Abstract
We identified antibacterial components in human T and natural killer (NK) cells by using freshly isolated lymphocytes enriched for T and NK cells as starting material. After growing these lymphocytes for 5 days in the presence of interleukin (IL)–2, we isolated and characterized several antibacterial peptides/proteins from the supernatant—α-defensins (HNP 1-3), LL-37, lysozyme, and a fragment of histone H2B—although other active components were also present. We then used reverse transcriptase–polymerase chain reaction to search for expression of the gene coding for LL-37 in several B-cell lines, γδ T-cell lines, NK clones, and one monocytic cell line, with positive results, but found no expression in several αβ T-cell lines. The α-defensins (HNP 1-3) were also found to be expressed in several of these cell lines. To confirm the presence of these antibacterial peptides in lymphocytes, we localized them to NK, γδ T cells, B cells, and monocytes/macrophages by using double-staining immunohistochemical analysis of freshly isolated lymphocytes. We also found that primary cultures of lymphocytes transcribe and secrete LL-37 and that these processes are affected by IL-6 and interferon-γ. In addition, we demonstrated that LL-37 has chemotactic activity for polymorphonuclear leukocytes and CD4 T lymphocytes, whereas others have shown chemotactic activity for human α-defensins (HNP 1-2). These findings suggest that microbicidal peptides are effector molecules of lymphocytes and that antibacterial activity previously shown to be derived from T and NK cells may be partly mediated by the antibacterial peptides LL-37 and HNP 1-3.
Introduction
Antibacterial peptides are effectors of immediate defenses in innate immunity. In mammals, they are included in the barrier protection of epithelia, where their expression can be induced during infection and inflammation.1,2 These peptides also constitute part of the nonoxidative bactericidal armament of phagocytes such as neutrophils and macrophages, and they can be secreted, as was shown for the precursor of LL-37 (hCAP18).3 Several peptides with antibacterial activity have been identified in humans. These include the α-defensins (HNP 1-4), which are mainly found in neutrophils,4 and HD 5 and 6, which are expressed in Paneth cells of the small intestine5,6; the β-defensins HBD 1 and 2, which are mainly synthesized by epithelial cells7,8; and LL-37, which was first located in granulocytes9 but was subsequently found to be expressed in epithelial cells of the skin, lungs, and gut.2,10,11 The defensins are cysteine-containing peptides with 3 intrachain disulfide bridges folded in a β-sheet structure with amphipathic properties. The cathelicidins are precursor proteins with a conserved cathelin proregion and a variant C-terminal antibacterial domain.12The amphipathic α-helical LL-37 is the only cathelicidin peptide found in humans.9
The common amphipathic motif of these peptides is important for the affinity and disruption of bacterial membranes. In synergy with bactericidal proteins, such as bactericidal permeability-inducing protein, lactoferrin, lysozyme, and phospholipase A2, these peptides build a strong immediate defense system, constitutively expressed or induced, for eliminating invading microorganisms.13 The importance of the peptides in disease became apparent when it was shown that immunocompromise of the lungs with recurrent bacterial infections in patients with cystic fibrosis correlates with inactivation of peptide-dependent antibacterial activity.14 In addition, mice deficient in the metalloprotease matrilysin, which processes epithelial α-defensins (cryptdins) of the small intestine, are more sensitive to orally administered bacteria,15 further indicating the importance of the peptides in gut epithelia.
Innate defense effectors constitute a link to the specialized lymphocytes of adaptive immunity by chemotaxis. This important function has been reported of α-defensins, attracting T cells,16and C3d of the complement cascade that amplifies B-cell secretion of specific antibodies.17 The human β-defensins (HBD 1-2) are chemoattractants for dendritic cells and memory CD4 T cells that seem to be mediated through the chemokine receptor CCR6.18Here, we found that LL-37 has specific chemotactic activity, attracting polymorphonuclear (PMN) and CD4 T cells.
Antibacterial activity was previously detected in natural killer (NK) cells19 and T cells,20 but the effector molecules that mediate this activity were not systematically characterized. However, one candidate effector could be granulysin. Originally, the porcine counterpart of granulysin (NK-lysin) was characterized as an antibacterial and cytotoxic polypeptide expressed by NK and T cells.21 Granulysin was subsequently found to kill intracellular Mycobacterium tuberculosis in synergy with perforin.22 Our results indicate that the antibacterial peptides LL-37 and HNP 1 to 3 are additional candidate effectors for bactericidal activity in lymphocytes. Specifically, we found (1) expression of the genes coding for the antibacterial peptides LL-37 and HNP 1 to 3 in freshly isolated lymphocytes and in several different cell lines or clones originating from NK, γδ T cells, B cells, and monocytes; (2) immunohistochemical localization of LL-37 and α-defensins in freshly isolated NK cells, γδ T cells, B cells, and monocytes/macrophages from blood; (3) lymphocyte secretion of LL-37, α-defensins, lysozyme, a fragment of histone H2B, and other unidentified antibacterial components; and (4) that secretion of peptide LL-37 and transcription of the corresponding gene are affected by interleukin (IL)–6 and interferon-γ (IFN-γ) and antibacterial activity correlates with secreted LL-37.
Materials and methods
Cells
Buffy coats from healthy blood donors were obtained from Karolinska Hospital, Stockholm, Sweden, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient centrifugation (Pharmacia, Uppsala, Sweden). For further enrichment of T and NK cells, PBMC were separated over a nylon-wool column (Biotest, Dreieich, Germany) or by Dynabeads (Dynal, Oslo, Norway) that were coated with one of the following cell-type–specific monoclonal antibodies (mAbs): CD3 (T cells), CD19 (B cells), CD56 (NK cells), and CD14 (monocytes/macrophages).
PBMC isolated by Ficoll-Hypaque separation contain 2% to 3% granulocytes (according to the manufacturer). Fluorescence-activated cell-sorter (FACS) analysis of T and NK cells enriched by nylon-wool filtration detected no granulocytes when mAbs for CD11b were used. The Dynabead separations yielded cell populations that were more than 98% pure.
Cell lines
All cell lines were of human origin. The B-cell lines were BL-28, CIR, and JY; the αβ T-cell lines were Jurkat, J1, Molt 16, and Hut 78; the monocytic cell line was U937; and the γδ T cell lines were SE δ1/γ3, GN PBL γδ, and D768/6 (Vγ3/Vγ9). The NK cells used were selected clones. The NK clones were stimulated with glutaraldehyde-fixed bacteria (Escherichia coli D21) at concentrations comparable to 1 × 107 colony-forming units for 1 × 106 cells in 1 mL. An overnight culture of bacteria was fixed in 0.25% glutaraldehyde for 10 minutes. After centrifugation, the bacteria were washed twice in phosphate-buffered saline (PBS) and suspended in PBS.
Antibacterial polypeptides from supernatants of T and NK cells
T and NK cells (4.9 × 108) enriched from buffy coats were cultured in RPMI medium with human IL-2 (50 units/mL). The medium was free of serum and no antibiotics were included. After 5 days in culture, the cells were removed by centrifugation and the supernatant was applied to Sep-Pak C18 cartridges (Waters, Milford, MA) equilibrated with 0.1% trifluoroacetic acid (TFA) and then washed with 0.1% TFA and 10% acetonitrile in 0.1% TFA. Peptides/proteins were eluted with 80% acetonitrile in 0.1% TFA and lyophilized. The material recovered was dissolved in phosphate buffer (22.5 mmol/L; pH 6.4) and further purified on a cationic CM-22 column (Whatman, Kent, United Kingdom [UK]) equilibrated in the same phosphate buffer. Three fractions were collected: the flow-through fraction with acidic components; a fraction eluted with 0.2 mol/L sodium chloride (NaCl), containing neutral components; and a fraction of basic components eluted with 0.2 mol/L hydrochloric acid (HCl). The basic fraction was further processed by high-performance liquid chromatography (HPLC) fractionation with a reversed-phase column (Source 15 RPC; 1.6 × 10 cm; Pharmacia), and all separated fractions were collected, lyophilized, and dissolved in 100 μL water. From this, 3 μL was tested for antibacterial activity.
Antibacterial zone assay
Thin plates (1 mm) were made of 1% agarose in standard Luria Bertani (LB) broth containing approximately 6 × 104cells/mL of the test bacteria Bacillus megaterium Bm11 orE coli D21. The LB broth was used with or without the salt medium E.23 Small wells (3 mm) were punched in the plates and samples (3 μL) were placed in each well. After overnight incubation at 30°C, the diameters of the inhibition zones were recorded.
Structural analyses
A matrix-assisted laser desorption and ionization instrument (Lasermat 2000; Finnigan MAT, San Jose, CA) was used for mass determination. A 10 mg/mL solution of α-cyano-4-hydroxy-cinnamic acid (Sigma Chemical, St Louis, MO) in 70% acetonitrile containing 0.1% TFA was used as matrix. Edman degradation of the isolated peptides was carried out in an Applied Biosystems 470 A instrument and in a PE-ABI Procise HT 494 protein sequencer (PE Applied Biosystems, Foster City, CA).
Dot-blot and Western blot analyses
LL-37 immunoreactivity in chromatographic fractions was detected with a dot-blot assay using the rabbit polyclonal antibody specific for LL-37.9 The second antibody was antirabbit IgG conjugated with alkaline phosphatase (Sigma Chemical). The filter was stained for enzymatic activity in 100 mmol/L Tris-HCl (pH 9.5), 100 mmol/L NaCl, and 5 mmol/L magnesium chloride containing 4-nitro blue tetrazolium chloride (0.2 mg/mL) and 5-bromo-4-chloro-3-indolyl phosphate (0.1 mg/mL), both from Boehringer Mannheim (Germany).
Mature LL-37 in chromatographic fractions that were positive in the dot-blot analysis or in different cell supernatants were identified by Western blot analysis. Material was separated by discontinuous sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis using 10% to 20% Tricine Ready Gels (Novex, San Diego, CA) and further blotted on polyvinylidene difluoride membranes by electrophoretic transfer, as described previously.24 Immunoreactivity was detected with the LL-37–specific antiserum and antirabbit Ig conjugated with horseradish peroxidase (Amersham, Little Chalfont, Buckinghamshire, UK). An electrogenerated chemiluminescence Western blotting detection system (Amersham) was used to visualize the results.
RNA preparation and reverse transcriptase–polymerase chain reaction
Total RNA was prepared from cell lines, freshly isolated PBMC, or Dynabead-isolated lymphocytes by using RNAzol B (Tel Test, Inc, Friendswood, TX) according to the manufacturer's instructions. All RNA material was denatured at 90°C for 5 minutes before the first-strand complementary DNA (cDNA) synthesis and then chilled to 4°C. Random hexamer primers and 200 units of Moloney leukemia virus reverse transcriptase (RT) (Gibco BRL, Gaithersburg, MD) were used in a reaction volume of 20 μL under conditions recommended for first-strand synthesis. The reaction was incubated at 40°C for 45 minutes and then heated at 95°C for 5 minutes and chilled on ice. The following primer pairs (0.5 μmol/L each) were used in separate polymerase chain reaction (PCR) reactions: 5′-TGAAGGTCGGAGTCAACGGATTTGGT and 5′-CATGTGGGCCATGAGGTCCACCAC (Clontech, Palo Alto, CA) for glyceraldehyde-3-phosphate dehydrogenase (G3PDH), 5′-GAAGACCCAAAGGAATGGCC and 5′-TCAGAGCCCAGAAGCCTGAG for the CAMP gene transcript that codes for the antibacterial peptide LL-37, and 5′-CTGAGCCACTCCAGGCAAGA and 5′-GCTCAGCAGCAGAATGCCCA for α-defensins (HNP 1-3). cDNA template concentrations were adjusted to yield similar signal strength for the housekeeping gene G3PDH. PCR amplifications were performed with the following thermal-cycle profile: 3 minutes of denaturation at 94°C, 40 cycles of annealing for 30 seconds, extension at 72°C for 1 minute, denaturation at 94°C for 30 seconds, and an extension step at 72°C for 7 minutes. The annealing temperatures in the PCR reactions were 60°C for G3PDH, 62°C for LL-37, and 55°C for the cDNAs of the α-defensins (HNP 1-3).
The reaction mixtures were analyzed in 1.4% agarose gel, and the DNA was blotted on a Hybond N nylon filter (Amersham) according to standard procedure.25 The filters were then prehybridized for 4 hours in 6 × standard saline citrate (SSC), 5 × Denhardt solution, 1% SDS, and denatured salmon sperm DNA (100 μg/mL) at 64°C. Hybridizations were done overnight with defined probes under the same conditions as the prehybridization. Cloned cDNAs were used as probes for the antibacterial peptide coding genes; the G3PDH probe was purchased from Clontech. All probes were labeled with phosphorus 32 (32p) by using a Rediprime labeling kit (Amersham). After the hybridizations, the filters were washed several times with 2 × SSC and 0.1% SDS; finishing was done with 0.1 × SSC at 64°C for 15 minutes. The results were analyzed with a PhosphorImager 445 SI (Molecular Dynamics, Sunnyvale, CA).
cDNA cloning and nucleotide-sequence analysis
Total RNA was isolated from lymphocytes derived from buffy coats from a healthy blood donor. A 3′ rapid amplification of cDNA ends (RACE)–PCR approach26 was used to clone α-defensin cDNA. For first-strand synthesis in the 3′ RACE, the primer 5′-TCGAATTCCTCGAGAAGC(T18) was used. The primers used for amplification were 5′-TCGAATTCCTCGAGAAGC and the α-defensin–specific primer 5′-GCCATGAGGACCCTCGCCAT. A nested PCR was performed with α-defensin–specific primers (5′-CTGAGCCACTCCAGGCAAGA and 5′-GCTCAGCAGCAGAATGCCCA). A band of the expected size (231 base pairs) was obtained, subcloned into pCRscript (Stratagene, La Jolla, CA), and sequenced with a BigDye terminator kit (PE Applied Biosystems). Cloning of the CAMP gene cDNA coding for LL-37 was described previously.23
Immunohistochemical analyses
A double-staining protocol using LL-37 polyclonal rabbit antiserum or α-defensin mAbs specific for HNP 1-3 (Biogenesis Ltd, Poole, UK), together with mAbs for different surface markers (CD3, CD14, CD19, CD56, and γδ T-cell receptor), was used to detect LL-37 and α-defensins in different lymphocytes. The starting material was PBMC (1 × 106 for each sample) that was fixed for 10 minutes in 2% formaldehyde. After 2 washes in PBS with 0.1% bovine serum albumin (BSA), the cells were incubated with the primary antibody (ie, specific for LL-37 or α-defensins) in the same buffer, supplemented with 0.1% saponin, for 30 minutes in 4°C. The LL-37 antiserum was diluted in PBS, 0.1% BSA, and 0.1% saponin to a concentration of 20 μg/mL, whereas the α-defensin antiserum was diluted 1:50 in the same buffer. The secondary antibody was a Texas red–conjugated donkey α-rabbit IgG (Jackson ImmunoResearch Lab, Inc, West Grove, PA) for LL-37 in a dilution of 1:50 in the same buffer used for the primary antibody. For the α-defensins, a donkey α-mouse IgG conjugated with indocarbocyanine was used as secondary antibody in a concentration of 20 μg/mL in PBS, 0.1% BSA, and 0.1% saponin. After incubation for 30 minutes in 4°C, the cells were washed twice in PBS. For identification of different cell types, the PBMC were incubated for 30 minutes at 4°C with mAbs conjugated with fluorescein. The mAbs used were directed to CD3 (F0818; DC A/S, Glostrup, Denmark) for identification of T cells, CD14 (F0844; DC) for monocytes/macrophages, CD19 (F0768; DC) for B cells, CD56 (340410; Becton Dickinson, San Jose, CA) for NK cells, and γδ chain of the T-cell receptor (MCA992F; Serotec, Oxford, UK) for γδ T cells. After the cells were rinsed twice in PBS, they were applied to microscope slides and mounted with fluorescent mounting medium (DC).
Stimulation of PBMC with IL-6 and IFN-γ
Two defined lymphocyte groups from fresh blood, PBMC, and enriched T and NK cells were cultured in RPMI without serum and antibiotics. Both groups were treated with IL-6 (3 ng/mL), IFN-γ (250 units/mL), or both. The cells from each stimulation experiment were harvested after 6, 15, or 24 hours and used for RNA preparation and subsequent RT-PCR analyses for G3PDH and LL-37. The supernatants were enriched for peptides/proteins by using Oasis TM cartridges (Waters), using the same protocol as for the Sep-Pak C18concentration. The lyophilized material from each supernatant was analyzed for the presence of LL-37 by Western blotting.
Chemotaxis assay
Migration of PMN leukocytes and PBMC was assayed in a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MD). The upper and lower chambers were separated by a polycarbonate membrane (3- to 5-μm pore size). LL-37 was diluted in RPMI to various concentrations and added to the lower well (30 μL). Human PMN cells or PBMC isolated by single-step centrifugation over a leukocyte separation medium were added to the upper chamber (40 μL; 5 × 106 cells/mL). The chamber was then incubated in humidified air at 37°C for 40 minutes for PMN cells and 4 hours for PBMC. After incubation, cells on the underside of the filter were fixed, stained with Giemsa stain (diluted 1:20) for 5 minutes, and counted under a light microscope (magnification, × 400). The total number of cells in 5 microscopical fields was counted for each well, and the total number of cells on the underside of the filter was calculated. The percentage of transmigrated cells was determined by dividing the calculated total number of transmigrated cells by the number of cells added to each well. Each experiment was performed in triplicate (3 wells). In some experiments, the cells on the underside of the filter were collected and stained for immunofluorescent flow cytometric analysis (FACS). These cells were incubated with fluorescein isothiocyanate–conjugated (FITC) anti-CD4 or anti-CD8 mAbs for 20 minutes at 4°C in the dark. After fixation, the specific fluorescence of 1 × 104 cells was assessed by a FACS analyzer. Data are expressed as the mean ± SD value from 4 separate experiments.
Results
Isolation and characterization of antibacterial components from enriched T and NK cells
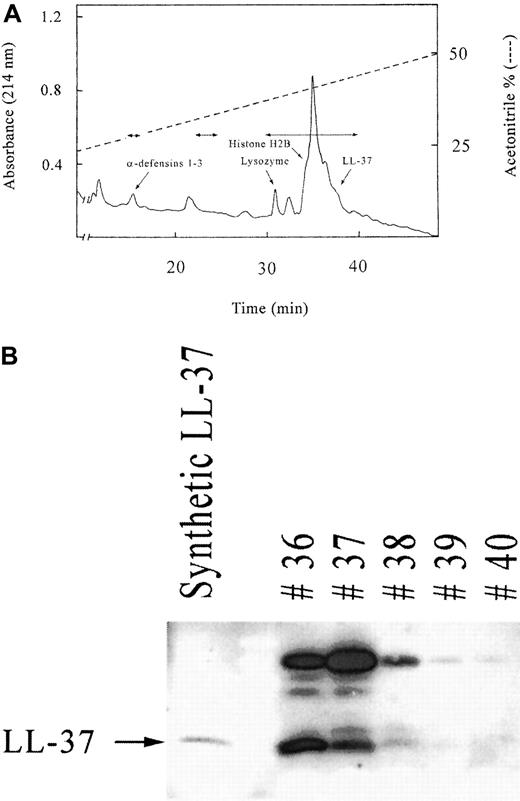
PBMC were enriched for T and NK cells by nylon-wool filtration and cultured for 5 days in the presence of IL-2 for further stimulation of NK cells. The cells were harvested by centrifugation, and peptides/proteins in the supernatant (240 mL) were concentrated on Sep-Pak C18 cartridges and lyophilized. The yield was 207 mg, and with further separation on a cationic exchange column, 3 fractions were collected—1 acidic, 1 neutral, and 1 basic. Because most known antibacterial peptides have a basic character, the basic fraction was selected for purification by reversed-phase HPLC in 0.1% TFA and acetonitrile. A flow rate of 7 mL/min and gradients of 0% to 20%, 20% to 50%, 50% to 80%, and 80% to 100% acetonitrile were used for 5 minutes, 45 minutes, 5 minutes, and 5 minutes, respectively. All fractions of 7 mL were collected, lyophilized, redissolved in 100 μL water, and screened for antibacterial activity. The chromatographic profile is shown in Figure1A, with the distribution of antibacterial activity indicated by arrows. Detailed characterization identified 6 peptides/proteins with antibacterial activity: 3 α-defensins (HNP 1-3); lysozyme; a fragment of histone H2B, identified by sequence analysis, mass spectrometry, or both; and LL-37, identified by dot-blot and Western blot analyses. The H2B fragment was identified after a second reversed-phase HPLC purification of fraction 35 (Figure 1A). The mature peptide LL-37 was found in fractions 36 to 38 together with a protein in the 18-kd region (Figure 1B) that may represent either the cathelin proform or the tetrameric form of LL-37 (4.5 kd). Because LL-37 is known to oligomerize,27 the coeluting protein could represent the tetramer.
Antibacterial components in T cells and natural killer (NK) cells.
(A) Results of reversed-phase chromatography with the supernatant from T cells and NK cells stimulated with interleukin (IL)–2. The distribution of the antibacterial activity is indicated in the chromatographic profile by double-headed arrows. The characterized antibacterial components were α-defensins (HNP 1-3), lysozyme, a fragment of histone H2B, and LL-37. (B) Western blot analysis for the presence of LL-37 in chromatographic fractions. The chromatographic fractions 36 to 40, which were positive for LL-37 in dot-blot analysis, were further characterized by Western blotting. The lower band in fraction 36 to 38 corresponds to the mature LL-37, whereas the higher bands probably correspond to oligomeric forms.
Antibacterial components in T cells and natural killer (NK) cells.
(A) Results of reversed-phase chromatography with the supernatant from T cells and NK cells stimulated with interleukin (IL)–2. The distribution of the antibacterial activity is indicated in the chromatographic profile by double-headed arrows. The characterized antibacterial components were α-defensins (HNP 1-3), lysozyme, a fragment of histone H2B, and LL-37. (B) Western blot analysis for the presence of LL-37 in chromatographic fractions. The chromatographic fractions 36 to 40, which were positive for LL-37 in dot-blot analysis, were further characterized by Western blotting. The lower band in fraction 36 to 38 corresponds to the mature LL-37, whereas the higher bands probably correspond to oligomeric forms.
Expression of LL-37 and α-defensins in cell lines and clones
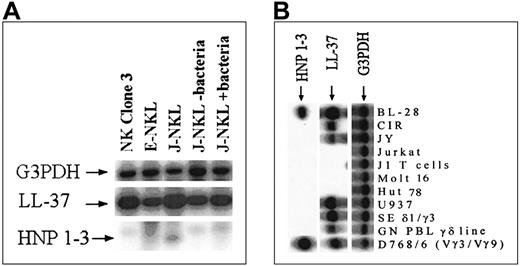
We screened different clones of human NK cells for expression of genes coding for the antibacterial peptides LL-37 and α-defensins (HNP 1-3). RNA was isolated from these clones and analyzed by RT-PCR with 3 specific primer sets (Figure 2A). The first primer set was specific for transcripts of the housekeeping gene coding for G3PDH, whereas the 2 other were specific for transcripts corresponding to LL-37 and α-defensins (HNP 1-3). The identity of the amplified fragments was confirmed by Southern blot analyses using characterized probes. The expression level for the housekeeping gene was similar in all the clones. All the analyzed clones derived from NK cells expressed the gene coding for LL-37, whereas signals for α-defensins were absent or very weak (Figure 2A). No difference in LL-37 expression was detected when the NK-cell clone J-NKL was treated with bacteria. Additional analyses included cell lines originating from B cells, αβ T cells, γδ T cells, and the monocytic cell line U937 (Figure 2B). LL-37 was expressed in all cell lines derived from B cells, γδ T cells, and monocytes but not in cells of αβ T-cell origin. The expression of α-defensin was more restricted in 1 of the 3 B-cell lines and 1 of the 3 γδ T-cell lines, and no signal was detected in the monocytic cell line (Figure 2B).
Southern blot analysis of reverse transcriptase–polymerase chain reactions (RT-PCRs) for the gene transcripts corresponding to G3PDH, LL-37, and HNP 1-3.
The amplified bands were the expected sizes, and the identities of these bands were confirmed by hybridization with phosphorus 32 (32P)–labeled radioactive probes. (A) Loaded material was from the different NK-cell clones indicated on top of each lane; the complementary DNA identity is indicated on the left. One NK-cell clone was stimulated with bacteria. (B) The origin of the loaded material is as follows. BL-28, CIR, and JY are of B-cell origin. Jurkat, J1 T cells, Molt 16, and Hut 68 are derived from αβ T cells. U937 is a monocytic cell line, and SE δ1/δ3, GN PBL γδ, and D768/6 (Vγ3/Vγ9) are of γδ T-cell origin.
Southern blot analysis of reverse transcriptase–polymerase chain reactions (RT-PCRs) for the gene transcripts corresponding to G3PDH, LL-37, and HNP 1-3.
The amplified bands were the expected sizes, and the identities of these bands were confirmed by hybridization with phosphorus 32 (32P)–labeled radioactive probes. (A) Loaded material was from the different NK-cell clones indicated on top of each lane; the complementary DNA identity is indicated on the left. One NK-cell clone was stimulated with bacteria. (B) The origin of the loaded material is as follows. BL-28, CIR, and JY are of B-cell origin. Jurkat, J1 T cells, Molt 16, and Hut 68 are derived from αβ T cells. U937 is a monocytic cell line, and SE δ1/δ3, GN PBL γδ, and D768/6 (Vγ3/Vγ9) are of γδ T-cell origin.
Expression of LL-37 and α-defensins in isolated lymphocytes from fresh blood
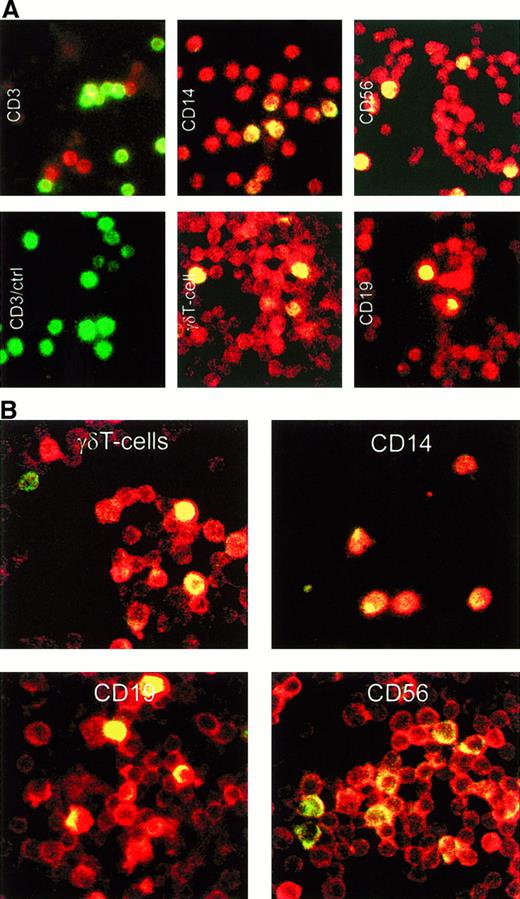
To follow up on our studies of the cell lines and clones, we analyzed freshly isolated lymphocytes for the expression of LL-37 and α-defensins. Isolated PBMC were prepared for immunohistochemical analysis by using LL-37 antiserum in combination with the following surface-specific antigens: CD3 for T cells, γδ chain of the T-cell receptor for γδ T cells, CD14 for monocytes/macrophages, CD19 for B cells, and CD56 for NK cells. Colocalization of the surface-specific antigen and LL-37 was detected in γδ T cells, monocytes, B cells, and NK cells (Figure 3A). The same procedure was done with α-defensin–specific mAb (recognizing HNP 1-3) and surface-specific markers for CD14, CD19, CD56, and γδ T cell receptor. Clear α-defensins localization was detected in γδ T cells, B cells, NK cells, and monocytes/macrophages (Figure 3B).
Immunohistochemical studies detecting LL-37 and α-defensins.
(A) Detection of LL-37 in different lymphocytes by double-staining immunohistochemical analysis. For identification of cell types, the following fluorescein isothiocyanate–conjugated monoclonal antibodies against cell-surface markers were used: CD3 for T cells, γδ chain of T-cell receptor for γδ T cells, CD14 for monocytes/macrophages, CD19 for B cells, and CD56 for NK cells. The LL-37 antibody was detected with a donkey α-rabbit IgG conjugated with Texas red. The cell types appear green; LL-37, red; and doubly stained cells, yellow. (B) Detection of α-defensins in different lymphocytes by double-staining immunohistochemical analysis. The monoclonal antibodies were the same as in A, except for CD3, and the α-defensin antibody was detected with a donkey α-mouse IgG conjugated with indocarbocyanine (red appearance).
Immunohistochemical studies detecting LL-37 and α-defensins.
(A) Detection of LL-37 in different lymphocytes by double-staining immunohistochemical analysis. For identification of cell types, the following fluorescein isothiocyanate–conjugated monoclonal antibodies against cell-surface markers were used: CD3 for T cells, γδ chain of T-cell receptor for γδ T cells, CD14 for monocytes/macrophages, CD19 for B cells, and CD56 for NK cells. The LL-37 antibody was detected with a donkey α-rabbit IgG conjugated with Texas red. The cell types appear green; LL-37, red; and doubly stained cells, yellow. (B) Detection of α-defensins in different lymphocytes by double-staining immunohistochemical analysis. The monoclonal antibodies were the same as in A, except for CD3, and the α-defensin antibody was detected with a donkey α-mouse IgG conjugated with indocarbocyanine (red appearance).
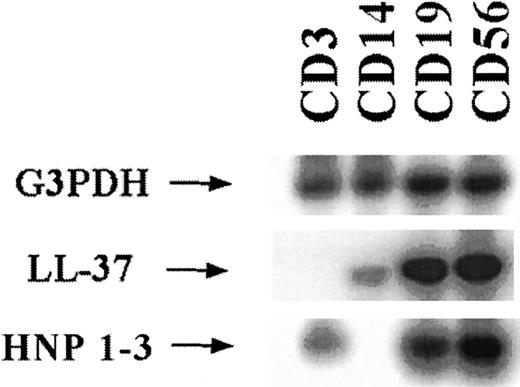
The PBMC were also separated according to their surface markers by using Dynabead-coupled antibodies against CD3, CD14, CD19, or CD56. RNA was extracted from the cells and expression analysis was done by using RT-PCR and Southern blotting (Figure 4). Expression of both α-defensins and LL-37 was detected in CD19-positive and CD56-positive cells. CD3 cells expressed lower levels of α-defensin and no expression of LL-37 was detected. In CD14 cells, expression of LL-37 was observed but not expression of α-defensin.
Southern blot analysis of RT-PCRs for the gene transcripts corresponding to glyceraldehyde-3-phosphate dehydrogenase (G3PDH), LL-37, and HNP 1-3.
Loaded material was derived from RNA that was directly prepared from lymphocytes separated with Dynabeads according to surface antigens and representing different cell types. The amplified bands were the expected sizes. The probes were labeled radioactively with32P.
Southern blot analysis of RT-PCRs for the gene transcripts corresponding to glyceraldehyde-3-phosphate dehydrogenase (G3PDH), LL-37, and HNP 1-3.
Loaded material was derived from RNA that was directly prepared from lymphocytes separated with Dynabeads according to surface antigens and representing different cell types. The amplified bands were the expected sizes. The probes were labeled radioactively with32P.
Taken together, these results demonstrate that B cells and NK cells express both LL-37 and α-defensin. The double-staining analysis showed expression in γδ T cells and monocytes/macrophages, but the faint or absent signal in the RT-PCR indicates that the levels of messenger RNA (mRNA) were low because of either a low number of cells (likely for γδ T cells in the CD3 population) or a low number of transcript copies.
Expression of LL-37 in lymphocytes stimulated with IL-6, IFN-γ, or both
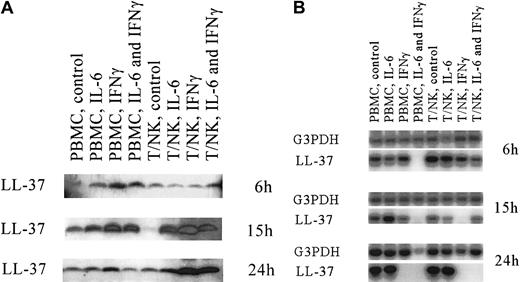
In previous studies of the structure of the LL-37 gene, we identified binding sites for expression of nuclear factor for IL-6 (NF-IL-6), a finding indicating that the cytokine IL-6 might influence gene expression.9 In addition, IFN-γ is known to stimulate antibacterial defenses.28 After showing expression of LL-37 in lymphocytes (ie, NK cells, B cells, and γδ T cells), we investigated whether IL-6, IFN-γ, or both affected expression and secretion of LL-37, in addition to secretion of total antibacterial activity, from these cells at different time points (Figure 5 and Table1). Thus, lyophilized material (40 μg) from all cell supernatants of PBMC and T and NK cells was analyzed by Western blotting for the presence of LL-37. The peptide was detected in both PBMC and T and NK cells at all time points (Figure5A). At 6 hours, both IL-6 and IFN-γ enhanced secretion of LL-37 in PBMC, but no enhancement was detected in the T and NK cells. The most prominent effect was noted after 15 hours of stimulation, when both IL-6 and IFN-γ increased secretion of LL-37 in both cell groups. At 24 hours, the stimulatory effect was noted only in the T and NK cells when IFN-γ was present.
Expression of LL-37 in stimulated lymphocytes.
(A) Western blot analyses for the presence of LL-37 in supernatants from enriched peripheral blood mononuclear cells (PBMC) and T and NK cells. The 2 cell groups were grown and stimulated with IL-6, interferon γ, or both for 6, 15, and 24 hours (indicated on right). The origin of the loaded material is shown on the top of each lane. The results were obtained with use of an electrogenerated chemiluminescence detection system. (B) RT-PCR and Southern blot analyses for expression of G3PDH and LL-37. The cells used for expression analyses at the transcriptional level were the same as those used in the studies shown in A.
Expression of LL-37 in stimulated lymphocytes.
(A) Western blot analyses for the presence of LL-37 in supernatants from enriched peripheral blood mononuclear cells (PBMC) and T and NK cells. The 2 cell groups were grown and stimulated with IL-6, interferon γ, or both for 6, 15, and 24 hours (indicated on right). The origin of the loaded material is shown on the top of each lane. The results were obtained with use of an electrogenerated chemiluminescence detection system. (B) RT-PCR and Southern blot analyses for expression of G3PDH and LL-37. The cells used for expression analyses at the transcriptional level were the same as those used in the studies shown in A.
Antibacterial activity in supernatants of stimulated PBMC and T/NK cells
| Sample . | Time of stimulation (hours) . | Zone diameter/amount applied (mm/mg) . |
|---|---|---|
| T/NK cells (control) | 15 | 18.0 |
| T/NK cells + IL-6 | 15 | 17.1 |
| T/NK cells + IFN-γ | 15 | 23.5 |
| T/NK cells + IL-6/IFN-γ | 15 | 18.7 |
| PBMC (control) | 24 | 22.7 |
| PBMC + IL-6 | 24 | 25.7 |
| PBMC + IFN-γ | 24 | 29.2 |
| PBMC + IL-6/IFN-γ | 24 | 21.0 |
| T/NK cells (control) | 24 | 17.0 |
| T/NK cells + IL-6 | 24 | 17.0 |
| T/NK cells + IFN-γ | 24 | 20.7 |
| T/NK cells + IL-6/IFN-γ | 24 | 19.0 |
| Sample . | Time of stimulation (hours) . | Zone diameter/amount applied (mm/mg) . |
|---|---|---|
| T/NK cells (control) | 15 | 18.0 |
| T/NK cells + IL-6 | 15 | 17.1 |
| T/NK cells + IFN-γ | 15 | 23.5 |
| T/NK cells + IL-6/IFN-γ | 15 | 18.7 |
| PBMC (control) | 24 | 22.7 |
| PBMC + IL-6 | 24 | 25.7 |
| PBMC + IFN-γ | 24 | 29.2 |
| PBMC + IL-6/IFN-γ | 24 | 21.0 |
| T/NK cells (control) | 24 | 17.0 |
| T/NK cells + IL-6 | 24 | 17.0 |
| T/NK cells + IFN-γ | 24 | 20.7 |
| T/NK cells + IL-6/IFN-γ | 24 | 19.0 |
PBMC indicates peripheral blood mononuclear cells; T/NK, T cells and natural killer cells; IL-6, interleukin-6; and IFN-γ, interferon-γ.
The remaining lyophilized peptide/protein material (260-300 μg) was used to study the antibacterial activity. The total secreted antibacterial activity was analyzed, and the greatest activity was detected in the supernatants of PBMC after 24 hours of culture, with the highest value in the IFN-γ–stimulated cells (Table 1). Interestingly, the antibacterial activity correlated positively with the quantity of LL-37 detected by Western blot analysis. Our data also showed that IFN-γ is a good stimulator of the release of antibacterial components from lymphocytes.
The cells cultured for activity and Western blot analysis were also analyzed by RT-PCR for expression of LL-37 at the transcriptional level. In contrast with peptide secretion, which increased, transcription of the gene encoding LL-37 was negatively affected by the cytokines IL-6 and IFN-γ. The decrease in mRNA expression was detected earlier in PBMC than in T and NK cells (Figure 5B). The down-regulation was already detectable at 6 hours in the presence of both IL-6 and IFN-γ, indicating synergy between the 2 cytokines. At 24 hours, IFN-γ alone down-regulated LL-37 expression in PBMC. IFN-γ was also the main effector of the down-regulation in the T and NK cells, which was first detected at 15 hours (Figure 5B).
Chemotactic activity of LL-37
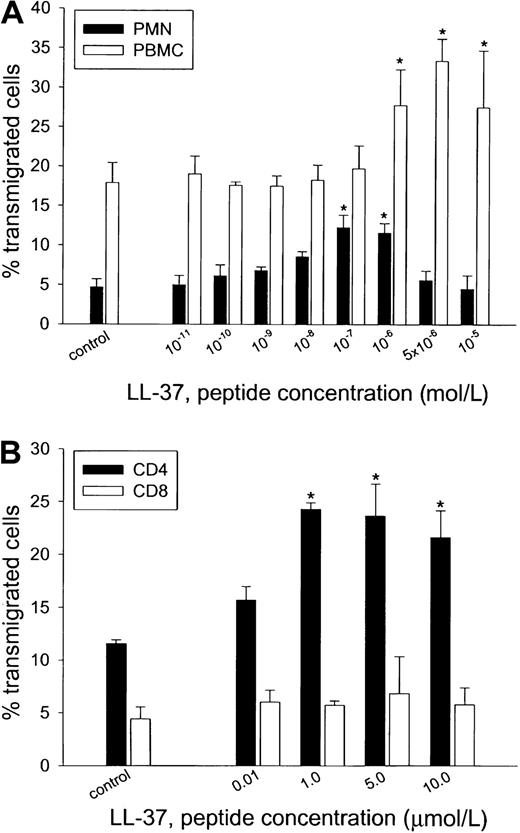
When placed in the lower chamber of the chemotaxis device, LL-37 induced migration of PMN leukocytes and PBMC (Figure6A). The chemotactic activity of LL-37 was dose dependent, reaching a maximum at concentrations of 0.1 μmol/L for PMN cells and 5 μmol/L for PBMC. At these concentrations, 12.2% ± 1.6% of PMN cells and 33.6 ± 2.8% of PBMC transmigrated (compared with 4.7 ± 1.0% and 18.0 ± 2.5%, respectively, under control conditions). To investigate a possible, selective chemotactic effect of LL-37 on lymphocyte subsets, the numbers of CD4 and CD8 cells were analyzed in several experiments. In response to medium, transmigration of CD4 and CD8 cells reflected their relative ratio in the original population (Figure 6B). However, when LL-37 was added to the lower chamber, transmigration of CD4 cells increased significantly, whereas transmigration of CD8 cells remained unchanged.
Chemotactic activity for various concentrations of LL-37.
Polymorphonuclear (PMN) leukocytes and PBMC (A) and CD4+and CD8+ lymphocytes (B) were assessed for chemotactic response by using a 48-well microchemotaxis chamber. PMN cells and PBMC both showed concentration-dependent migration to LL-37, over a range of 0.1 μmol/L to 1 μmol/L (PMN cells) or 1 μmol/L to 10 μmol/L (PBMC). Typing of PBMC subtypes revealed a chemotactic response of CD4 lymphocytes to LL-37, whereas the peptide was ineffective in activating CD8 cells for migration. The results shown are the mean ± SD values of triplicates in 4 to 5 assays performed with leukocytes from different donors. * indicates significant difference from leukocytes not exposed to peptide (P = .01).
Chemotactic activity for various concentrations of LL-37.
Polymorphonuclear (PMN) leukocytes and PBMC (A) and CD4+and CD8+ lymphocytes (B) were assessed for chemotactic response by using a 48-well microchemotaxis chamber. PMN cells and PBMC both showed concentration-dependent migration to LL-37, over a range of 0.1 μmol/L to 1 μmol/L (PMN cells) or 1 μmol/L to 10 μmol/L (PBMC). Typing of PBMC subtypes revealed a chemotactic response of CD4 lymphocytes to LL-37, whereas the peptide was ineffective in activating CD8 cells for migration. The results shown are the mean ± SD values of triplicates in 4 to 5 assays performed with leukocytes from different donors. * indicates significant difference from leukocytes not exposed to peptide (P = .01).
Taken together, these data demonstrate that LL-37 has a chemotactic function in both PMN and CD4 lymphocytes. The finding that CD4 lymphocytes were selectively recruited by the peptide may indicate that there are mechanisms whereby LL-37–producing cells within tissues regulate the accumulation of specific lymphocyte subsets.
Discussion
Extracellular antibacterial activity secreted from human mononuclear leukocytes has been shown to originate from NK cells,19 T cells,20 and monocytes.29 The most detailed studies on NK cells were performed a decade ago,19,30 but the effector molecules that mediate the activity were not then characterized. Granulysin, a more recently characterized antibacterial polypeptide of T and NK cells, could be one effector. Granulysin was shown to kill M tuberculosis in synergy with perforin,22 and it can also kill several gram-negative bacteria, gram-positive bacteria, and fungi. In this study, our starting point was to identify antibacterial components included in secreted material from NK cells. For this process, we used a cell population enriched for T and NK cells that were then treated with IL-2, which further stimulated and enhanced the NK-cell fraction. The active components found in the supernatants of these stimulated cells were the antibacterial peptides LL-37 and α-defensins (HNP 1-3), lysozyme, a fragment of histone H2B, and several uncharacterized components. No indication of the presence of granulysin was observed. Except for the histone H2B fragment, all components found are well-described antibacterial peptides/proteins in professional phagocytes or at sites exposed to bacteria. Histones frequently appear as active components in antibacterial assays,31 but a physiologic role for them in bacterial killing is uncertain, although it has been suggested that histone fragments may have a bactericidal role when released from apoptotic epithelial cells in the gastrointestinal tract.32 Thus, our studies detected a multicomponent antibacterial system in lymphocytes that might contribute to the elimination of bacteria.
To confirm that the antibacterial peptides LL-37 and α-defensins are derived from NK cells, we screened clones of NK cells for expression of these peptides. Clear expression of LL-37 was observed in all NK-cell clones, but expression of amplified α-defensin cDNA was weak. Interestingly, we also noted expression of LL-37 in cell lines of γδ T cells, B cells, and monocytes, whereas expression of α-defensins in these cell lines was more restricted. Because neither the cloned NK cells nor the transformed cell lines necessarily reflect the in vivo situation, we used double-staining immunohistochemical analysis for cellular localization of LL-37 and α-defensins in freshly isolated PBMC. These peptides were found in NK cells, γδ T cells, B cells, and monocytes/macrophages. Furthermore, RT-PCR was employed to detect expression at the mRNA level in fresh blood in studies using immobilized mAbs against cell-specific surface antigens for affinity purification. Pronounced expression of both LL-37 and α-defensin was detected in B cells (CD19) and NK cells (CD56). Taken together, our results confirm expression of these peptides in NK and B cells.
The discrepancies observed between the results with the immunohistochemical analyses and those with the cell-specific RT-PCRs were most likely due to low transcript quantities for LL-37 in CD3 cells and α-defensin in CD14 cells. The low or absent signal in RT-PCR may indicate a low mRNA concentration for LL-37 transcripts, since γδ T cells represent a small fraction of CD3 cells. Similarly, in the CD14 cells, the RT-PCR signals that reflected low transcriptional activity may be connected to the status of differentiation or activation of these cells. Notably, despite the cellular colocalization of LL-37 and α-defensins, which also applies to granulocytes, the genes appeared not to be strictly coregulated in the lymphocytes, since we found that some clones and cell lines expressed LL-37 but not α-defensin. Freshly isolated NK cells actively transcribed the genes for α-defensins (HNP 1-3) (Figure 4), but with time, the NK clones may lose this ability. In primary lymphocyte cultures of several days' duration, we also observed disappearance of α-defensin transcript. An explanation for this could be instability of an important transcription factor needed for α-defensin expression.
Mammalian antibacterial peptides were originally discovered in granulocytes and macrophages (professional phagocytes).4Later, they were traced in mucosal epithelia33 and found to be induced in keratinocytes in the skin during inflammation.2,8 Consequently, they are considered active effectors in epithelial barriers.1 Here, we showed expression of LL-37 and α-defensin in NK cells. It is therefore conceivable that when recruited to sites of infection, NK cells contribute directly to bacterial killing, as has been documented of macrophages. Furthermore, B cells and γδ T cells also synthesize these peptides and might therefore participate in direct killing of bacterial intruders in addition to their adaptive capacity (ie, production of antibodies and specific recognition of presented foreign antigens). Thus, the mononuclear leukocytes can kill bacteria directly and are therefore part of a second defense barrier against intruders that cross the primary surface defenses.
The secreted proform for LL-37 (hCAP18) was previously detected in high concentrations in plasma.3 In blood, the origin of LL-37 has been considered to be granulocytes, but because we here found its expression in mononuclear leukocytes, the secreted proform of LL-37 might also be derived from these cells. The expression sites for the peptides are strategically located for defenses in phagocytes or at surfaces where the initial microbe contact takes place. The presence of the precursor protein in plasma is likely relevant for rapid defense response, since only protein cleavage is needed for immediate activation when bacterial intruders enter the blood.
The promoter of the gene encoding LL-37 contains potential binding sites for NF-IL-6 and acute phase response factor, which is also called signal transducer and activator of transcription (STAT) 3.9 Control of the gene might therefore be affected by the cytokines IL-6 or IFN-γ through a JAK/STAT pathway or aras-dependent mitogen-activated protein kinase cascade that activates NF-IL-6.34 Early in infection and inflammation, both these cytokines have diverse effects on lymphocytes, and IFN-γ has been designated a central effector role in antibacterial defenses.28 Thus, we analyzed the effect of these cytokines on the expression and secretion of LL-37 in PBMC and enriched T and NK cells. IFN-γ enhanced secretion of the mature peptide (probably involving both vacuolar release and processing of the precursor protein) but simultaneously down-regulated transcription of the gene encoding LL-37. Furthermore, IL-6 appeared to have synergistic effects leading to faster down-regulation. The early release of bactericidal peptides might be one relevant effector function for IFN-γ in initial antibacterial responses. LL-37 is cytotoxic at enhanced concentrations27; therefore, the transcriptional down-regulation could be important in balancing the concentration levels. The effects of the down-regulation at the peptide level were observed at 24 hours (Figure 5A), when the peptide levels were decreased, reflecting the blockage of de novo synthesis. Notably, the importance of IFN-γ in bacterial infections has so far mainly been connected to intracellular bacteria, as in chlamydial35 and mycobacterial infections.36 However, knowledge of the role of antibacterial peptides in connection with protective immunity against intracellular bacteria is limited.
Antibacterial peptides have broad-spectrum antimicrobial activity against bacteria, viruses, and fungi.37 The chemotactic properties of the peptides have been studied,16,38 and several other activities have been attributed to these peptides.39 The basis of chemotactic activity is currently being investigated. The chemotactic properties of α-defensins are well characterized and include recruitment of granulocytes and T cells.16 In addition, α-defensins enhance systemic IgG levels through CD4 cytokines.38 Mucosal β-defensins (HBD 1-2) were shown to attract immature dendritic cells and memory T cells through interactions with the chemokine receptor CCR6.18Here, we showed that LL-37 has chemotactic properties, with the highest affinity for CD4 T cells. Thus, the antibacterial peptides are not only active bacterial eliminators but also seem to serve as early links to effector cells of adaptive immunity by means of recruitment and stimulation.
Acknowledgments
We thank Ghasem Ahangari and Dr Mahmood Therani for fruitful suggestions, Dr Kalle Söderström and Dr Dieter Kabelitz for providing cell lines, and Joakim Johansson for assistance with the confocal microscopy.
Supported by the Swedish Medical Research Council; the Swedish Cancer Society; the Swedish Society of Medicine; the Swedish Foundation for Strategic Research; the Swedish Foundation for Health Care Sciences and Allergy Research; Magnus Bergvall's Foundation; and Åke Wiberg's Foundation.
J.C. and J.W. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gudmundur H. Gudmundsson, Microbiology and Tumorbiology Center, Nobels väg 16, Karolinska Institutet, S-171 77 Stockholm, Sweden; e-mail: gudmundur.gudmundsson@mtc.ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal