (anti-CD44), and LAM 1-3 (anti-CD62L) were purchased from Serotec (Oxford, United Kingdom), Endogen (Woburn, MA), Ancell (Bayport, MN), and Coulter, respectively. ECM was prepared from ECs cultured for 1, 2, or 3 days by the method described by Cavender et al.16
Fixation of the EC monolayers
ECV-304 cell monolayers were washed twice in phosphate-buffered saline (PBS) and then incubated for 15 minutes at 37°C with 1% paraformaldehyde. To remove residual fixative, the monolayers were washed 5 times with PBS solution and incubated with RPMI and 10% FCS for 3 days at 37°C before use.
IL-6 content and neutralization of IL-6 activity in culture supernatants
IL-6 was measured in the ECCM using an ELISA assay (Genzyme) according to the manufacturer's instructions. To neutralize IL-6 activity, aliquots of ECCM were incubated with a polyclonal rabbit antihuman IL-6 antibody at final dilutions capable of neutralizing 0.1 to 50 ng hIL-6. Rabbit serum was used as a control. The reaction was carried out for 1 hour at 37°.
Immunofluorescent staining and cytometric analysis of apoptotic cells
Flow cytometry for surface staining was performed according to the published procedure.14 Apoptosis of lymphocytes was assessed by 4 flow cytometric methods—annexin-V binding and propidium iodide (PI) staining, as described17,18; whole-cell staining with PI, as described19; Nick translation assay (TUNEL), according to the manufacturer's instructions (Boehringer Mannheim, Mannheim, Germany); and modifications in scatter properties (FSC/SSC), as described.20 FSC–annexin-V fluorescence dot plot was used to discriminate debris from apoptotic cells. An appropriate scattering gate was then set around viable, early, and late apoptotic cells.
Phagocytosis assay
Purified nonapoptotic B-CLL cells (5 × 106) were labeled with 50 μg viable dye TAMRA 5-(&6)-carboxy tetramethylrhodamine, succinimide ester; Molecular Probes, Eugene, OR), washed and then added to monolayers of ECV-304 cells as previously described.21 In the FSC/SSC dot plot, ECs were localized on the basis of their medium-to-high FSC and medium-to-high SSC characteristics.
Flow cytometric assay of IL-6 receptors and competition experiments
We determined IL-6R expression by using the relative binding of Fluorokine IL-6 biotin and streptavidin-FITC, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN), performed with fresh B-CLL cells. By preliminary titration experiments, 10 ng biotin IL-6 saturated IL-6R of all B-CLL samples tested, and this concentration was used for all experiments. Competition experiments were performed after preincubating cells for 30 minutes at 4°C with different amounts (0-100 ng) of IL-6 dimers or monomers.
Heat inactivation and size fractionation of ECCM (S-200)
Aliquots of ECCM were heated at either 60°C or 90°C for 15 minutes to assess the effect of heat treatment on inhibitory activity.
To characterize the size of the inhibitory factor(s) present in the ECCM, we concentrated this medium 30-fold with an XM50 Amicon membrane (Amicon, Beverly, MA) before chromatography. A 1.5-mL aliquot of concentrated supernatant was layered onto a Sephacryl S-200 (Pharmacia LKB Biotechnology, Uppsala, Sweden) column (500 mL; 2.5 cm × 100 cm). The column was run at a constant flow rate of 60 mL/h. Fractions of 5 mL were collected. Protein elution was monitored at 280 nm, and fractions were stabilized with 10% FCS (vol/vol). Analysis of activity was measured in every fraction. The presence of IL-6 was determined by ELISA. hrIL-6 preparations derived from E coli or CHO cells (10 μg hrIL-6/200 μL) were applied onto a Sephacryl S-200 column (20 mL; 0.8 cm × 40 cm) and eluted at a constant flow rate of 5 mL/h. Fractions (200 μL) were stabilized with FCS (10% vol/vol) and analyzed for IL-6 content by ELISA.
Immunoprecipitation of metabolically labeled IL-6
ECV-304 cells were cultured for 2 days at 105/mL in CM in a 24-well plate (Costar). Cells were washed twice in methionine-free DMEM medium and resuspended in the same medium supplemented with dialyzed FCS. After 1 hour, 25 μCi/mL35S-methionine was added to each well. Methionine-labeled supernatants were collected after 20 hours of incubation. Protease inhibitors (phenylmethylsulfonyl fluoride 200 μM; EDTA 2 mM; pepstatin A 0.7 μg/mL; leupeptin 0.5 μg/mL) were added. Supernatants were then incubated with 5 μL normal rabbit serum (Sigma). After 2 hours, 10 μL protein G-Sepharose (Pharmacia) was added to each one. Samples were then incubated for 1 hour at 4°C with gentle agitation and centrifuged for 5 minutes at 2000g. Precleared supernatants were mixed with a polyclonal rabbit antihuman IL-6 (10 μg/mL) (Genzyme) for 3 hours at 4°C. The immune complexes were adsorbed onto protein G-Sepharose for 1 hour at 4°C and centrifuged at 12 000 rpm. The beads were sequentially washed 3 times and boiled in reducing (10% β-mercaptoethanol) or nonreducing (without β-mercaptoethanol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. SDS-PAGE and autoradiography were used to analyze the material eluted from the beads.
Statistical evaluation
The data are presented as mean ± SEM of nexperiments. Statistical significance was evaluated using paired Student t test for comparisons between 2 means and analysis of variance for more than 2 means. P < .05 indicated statistical significance.
Results
ECCM inhibits apoptosis of B-CLL cells
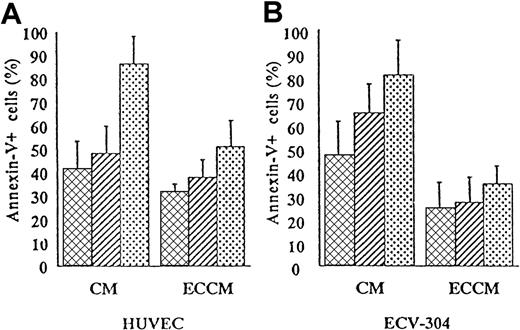
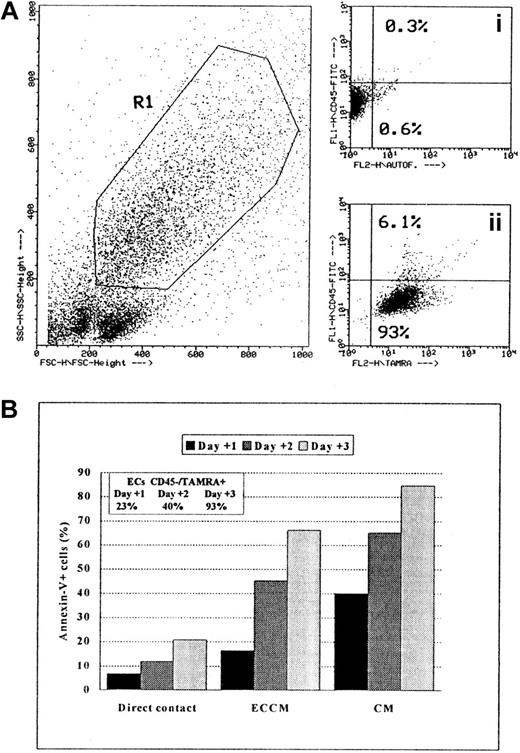
To determine whether conditioned medium derived from HUVEC had antiapoptotic activity, B-CLL cells were cultured for 1, 2, or 3 days in CM or ECCM. For this purpose, we examined cell death by double staining with annexin-V and PI. As shown in Figure1A, the percentage of apoptotic cells was substantially reduced in ECCM throughout the culture period. Because the isolation and culture of fresh HUVEC is laborious and their expansion and maintenance are limited, medium conditioned by the human umbilical cord-derived ECV-304 cell line was tested for its ability to inhibit B-CLL apoptosis. As can be seen in Figure 1B, ECCM derived from ECV-304 cells supported the survival of B-CLL cells with efficiency at least similar to that of conditioned medium derived from fresh HUVEC. Consequently, all future experiments were performed with ECCM derived from ECV-304 cells. Thus, in the presence of ECCM, the percentage of apoptotic B-CLL cells was significantly reduced from 35% ± 6% to 17% ± 4% on day 1 (n = 30, P < 10−7), 57% ± 8% to 32% ± 8% on day 2 (n = 30;P < 10−6), and 73% ± 9% to 49% ± 9% on day 3 (n = 28; P < 10−5). The ECCM-mediated prevention of in vitro apoptosis was observed in all patients tested regardless of their age, Rai stage, white cell count, or lymph node involvement. In contrast to B-CLL cells, the apoptotic kinetics of normal PBMCs and tonsillar B cells were not modified by the ECCM (data not shown).
ECCM inhibits spontaneous in vitro B-CLL cell apoptosis.
Purified tumoral cells were cultured either with CM or ECCM derived from fresh HUVEC (A) or ECV-304 cells (B). After 1 (▩), 2 (▨), or 3 (░) days of culture, B-CLL cells were removed from the well by pipetting and were stained with annexin-V–FITC and PI for flow cytometry analysis. Conditioned medium from HUVEC or ECV-304 cells was collected after 7 and 3 days of culture, respectively. Results are expressed as the mean ± SEM of 5 independent experiments and represent the percentage of apoptotic cells.
ECCM inhibits spontaneous in vitro B-CLL cell apoptosis.
Purified tumoral cells were cultured either with CM or ECCM derived from fresh HUVEC (A) or ECV-304 cells (B). After 1 (▩), 2 (▨), or 3 (░) days of culture, B-CLL cells were removed from the well by pipetting and were stained with annexin-V–FITC and PI for flow cytometry analysis. Conditioned medium from HUVEC or ECV-304 cells was collected after 7 and 3 days of culture, respectively. Results are expressed as the mean ± SEM of 5 independent experiments and represent the percentage of apoptotic cells.
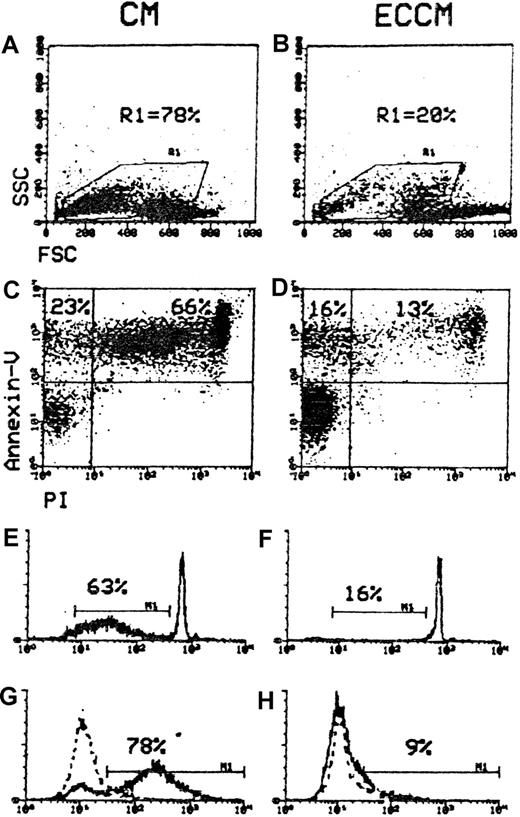
Moreover, the incidence of hypodiploid and TUNEL+ cells and of B-CLL lymphocytes with morphologic alterations agreed closely with the results of annexin-V/PI analysis (Figure2). In comparison, however, the annexin-V assay was able to detect early phases of B-CLL apoptosis. For this reason, the annexin-V/PI method was chosen to quantify apoptotic leukemic cells. These results suggest that ECCM transduces a survival signal to B-CLL cells.
Comparison of cytofluorometric methods for quantifying apoptotic cells.
B-CLL cells were subjected to one of the 4 assays for apoptosis quantitation after incubation for 48 hours in CM and ECCM. (A, B) Gate R1 was drawn around apoptotic cells, which can be distinguished from living cells through their decreased FSC and increased SSC characteristics. (C, D) The upper left quadrant (annexin-V+/PI−) represents the early apoptotic cells, and the upper right quadrant (annexin-V+/PI) contains the late apoptotic cells. M1 defines the percentages of B-CLL cells with hypodiploid DNA content (E, F) and TUNEL+ signal (G, H). Dotted lines show control staining. Numbers at the top of each cytogram represent the percentages of apoptotic cells. Data are representative of 5 experiments.
Comparison of cytofluorometric methods for quantifying apoptotic cells.
B-CLL cells were subjected to one of the 4 assays for apoptosis quantitation after incubation for 48 hours in CM and ECCM. (A, B) Gate R1 was drawn around apoptotic cells, which can be distinguished from living cells through their decreased FSC and increased SSC characteristics. (C, D) The upper left quadrant (annexin-V+/PI−) represents the early apoptotic cells, and the upper right quadrant (annexin-V+/PI) contains the late apoptotic cells. M1 defines the percentages of B-CLL cells with hypodiploid DNA content (E, F) and TUNEL+ signal (G, H). Dotted lines show control staining. Numbers at the top of each cytogram represent the percentages of apoptotic cells. Data are representative of 5 experiments.
Antiapoptotic effect mediated by EC/B-CLL cell contacts is more apparent than real
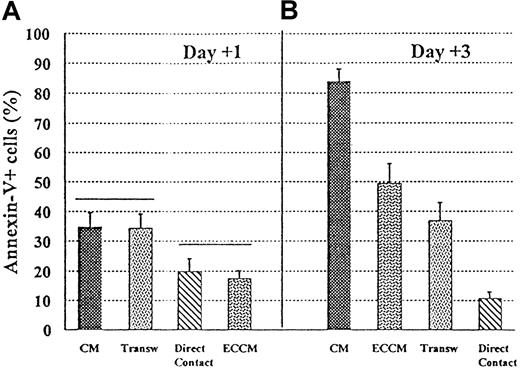
We next performed experiments to determine the role of direct contact with endothelium versus the effect of soluble factors produced by ECs in mediating this B-CLL apoptosis inhibition. As can be seen in Figure 3, apoptosis was almost completely inhibited when B-CLL cells were plated in direct contact with the endothelium. This antiapoptotic effect was also observed when B-CLL cells were physically separated from ECs by transwell inserts, though to a lesser extent than that observed in endothelium-contact cultures. It should be noted that the viability observed in endothelium-prevented contact cultures was commonly lower than that detected with conditioned medium, at least in the first 2 days of culture (Figure 3A). After 3 days of culture, however, the transwell system was more effective in inhibiting B-CLL apoptosis than ECCM (Figure 3B). These results appear to indicate the consumption of soluble factor(s) in the ECCM after 3 days of culture. Taken together, these data also apparently suggest that B-CLL cells were protected from spontaneous apoptosis not only by soluble factor(s) but also through cell-cell interactions.
Requirements for direct contact with ECV-304 endothelial cells for the inhibition of apoptosis in B-CLL cells.
B-CLL cells were cultured for 1 and 3 days in 4 conditions: CM, ECCM, ECV-304 cells + B-CLL cells (direct contact), ECV-304 cells and B-CLL cells separated in a transwell (Transw) system. A gate for lymphoid cells was established to exclude contaminating ECs. Statistical analysis was carried out using analysis of variance with n = 9, and P < .05.
Requirements for direct contact with ECV-304 endothelial cells for the inhibition of apoptosis in B-CLL cells.
B-CLL cells were cultured for 1 and 3 days in 4 conditions: CM, ECCM, ECV-304 cells + B-CLL cells (direct contact), ECV-304 cells and B-CLL cells separated in a transwell (Transw) system. A gate for lymphoid cells was established to exclude contaminating ECs. Statistical analysis was carried out using analysis of variance with n = 9, and P < .05.
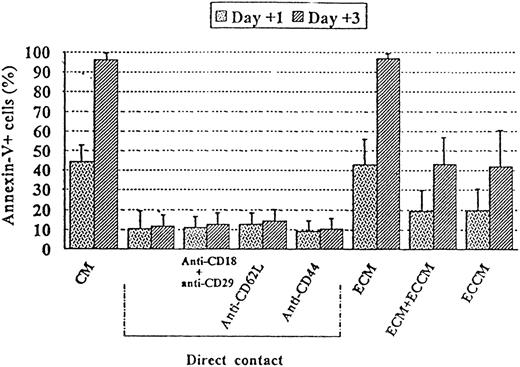
To determine whether adhesion proteins or their cognate ligands (ECM) were involved in this process,22 neutralizing mAb antiadhesion molecules expressed by B-CLL cells were added to endothelium-contact cultures. As shown in Figure4, the addition of blocking mAbs, alone or in combination, against CD29, CD18, CD44, or CD62L did not interfere with the protective capacity of ECs to prevent apoptosis in leukemic cells. A complex ECM obtained by endothelial-cell lysis with 0.5% sodium deoxycholate after 3 days of culture was also unable to inhibit tumoral B-cell apoptosis (Figure 4). Interestingly, paraformaldehyde fixation of ECs abrogated their ability to prevent B-CLL apoptosis: the percentages of apoptotic cells after 1 and 3 days of culture were 30% ± 7% and 70% ± 8% in the presence of fixed ECs versus 32% ± 9% and 69% ± 9% in CM (n = 4). A synergistic effect mediated by physical interactions and soluble factors was ruled out because fixed ECV-304 cells with ECCM prevented B-CLL apoptosis in a manner similar to that observed with ECCM alone (data not shown).
Role of adhesive interactions and ECM components in the apoptosis of B-CLL cells.
B-CLL cells were cultured for 1 and 3 days either with ECV-304 cells in the presence or absence of 10 μg/mL of the indicated mAbs or with a complex ECM obtained by EC lysis with 0.5% sodium deoxycholate. The results are compared with the percentage of apoptosis observed in the absence (CM) or presence (ECCM) of soluble factors. Data are presented as the mean ± SEM and are representative of at least 3 experiments.
Role of adhesive interactions and ECM components in the apoptosis of B-CLL cells.
B-CLL cells were cultured for 1 and 3 days either with ECV-304 cells in the presence or absence of 10 μg/mL of the indicated mAbs or with a complex ECM obtained by EC lysis with 0.5% sodium deoxycholate. The results are compared with the percentage of apoptosis observed in the absence (CM) or presence (ECCM) of soluble factors. Data are presented as the mean ± SEM and are representative of at least 3 experiments.
These results indicate 2 apparently contradictory facts: the direct contact with ECs seemed to be involved in the prevention of B-CLL apoptosis (Figure 3), and the protective role of ECs was not mediated by adhesion molecules or by ECM proteins (Figure 4). With regard to this paradox, we observed that significantly fewer B-CLL cells were recovered from cultures in which leukemic cells were cultured in contact with endothelium than when these cells were cultured either separated from endothelium by a transwell insert or with ECCM (data not shown). Because the role of ECs in recognizing and phagocytosing apoptotic cells is well established,23,24 we predicted that apoptotic B-CLL cells would be engulfed by ECs. To test this hypothesis, the percentage of ECs with phagocytosed apoptotic cells was determined at different time intervals using a fluorescence-based phagocytosis assay in which fresh B-CLL cells were previously labeled with TAMRA.21 As shown in Figure5 from a representative experiment, the percentage of ECs labeled with TAMRA after 24 hours of coculture with B-CLL cells was 26% ± 8%, and this increased to 45% ± 6% and 95% ± 4% after 48 and 72 hours, respectively (n = 5). Moreover, most ECs were TAMRA+/CD45−, clearly indicating that apoptotic leukemic cells were removed from the surface of ECs by an internalization process. In conclusion, the survival effect mediated by cell-cell contacts was more apparent than real because the number of B-CLL cells remaining in culture after 1, 2, or 3 days was overestimated owing to the selective disappearance of apoptotic cells. Taken together, these experiments demonstrate that soluble factor(s) produced by ECs, rather than contact-mediated events, are responsible for the antiapoptotic effect of ECs on B-CLL cells.
Phagocytic capacity of ECs measured by flow cytometry.
Nonapoptotic B-CLL cells were labeled with the fluorophore TAMRA, as described in “Materials and methods.” (A) Dot-plot (FSC/SSC) of ECs and TAMRA-labeled B-CLL cells after 3 days of coculture is shown. Gate R1 was drawn around ECs according to FSC/SSC criteria. Dot plots of FL1 (y-axis)/FL2 (x-axis): (Ai) CD45 expression (FL1) and FL2-autofluorescence of ECs only; (Aii) CD45 and TAMRA (FL2) signals on ECs gated on R1 after 3 days of coculture. (B) Ingestion kinetics of apoptotic cells by ECs after 1, 2, and 3 days of coculture. Values represent means of triplicate samples from one representative experiment. SEM was always less than 5%.
Phagocytic capacity of ECs measured by flow cytometry.
Nonapoptotic B-CLL cells were labeled with the fluorophore TAMRA, as described in “Materials and methods.” (A) Dot-plot (FSC/SSC) of ECs and TAMRA-labeled B-CLL cells after 3 days of coculture is shown. Gate R1 was drawn around ECs according to FSC/SSC criteria. Dot plots of FL1 (y-axis)/FL2 (x-axis): (Ai) CD45 expression (FL1) and FL2-autofluorescence of ECs only; (Aii) CD45 and TAMRA (FL2) signals on ECs gated on R1 after 3 days of coculture. (B) Ingestion kinetics of apoptotic cells by ECs after 1, 2, and 3 days of coculture. Values represent means of triplicate samples from one representative experiment. SEM was always less than 5%.
ECCM does not affect either proliferation or differentiation of B-CLL cells
Our next objective was to find out whether the inhibitory effect of ECCM on the apoptosis of B-CLL cells was mediated by proliferative signals. For this purpose, we measured viability and the cell-cycle state of B-CLL cells that had been cultured with ECCM after 48 hours in comparison with fresh B-CLL cells. Results from 5 experiments showed that monoclonal B-lymphocytes rescued from apoptosis were mostly noncycling tumor cells after 2 days of culture with ECCM (98% ± 2% of B-CLL cells in G0/G1 phase of the cell cycle versus 97% ± 2% of fresh tumoral B cells; P > .05). Therefore, the persistence of B-CLL cells cultured in ECCM was not mediated by an increase in cell proliferation. We also confirmed that the antiapoptotic effect observed with the ECCM was not mediated by a differentiation signal because the percentage of more mature cells (CD38++) was undetectable after 2 days of culture (1% ± 1%; n = 5) and because the phenotypic profile was identical to that observed in freshly isolated B-CLL cells (CD20low, CD23+, CD5+, surface Iglow).
Antiapoptotic factor contained in the ECCM is a dimeric form of IL-6
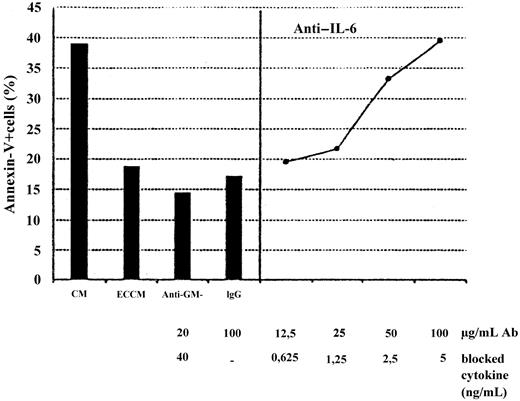
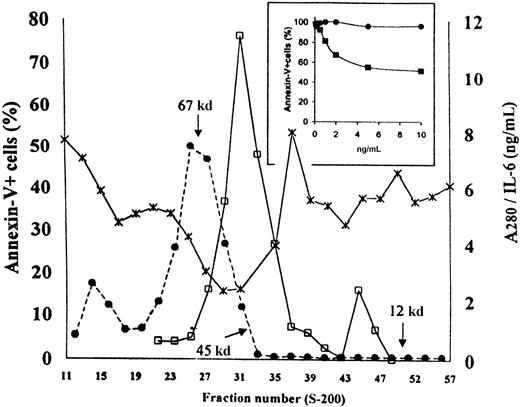
To identify the antiapoptotic soluble factor(s) produced and released by ECV-304 cells, we performed 2 parallel sets of experiments. First, the effect of neutralizing polyclonal antibodies against several well-known soluble factors produced by human ECs was tested. Second, concentrated ECCM was analyzed by Sephacryl S-200 gel filtration to investigate the approximate molecular weight of the active soluble factor(s). As can be seen in Figure 6, antiapoptotic activity in the crude ECCM was completely neutralized by anti–IL-6 polyclonal antibodies in a dose-dependent manner. In contrast, the addition of anti–GM-CSF, anti–IL-4, anti–IL-8, or anti–IL-13 neutralizing antibodies did not modify the kinetics of entry of the B-CCL cell into apoptosis in these cultures (n = 5;P > .05). These results were in close agreement with the fact that the highest antiapoptotic activity was always found in the ECCM collected from ECV-304 monolayer after 3 days of culture—IL-6 content was 1 ± 0.4 ng/mL at day 1 and 3 ± 1 ng/mL at day 2, peaking at day 3 (11 ± 7 ng/mL) and declining thereafter, as measured by ELISA. Moreover, fractionation of concentrated ECCM (the eluate obtained after size separation by Amicon ultrafiltration had no antiapoptotic activity; data not shown) showed that the apoptosis-inhibiting agent was a molecule(s) with a relative molecular mass in the range of 45 to 55 kd (Figure7). Human IL-6 is a protein with a molecular mass ranging from 21 to 28 kd, depending on the cellular source and preparation.25 On the basis of the apparent molecular weight of the soluble factor identified in the above experiment, however, ECs secreted a form of IL-6 with a molecular mass ranging from 45 to 55 kd. To confirm this hypothesis, we used a sensitive ELISA to detect the IL-6 eluting from the column. As is apparent from Figure 7, one major immunoreactive peak was detected, corresponding to 45- to 55-kd fractions, whereas a minor peak was detected between fractions 44 and 47 (22 to 25 kd) that had no effect as survival factor. The dimer-monomer ratio was 10:12/1 (n = 3). Thus, the elution of bioactivity correlates with the elution of IL-6 immunoreactivity, confirming the findings of previous reports26 demonstrating that the native IL-6 exists as a dimer. It is of note that the concentration of IL-6 in the 22- to 25-kd fractions was much lower than that in the 45- to 55-kd fractions. Consequently, the failure of IL-6M to prevent B-CLL cell apoptosis could be due to insufficient amounts of monomer. To exclude this possibility, the 22- to 25-kd fractions and the 45- to 55-kd fractions were pooled and further concentrated to explore their antiapoptotic activity. As shown in Figure 7, IL-6Dinhibited apoptosis in a dose-dependent manner. In contrast, identical concentrations of IL-6M added to B-CLL cultures were inactive. In addition, the survival effect mediated by IL-6D was fully inhibited by anti–IL-6 neutralizing antibodies: the percentages of annexin-V+ cells after 2 days of culture were 53% ± 8%, 27% ± 6%, and 51% ± 8% in CM, IL-6D (10 ng/mL), and IL-6D + anti–IL-6 antibodies, respectively (n = 3).
Effects of neutralizing antibodies on the apoptosis of B-CLL cells supported by the ECCM.
B-CLL cells were incubated with CM, ECCM, or ECCM preincubated with anti–GM-CSF (A) or anti–IL-6 neutralizing antibodies (B), which sufficiently neutralize 40 ng GM-CSF and 5 ng IL-6, respectively. The dose-dependent inhibition mediated by anti–IL-6 antibodies is shown. ECCM was also preincubated with IgG (100 μg/mL) from preimmune rabbit serum as a control for nonspecific antibody effects. Percentages of apoptotic cells were measured in a 48-hour assay.
Effects of neutralizing antibodies on the apoptosis of B-CLL cells supported by the ECCM.
B-CLL cells were incubated with CM, ECCM, or ECCM preincubated with anti–GM-CSF (A) or anti–IL-6 neutralizing antibodies (B), which sufficiently neutralize 40 ng GM-CSF and 5 ng IL-6, respectively. The dose-dependent inhibition mediated by anti–IL-6 antibodies is shown. ECCM was also preincubated with IgG (100 μg/mL) from preimmune rabbit serum as a control for nonspecific antibody effects. Percentages of apoptotic cells were measured in a 48-hour assay.
Survival-promoting activity in size-fractionated ECCM.
ECCM was concentrated with an Amicon filtration device and then passed through a Sephacryl S-200 gel filtration column equilibrated with PBS. Each fraction was assayed at dilution 1:2 on B-CLL cells to determine their antiapoptotic activity. IL-6 content (■) was measured as described in “Materials and methods.” The Mr standards are indicated by arrows; ●, A280; ×, annexin-V. A representative experiment of 3 similar elution profiles is shown. The inset shows the antiapoptotic activity of monomeric and dimeric forms of endothelial IL-6. B-CLL cells were incubated with various concentrations of IL-6D (■) or IL-6M (●). Percentages of apoptotic cells were measured in a 48-hour assay. These results were confirmed in 2 additional experiments. Results are expressed relative to the percentage of apoptosis observed in the CM.
Survival-promoting activity in size-fractionated ECCM.
ECCM was concentrated with an Amicon filtration device and then passed through a Sephacryl S-200 gel filtration column equilibrated with PBS. Each fraction was assayed at dilution 1:2 on B-CLL cells to determine their antiapoptotic activity. IL-6 content (■) was measured as described in “Materials and methods.” The Mr standards are indicated by arrows; ●, A280; ×, annexin-V. A representative experiment of 3 similar elution profiles is shown. The inset shows the antiapoptotic activity of monomeric and dimeric forms of endothelial IL-6. B-CLL cells were incubated with various concentrations of IL-6D (■) or IL-6M (●). Percentages of apoptotic cells were measured in a 48-hour assay. These results were confirmed in 2 additional experiments. Results are expressed relative to the percentage of apoptosis observed in the CM.
Moreover, IL-6D contained in the ECCM were heat stable because heat treatment at 60°C or 90°C for 15 minutes did not alter their antiapoptotic activity: the percentages of apoptotic cells after 3 days of culture were 68% ± 10%, 44% ± 7%, and 47% ± 8% in CM, ECCM, and heat-treated ECCM, respectively (n = 5).
To determine the electrophoretic behavior of this IL-6, ECV-304 endothelial cells were initially labeled with35S-methionine, and IL-6 products were immunoprecipitated from conditioned medium. Subsequent separation of this immunoprecipitate by SDS-PAGE led to the appearance of a single band corresponding to Mr 25 000 under both nonreducing and reducing conditions (Figure 8, lanes 2 and 4). In contrast, no band corresponding to the dimeric form detected in gel filtration analysis was found. These results suggested that IL-6D contained in the ECCM could be formed by a noncovalent association.
SDS-PAGE analysis of IL-6 immunoprecipitates of ECCM.
Control immunoprecipitation with normal rabbit serum (lanes 1, 3) and IL-6 immunoprecipitation with an antihuman IL-6 rabbit polyclonal antibody (lanes 2, 4) under nonreducing (lanes 1, 2) and reducing (lanes 3, 4) conditions. Samples were analyzed on an SDS–12% polyacrylamide gel. The positions of molecular weight markers are indicated on the right.
SDS-PAGE analysis of IL-6 immunoprecipitates of ECCM.
Control immunoprecipitation with normal rabbit serum (lanes 1, 3) and IL-6 immunoprecipitation with an antihuman IL-6 rabbit polyclonal antibody (lanes 2, 4) under nonreducing (lanes 1, 2) and reducing (lanes 3, 4) conditions. Samples were analyzed on an SDS–12% polyacrylamide gel. The positions of molecular weight markers are indicated on the right.
Ability of various recombinant IL-6 (rIL-6) proteins to prevent B-CLL apoptosis
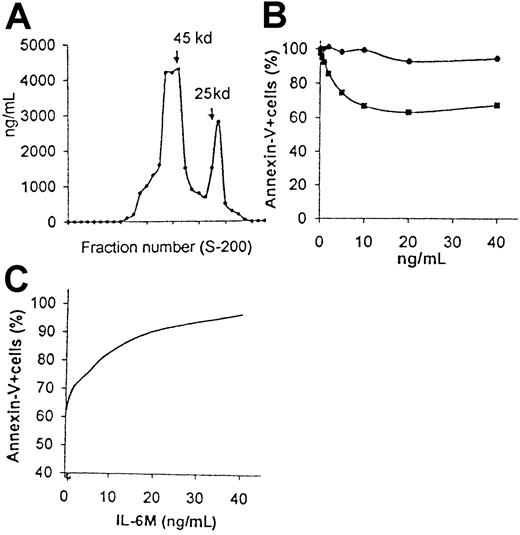
Having determined the antiapoptotic effect mediated by natural IL-6D produced by ECs, we determined whether other IL-6 protein preparations showed a similar effect with regard to their ability to inhibit B-CLL apoptosis. Crude hrIL-6 preparations derived from E coli had little, if any, effect on B-CLL apoptosis (percentages of apoptotic cells after 2 days of culture were 56% ± 8% and 50% ± 6% in CM and CM+hrIL-6, respectively; n = 9; P > .05). For this reason, hrIL-6 was fractionated, as has been described previously for endothelial IL-6. When subjected to gel filtration, this IL-6 preparation, eluted in 2 peaks, distributed in a manner similar to that observed in the ECCM, indicating that it has similar molecular sizes in solution (Figure9A). The dimer-monomer ratio was 2:6/1 (n = 3). To investigate the ability of both forms of hrIL-6 to prevent B-CLL apoptosis, fractions containing either monomers or dimers were pooled: hrIL-6D prevented in a dose-dependent manner the apoptosis of B-CLL cells, whereas monomers did not (Figure 9B). This hrIL-6D–mediated effect was reversed by the addition of blocking anti–IL-6 antibodies (percentage of apoptotic cells, 94% ± 5%; n = 3); hrIL-6D derived from CHO cells prevented B-CLL apoptosis in a concentration-dependent manner, similar to that observed in response to hrIL-6D derived fromE coli (data not shown).
hrIL-6M derived from
E coli inhibit hrIL-6D–prevented B-CLL apoptosis. (A) hrIL-6 derived from E coli was filtered through a Sephacryl G-200 column, and fractions were analyzed for IL-6 content by ELISA. The Mr standards are indicated by arrows. (B) B-CLL cells were cultured in the presence of various concentrations of hrIL-6D (■) or hrIL-6M (●) derived fromE coli. hrIL-6D exhibited saturation of B-CLL survival at 10 ng/mL. Results are expressed relative to the percentage of apoptosis observed in the CM. (C) In the same experiment, inhibition of hrIL-6D–prevented B-CLL apoptosis by hrIL-6M was measured by mixing varying concentrations of hrIL-6M with an optimal concentration (10 ng/mL) of hrIL-6D. Similar results were observed in 2 additional experiments. Percentages of apoptotic cells were measured in a 48-hour assay.
hrIL-6M derived from
E coli inhibit hrIL-6D–prevented B-CLL apoptosis. (A) hrIL-6 derived from E coli was filtered through a Sephacryl G-200 column, and fractions were analyzed for IL-6 content by ELISA. The Mr standards are indicated by arrows. (B) B-CLL cells were cultured in the presence of various concentrations of hrIL-6D (■) or hrIL-6M (●) derived fromE coli. hrIL-6D exhibited saturation of B-CLL survival at 10 ng/mL. Results are expressed relative to the percentage of apoptosis observed in the CM. (C) In the same experiment, inhibition of hrIL-6D–prevented B-CLL apoptosis by hrIL-6M was measured by mixing varying concentrations of hrIL-6M with an optimal concentration (10 ng/mL) of hrIL-6D. Similar results were observed in 2 additional experiments. Percentages of apoptotic cells were measured in a 48-hour assay.
To further understand the mechanism responsible for the increased effect of hrIL-6D, compared with hrIL-6M, on B-CLL apoptosis, we tested the capacity of both monomeric and dimeric forms of hrIL-6 derived from E coli for binding to IL-6R (CD126). Initially, we performed experiments on the binding of biotin–IL-6 to human B-CLL cells. The percentage of leukemic cells capable of binding IL-6 was 83% ± 13% (n = 10). The specificity of the binding was demonstrated by the achievement of more than 95% inhibition of the binding by preincubating biotin–IL-6 with an inhibitory antihuman IL-6 antibody. Both monomers and dimers were then examined for their ability to inhibit binding of biotin–IL-6 to B-CLL cells. The IC50 values for either hrIL-6M or hrIL-6D (ie, concentration of competitor inhibiting binding of biotin–IL-6 by 50%) were similar and ranged from 75 to 100 ng/mL (n = 3). These results not only suggest that monomers and dimers had similar affinity for the IL-6R but also that the bioactivity of the 2 forms of IL-6 did not correlate with their receptor-binding ability. Combining the data from the binding studies with the lack of activity of crude hrIL-6 preparations, we interpret that the monomers would interfere with the antiapoptotic effect mediated by the dimers. To confirm this possibility, varying concentrations of hrIL-6Mwere incubated for 48 hours with B-CLL cells in the presence of an optimal concentration (10 ng/mL) of hrIL-6D. As shown in Figure 9C, hrIL-6M inhibited, in a dose-dependent manner, the antiapoptotic effect of hrIL-6D on B-CLL cells. These results clearly suggest that IL-6M specifically inhibits the survival effect mediated by IL-6D.
Discussion
This study shows for the first time that IL-6Dsecreted by ECs promotes B-CLL cell survival in vitro. B-CLL is characterized by the accumulation in the blood, bone marrow, lymph nodes, and spleen of a clonal population of B-lymphocytes. Interestingly, ECs cover the inner surface of blood vessels and are an important component of all of these sites in vivo.12Therefore, the ability of ECs to support B-CLL viability may be relevant in the pathogenesis of this disease.
In contrast to our results, previous studies using the pre-established EA.hy 926 endothelial cell line found that its conditioned medium was unable to prevent B-CLL apoptosis.27 Although the mechanism of this difference remains to be elucidated, a possible explanation is the different nature of these cell lines: ECV-304 is a spontaneously transformed human umbilical endothelial cell line,28 whereas EA.hy 926 is a cell line produced by hybridizing HUVECs with the human epithelial cell line A549.27 Indeed, it has been published that EA.hy 926 cells appear to have lost the ability to respond to IL-4 or IFN stimulation,29 whereas ECV-304 cells have not.28 It is possible, therefore, that these cell lines differ not only in their response to several cytokines but also in the synthesis and secretion of survival factors.
Our initial results suggested that contact-mediated events were also responsible for the antiapoptotic effect of ECs on B-CLL cells. We have demonstrated that this apparent survival effect was due to the phagocytic capacity of ECs, which rapidly internalized apoptotic cells. On the one hand, the fact that ECs, and other nonprofessional phagocytes, engulf apoptotic cells is not surprising because this phenomenon has already been described by several groups of researchers.15,16,30 On the other hand, the use of live feeder cells in assays to determine cell-contact–dependent survival effects has been widely studied.31,32 Indeed, 2 reports have shown that ECs are useful as feeder cells in contact-mediated survival systems for B-CLL cells.27 33 Many of these system cocultures, however, are performed without excluding the probable contribution to this phenomenon of the phagocytic activity displayed by these cells. Therefore, it is likely that, at least in some cases, the use of cell-contact–dependent assays greatly overestimates the antiapoptotic effect mediated by the feeder cell owing to its phagocytic activity.
Tumorigenesis can be induced by activating cell proliferation, by inhibiting metabolic pathways regulating programmed cell death, or both.34 However, it is difficult to separate the antiapoptotic activity mediated by soluble factors from their ability to promote proliferation.35 Our results consistently showed that most malignant cells remained in the G0/G1 phase after 2 days of culture. Moreover, we also discarded the possibility that EC triggered a differentiation signal, on receipt of which the B-CLL cells were transformed into plasmacytoid-like cells. In this sense, our results also demonstrate that the signal mediated by ECCM is integrated into survival but not proliferation or differentiation pathways.
Here we present evidence that the antiapoptotic factor contained in the ECCM is a dimeric form of IL-6. The evidence that IL-6 forms dimers in solution at physiological concentrations is widely accepted.26,36,37 We also observed that the monomer association in IL-6 derived from ECs is noncovalent and does not involve disulfide linkage. This was not an unexpected finding because previous works have demonstrated that IL-6D derived from different sources is not stable under denaturing conditions.26 37
In our hands, crude commercially available hrIL-6 preparations had little, if any, effect on our culture system. In contrast, hrIL-6D obtained after size exclusion chromatography showed antiapoptotic activity, confirming that dimer formation is essential in the prevention of B-CLL apoptosis. Moreover, we also demonstrate that the monomeric form functions as a competitive inhibitor of the dimeric form. These results may explain why there is considerable confusion regarding the role of IL-6 in B-CLL cell apoptosis: most researchers have found no effect,5,6whereas Reittie et al38 have described the inhibition of apoptosis by IL-6. Because all the recombinant IL-6 proteins appear as 2 components on gel filtration, though the abundance of the 2 forms does vary between preparations, the dimer-monomer ratio could be related to the in vitro activity of hrIL-6. Alternatively, other disparities in the hrIL-6 proteins may account for the varying abilities to inhibit the apoptosis of B-CLL cells and may be unique for each protein expression system.39-41
This study also shows that IL-6D contained in the ECCM was clearly more active than rIL-6D derived from E coli. Although it is uncertain from the current work whether the difference in the magnitude of survival effect mediated by EC (natural) and E coli–derived IL-6D could result from their glycosylated and unglycosylated nature, respectively, this hypothesis is unlikely to be correct. Indeed, glycosylated hrIL-6D obtained from CHO cells was active in inhibiting B-CLL apoptosis with a dose-response curve similar to that of hrIL-6D derived from E coli (data not shown). Differences in the mode of dimerization that affects the stability of the IL-6D can be predicted to decrease the mediation of survival activity, as has been described for the noncovalently associated hrSCF dimer, which can undergo spontaneous rapid monomer dissociation.42 Accordingly, it is also possible that IL-6D derived from ECV-304 cells exhibit more stability than hrIL-6D derived from E coli. Indeed, IL-6D contained in the ECCM were heat stable. This is in contrast to hrIL-6D derived from E coli, which are rapidly dissociated into monomers at 37°C.43
The most intriguing question raised by this work is why dimeric, but not monomeric, form of IL-6 shows antiapoptotic activity. Our results are, indeed, in contrast to those of May et al,26 who have reported that IL-6M is more active than or as active as IL-6D in the B9 hybridoma growth factor and Hep3B hepatocyte-stimulating factor assays, respectively. It is well known that IL-6 exerts its biologic activities through the IL-6–specific receptor complex that consists of the IL-6–binding subunit (IL-6R) and the signal transducing subunit (gp130).44 Moreover, several reports demonstrate that clustering of membrane-bound cytokine receptors is important in establishing a downstream signal. In fact, dimerization of several cytokine receptors is sufficient to elicit a biologic response.45,46 Because our competition-binding experiments demonstrate that both monomeric and dimeric forms of hrIL-6 bind IL-6R with comparable affinity, it was reasonable to assume that IL-6M could induce only IL-6R occupancy, not receptor clustering, and could exhibit partial signaling in comparison to the complete signaling generated by IL-6D. This hypothesis, however, is unlikely because monomeric IL-6 is dimerized at least at the receptor site and can induce intracellular signal transduction pathways by inducing the homodimerization of gp130, as previously described.26 As a possible explanation, Ward et al37 demonstrate that IL-6M is more potent than IL-6D in a STAT3 phosphorylation assay, suggesting that both IL-6 forms mediate different intracellular signals. Moreover, Van den Berghe et al47 have found recently that although monomers and dimers of the fibroblast growth factor-2 (FGF-2), another well-known dimeric soluble factor, show similar receptor binding capacity, their biologic capacities are different. Interestingly, monomeric FGF-2 induced cell differentiation but not cell proliferation. In contrast, dimeric FGF-2 was capable of inducing both biologic responses. The authors thus demonstrate the uncoupling between proliferation and differentiation FGF-2 signaling pathways and the essential role of dimerization in the mitogenic activity of this factor. It is of note that our results demonstrate that dimeric IL-6 form inhibits apoptosis but does not affect proliferation or differentiation status of B-CLL cells. This analogous behavior led us to speculate that similar uncoupled signals may operate in our system.
Moreover, neither Bcl-2 nor Bax expression correlated with the ability of ECCM to prevent B-CLL cell death (not shown). Because Bcl-xL or Mcl-1 proteins correlate with survival of human peripheral blood B-lymphocytes better than Bcl-2,48 experiments are in progress to determine whether the survival signal provided by the ECCM in human B-CLL cells could be mediated through the up-regulation of survival genes such as Bcl-xL or Mcl-1.
Acknowledgments
We thank Drs A. Silva, A. Garcı́a-Pardo, and M. T. de la Fuente (Centro de Investigaciones Biologicas) and Drs A. Durantez, J.A. Vargas and R. Castejon (Clı́nica Puerta de Hierro) for some helpful discussions. We also thank David Weston for the critical reading of the manuscript.
Supported by the Comunidad Autónoma de Madrid (grants 07/029/96 and 08.1/0012/97) and by Fondo de Investigaciones Sanitarias de la Seguridad Social (grant 96/1555).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ernesto Roldán Santiago, Servicio de Inmunologı́a, Hospital Ramón y Cajal, Carretera Colmenar Km 9.100, 28034 Madrid, Spain.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal