Abstract
Kaposi sarcoma–associated herpesvirus (KSHV), or human herpervirus 8 (HHV-8), is a γ-herpesvirus that infects human lymphocytes and is associated with primary effusion lymphoma (PEL). Currently, the role of viral infection in the transformation of PEL cells is unknown. One possibility is that KSHV, like the lymphotropic viruses Epstein-Barr virus (EBV) and human T-cell leukemia virus I (HTLV-I), activates the transcription factor NF-κB to promote survival and proliferation of infected lymphocytes. To examine this possibility, we assessed NF-κB activity in KSHV-infected PEL cell lines and primary tumor specimens by electrophoretic mobility shift assay (EMSA). We observed that NF-κB is constitutively activated in all KSHV-infected lymphomas, and consists of 2 predominant complexes, p65/p50 heterodimers and p50/p50 homodimers. Inhibition experiments demonstrated that Bay 11-7082, an irreversible inhibitor of IκBα phosphorylation, completely and specifically abrogated the NF-κB/DNA binding in PEL cells. PEL cells treated with Bay 11 demonstrated down-regulation of the NF-κB inducible cytokine interleukin 6 (IL-6), and apoptosis. These results suggest that NF-κB activity is necessary for survival of KSHV-infected lymphoma cells, and that pharmacologic inhibition of NF-κB may be an effective treatment for PEL.
Introduction
Primary effusion lymphoma (PEL) is a rare, distinct subtype of non-Hodgkin's lymphoma (NHL) that usually presents as lymphomatous effusions in body cavities.1,2 The neoplastic cells have a B-cell genotype, but lack surface expression of some B-cell–associated antigens and surface immunoglobulin. Many of the translocations and mutations associated with other B-cell malignancies are absent in these lymphomas, although the presence of mutations in the regulatory region of bcl-6 has been reported in a subset of cases.3,4 The most unique characteristic of these cells is their consistent infection with Kaposi sarcoma–associated herpesvirus (KSHV), also called human herpesvirus-8 (HHV-8).5 KSHV, a γ-herpesvirus, can infect multiple cell types, including B cells, which can provide a reservoir for latent virus.6-9 These and other circulating mononuclear cells carrying KSHV are thought to be responsible for viral dissemination, leading to infection of endothelial cells and development of Kaposi sarcoma.10-12
The consistent presence of KSHV in PEL suggests that infection with this virus may contribute to transformation of infected B cells. Like the other lymphomagenic viruses in humans, EBV and HTLV-1, KSHV encodes proteins that modulate and enhance the proliferation and survival of infected cells. Through expression of the viral transforming genesLMP-1 and Tax respectively, EBV and HTLV-1 subvert cellular signaling pathways to activate cellular transcription factors.13-18 Of these, NF-κB, a potent inducer of genes involved in the proliferative and survival responses of lymphocytes, is consistently activated and therefore may play an essential role in the transformation process.19-24 This transcription factor occurs as a dimer of rel family proteins that are maintained in an inactive form in the cytoplasm, and that, on activation, translocate to the nucleus and up-regulate gene expression. Among the NF-κB target genes are inflammatory cytokines, antiapoptotic proteins, and cell cycle regulators that promote cellular longevity.25-27
To evaluate the NF-κB activity in KSHV-infected PEL cells, we have examined 5 cell lines and 2 ex vivo PEL primary clinical specimens. All the cell lines and patient specimens demonstrated constitutive NF-κB activity comprised of identical NF-κB/rel protein dimers. The application of NF-κB inhibitors to the PEL cells resulted in decreased viability. These data suggest that KSHV infection leads to constitutive activation of NF-κB, which induces genes vital for the survival and perpetuation of PEL cells.
Materials and methods
Cell lines and patient specimens
The PEL cell lines BC1, BC2, BC4, and BC5 infected with both KSHV and EBV, and BC3 infected with KSHV but not EBV, were maintained in culture with RPMI 1640 (Gibco BRL, Grand Island, NY), supplemented with 20% heat-inactivated fetal bovine serum (FBS) (Gibco) and 50 mg/mL gentamicin (Sigma, St Louis, MO) at 37°C in 5% CO2. The B lymphoma cell line BJAB (KSHV−/EBV−) was maintained in RPMI supplemented with 10% FBS and gentamicin. For initial experiments involving a direct comparison of NF-κB activity between PEL and BJAB cells, all cells were cultured in RPMI with 10% FBS. The clinical pathology specimens were obtained from lymphomatous pleural effusions of 2 human immunodeficiency virus–positive (HIV+) patients. Mononuclear cells were isolated from the pleural fluid by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density gradient centrifugation, and cryopreserved using standard techniques.
Electrophoretic mobility shift assays and supershift analyses
For isolation of nuclear proteins, 5 × 106 cells from culture were washed in cold phosphate-buffered saline (PBS) before addition of 400 μL cold buffer A (10 mmol/L HEPES [pH 7.9], 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 2 μmol/L aprotinin, 2 μmol/L leupeptin, 2 μmol/L pepstatin A, 1 mmol/L DTT, 1 mmol/L PMSF, and 1 mmol/L Na3VO4). After a 15-minute incubation on ice, 25 μL of 10% Nonidet P-40 was added and samples vortexed vigourously. Nuclei were pelleted by centrifugation, washed once in buffer A, and repelleted before the addition of 50 μL buffer B (20 mmol/L HEPES [pH 7.9], 0.4 mol/L NaCl, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 2 μmol/L aprotinin, 2 μmol/L leupeptin, 2 μmol/L pepstatin A, 1 mmol/L DTT, 1 mmol/L PMSF, and 1 mmol/L Na3VO4). Nuclear extracts were collected after a 30-minute incubation on ice and subsequent 5-minute centrifugation. Protein was quantitated using a protein bioassay (Bio-Rad, Hercules, CA) with bovine serum albumin standard. For electrophoretic mobility shift assays (EMSAs), NF-κB (a gift from Dr Hsiou-Chi Liou, Cornell Medical College, NY), or Oct-1 (Promega, Madison, WI), specific oligonucleotides were end-labeled with T4 kinase and [γ-32P]ATP. Unincorporated ATP was removed with G-25 sephadex columns and centrifugation. Before addition of oligonucleotide probe, 4 μg of nuclear proteins were incubated with DNA binding mix (2 μg poly (dI-dC), 0.25% NP-40, 5% glycerol, 10 mmol/L Tris HCl, 50 mmol/L KCl, 1 mmol/L DTT, and 1 mmol/L EDTA) for 10 minutes at room temperature. 2 × 104 cpm of radiolabeled oligonucleotide were incubated with the reaction mixture for 15 minutes at room temperature. For cold competition experiments, 50-fold molar excess of either unlabeled Oct-1 or NF-κB oligonucleotides were added before the addition of labeled probe. Samples were separated on 7% acrylamide gels and assessed by autoradiography. For supershift experiments, nuclear proteins were incubated with human p65, p50, and c-rel antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 minutes on ice prior to addition of radiolabeled oligonucleotide and gel electrophoresis were subsequently performed, as described above.
Inhibition of NF-κB
BC3 cells were placed in culture at 5 × 105cells/mL and screened for inhibition of NF-κB after exposure to NF-κB inhibitors, obtained from Calbiochem (San Diego, CA), as follows: SN50 (0, 50, and 100 μg/mL), CAPE (0, 20, 40, and 80 mg/mL), aspirin (0, 2, 5, and 10 μmol/L), sodium salicylate (0, 2, 5, and 10 μmol/L), and Bay 11-7082 (0, 2, 4, 5, and 10 μmol/L) for 1 and 24 hours at 37°C. Concentration ranges were selected according to previous reports for effective doses of each inhibitor.28-32 Nuclear proteins were extracted as described above, and EMSAs performed using the NF-κB–specific oligonucleotide probe. For specificity experiments, nuclear proteins were extracted from 5 × 106 cells after 1 and 24 hours treatment with Bay 11 and evaluated by EMSA using NF-κB and Oct-1 radiolabeled oligonucleotides. For IL-6 assays, 200 μL of triplicate culture supernatants were used for human IL-6 enzyme-linked immunosorbent assays (ELISAs) (University of Maryland Cytokine Core Facility).
Immunoblot analyses
Whole cell extracts were generated from 5 × 106BC1, BC2, and BC3 cells treated with or without Bay 11 for 24 hours. Cells were washed once in PBS, followed by lysis in 500 μL of extraction buffer (50 mmol/L Tris-HCl, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L PMSF, 0.5 μg aprotinin, 0.5 μg leupeptin, 1 mmol/L Na3VO4, 1 mmol/L NaF) for 15 minutes at 4°C. Proteins were quantitated as described above and 30 μg of extract was mixed with 4X sample buffer, boiled for 5 minutes, and loaded on a 12.5% acrylamide gel. Membranes were first probed with phospho-specific p38 antibody (New England Biolabs, Beverly, MA) at 1:1000 dilution in 5% BSA in TBS with 0.1% Tween, overnight at 4°C. Detection was performed using HRP-conjugated rabbit Ig (Amersham, Piscataway, NJ) and chemiluminescence (Amersham). Blots were then stripped and reprobed with p38 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:1000 dilution in 1% milk in TBS for 1 hour at room temperature. Detection was repeated as described for phopho-p38.
Cell viability assays
For all assays, 2 × 105 cells were washed once in cold PBS before staining. Morphologic changes associated with apoptosis were evaluated with 4′-6′-diamino-2′-phenylindole dihydrochloride (DAPI, 1 mg/mL) (Sigma, St Louis, MO), and fluorescence microscopy (Axioplan 2, Zeiss). For annexin V binding assays, 5 μL of FITC-conjugated annexin V (Coulter-Immunotech, Miami, FL) in 200 μL binding buffer was added to the cells for 10 minutes at 4°C, and analyzed with a FACSCaliber using CellQuest software (Becton Dickinson, Franklin Lakes, NJ). The viability of cell populations was measured directly by hemocytometry and trypan blue exclusion.
Results
Constitutive activation of NF-κB in KSHV-infected primary effusion lymphoma cells
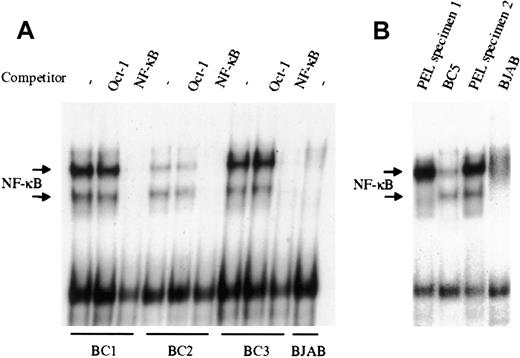
NF-κB activity was evaluated by EMSA in the KSHV-infected PEL derived cell lines BC1, BC2, and BC3. As shown in Figure1A, all 3 PEL cell lines demonstrate significantly greater NF-κB/DNA binding, compared to the uninfected B lymphoma cell line BJAB, although the degree of activity varied among the 3 lines evaluated. In all cases, protein/DNA binding was specific for NF-κB, as excess unlabeled NF-κB oligonucleotide could effectively compete and abrogate binding, whereas unlabeled Oct-1 oligonucleotide demonstrated no effect. Analyses of 2 additional KSHV-infected PEL cell lines BC4 (not shown) and BC5 (Figure 1B), recently established in our laboratory, also showed significant NF-κB activity. Overall, these results indicate that there is constitutive activation of the transcription factor in latently infected PEL cells. To determine that the observed NF-κB activity was not the result of cell culture, nuclear material from primary ex vivo PEL specimens was assessed by EMSA (Figure 1B). The 2 PEL specimens shown were obtained from KSHV-infected patients, and analyzed without culture. Both samples demonstrated greater NF-κB activity, compared with BJAB, and in one case, more activity was observed in the primary tumor cells (PEL specimen 2) than in the cell line that was subsequently established from this clinical specimen (BC5). This suggests that constitutive activation of NF-κB is an inherent property of KSHV-infected PEL cells, and not an artifact of culture.
NF-κB is constitutively activated in KSHV-infected PEL cell lines and patient specimens.
(A), Nuclear proteins extracted from latent cultures of BC1, BC2, and BC3 cells were examined by EMSA for DNA binding using a radiolabeled NF-κB–specific probe. All 3 PEL cell lines demonstrated protein/DNA binding with 2 distinct complex formations, compared with the uninfected negative control cell line BJAB. Cold competition using 50-fold molar excess of unlabeled NF-κB and Oct-1 oligonucleotides demonstrated the specificity of the protein/DNA binding complexes. (B), NF-κB activity in ex vivo KSHV-infected PEL cells was assessed by EMSA. Mononuclear cells were purified from 2 different patient effusions specimens, and the nuclear proteins were extracted and assessed for NF-κB/DNA binding. In one case, the amount of binding in the ex vivo specimen surpassed that of the BC5 cell line subsequently established from the same specimen. BJAB was used as the negative control for NF-κB activity.
NF-κB is constitutively activated in KSHV-infected PEL cell lines and patient specimens.
(A), Nuclear proteins extracted from latent cultures of BC1, BC2, and BC3 cells were examined by EMSA for DNA binding using a radiolabeled NF-κB–specific probe. All 3 PEL cell lines demonstrated protein/DNA binding with 2 distinct complex formations, compared with the uninfected negative control cell line BJAB. Cold competition using 50-fold molar excess of unlabeled NF-κB and Oct-1 oligonucleotides demonstrated the specificity of the protein/DNA binding complexes. (B), NF-κB activity in ex vivo KSHV-infected PEL cells was assessed by EMSA. Mononuclear cells were purified from 2 different patient effusions specimens, and the nuclear proteins were extracted and assessed for NF-κB/DNA binding. In one case, the amount of binding in the ex vivo specimen surpassed that of the BC5 cell line subsequently established from the same specimen. BJAB was used as the negative control for NF-κB activity.
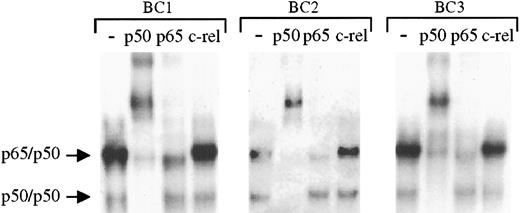
To determine the specific components of these NF-κB/DNA complexes, supershift experiments were performed whereby the nuclear proteins were incubated with antibodies to specific rel family proteins before performing EMSAs. These studies (Figure2) demonstrate identical shifting patterns in the 3 PEL cell lines. As shown, addition of p50 antibody resulted in the complete shift of the lower and a significant portion of the upper complexes, whereas antibody to rel A (p65) affected part of the upper complexes. In the same lysates, antibody to c-rel has no effect. On the basis of these analyses, we conclude that the protein/DNA complexes consist of NF-κB/rel family proteins bound to DNA with p50/p50 homodimers and p65/p50 heterodimers. Moreover, although the levels of activity varied among the cell lines, the same types of NF-κB complexes were present in each sample, suggesting a common source of NF-κB activation in the PELs.
The protein/DNA complexes in KSHV-positive PELs are primarily comprised of p65/p50 heterodimers and p50 homodimers.
To assess the components of the protein/DNA complexes, nuclear proteins extracted from latent cultures of BC1, BC2, and BC3 cells were incubated with antibodies to p50, p65, and c-rel before the addition of NF-κB–specific radiolabeled oligonucleotide. Compared with control samples in which no antibody was added (lanes 1, 5, and 9) addition of p50 antibody (lanes 2, 6, and 10) resulted in a complete shift of the lower complex and a large proportion of the upper complex in each cell line. Partial shifting of the upper complex was also seen on addition of p65 antibody, but no shifting of either complex was observed after addition of c-rel antibody.
The protein/DNA complexes in KSHV-positive PELs are primarily comprised of p65/p50 heterodimers and p50 homodimers.
To assess the components of the protein/DNA complexes, nuclear proteins extracted from latent cultures of BC1, BC2, and BC3 cells were incubated with antibodies to p50, p65, and c-rel before the addition of NF-κB–specific radiolabeled oligonucleotide. Compared with control samples in which no antibody was added (lanes 1, 5, and 9) addition of p50 antibody (lanes 2, 6, and 10) resulted in a complete shift of the lower complex and a large proportion of the upper complex in each cell line. Partial shifting of the upper complex was also seen on addition of p65 antibody, but no shifting of either complex was observed after addition of c-rel antibody.
Specific inhibition of NF-κB in KSHV-infected primary effusion lymphoma cells
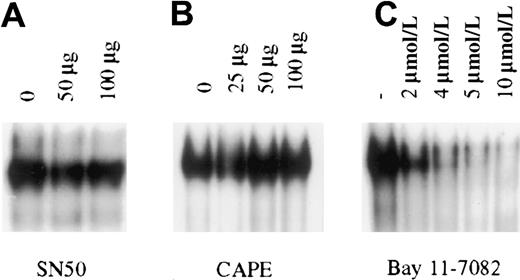
To determine the importance of constitutive NF-κB activity in PEL cell growth and survival, pharmacologic intervention was used for specific inhibition of NF-κB in PEL cells. NF-κB inhibitors that are currently available function by one of 3 broad mechanisms: inhibiting proteasome mediated degradation of IκB, inhibition of IκB phosphorylation, or inhibition of translocation of activated NF-κB dimers to the nucleus. In general, the most specific inhibitors are those that inhibit IκB phosphorylation and NF-κB translocation. These compounds abrogate NF-κB induction after stimulation by inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin 1 (IL-1).28-32 We therefore focused on a variety of these inhibitors to block the constitutive activity observed in the KSHV-infected PEL cells. For these studies, we used BC3 cells that are infected with only KSHV. The cells were treated with the IκB phosphorylation inhibitors aspirin, sodium salicylate, and Bay 11-7082, or with the nuclear translocation inhibitors SN50 and CAPE. These experiments revealed that only Bay 11, an irreversible inhibitor of IκB phosphorylation, could inhibit NF-κB/DNA binding in BC-3 cells (Figure 3). Studies extended for as long as 24 hours, with or without further addition of inhibitors, provided the same results, suggesting that functional inhibition of constitutive activity in PEL cells occurs only when signaling is irreversibly blocked upstream of NF-κB release and translocation to the nucleus.
Inhibition of constitutive NF-κB activity in BC3 cells.
BC3 cells placed in culture at 7.5 × 105 cells/mL were treated for 1 hour with the inhibitors SN50 (A), CAPE (B), and Bay 11-7082 (C). Of the inhibitors tested only Bay 11-7082 inhibited NF-κB/DNA binding within 1 hour of treatment. Inhibition observed with Bay 11 was dose dependent, with nearly complete inhibition at the 5 and 10 μmol/L concentrations. Additional studies in which cells were analyzed for up to 24 hours after treatment or were treated with the inhibitors SN50 and CAPE every 2 hours for a 6-hour period yielded the same results.
Inhibition of constitutive NF-κB activity in BC3 cells.
BC3 cells placed in culture at 7.5 × 105 cells/mL were treated for 1 hour with the inhibitors SN50 (A), CAPE (B), and Bay 11-7082 (C). Of the inhibitors tested only Bay 11-7082 inhibited NF-κB/DNA binding within 1 hour of treatment. Inhibition observed with Bay 11 was dose dependent, with nearly complete inhibition at the 5 and 10 μmol/L concentrations. Additional studies in which cells were analyzed for up to 24 hours after treatment or were treated with the inhibitors SN50 and CAPE every 2 hours for a 6-hour period yielded the same results.
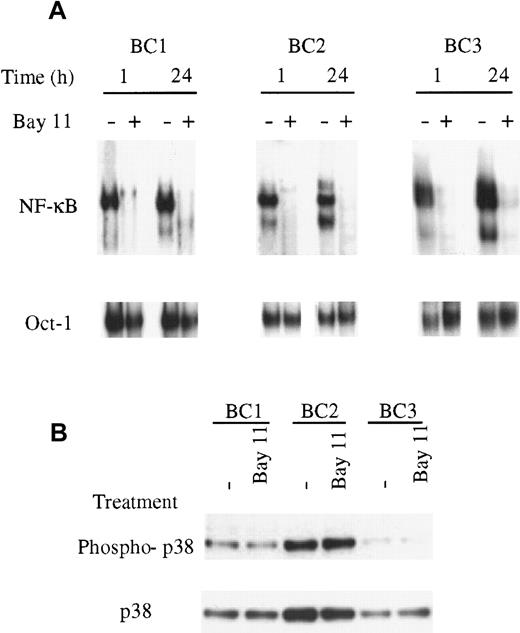
To assess the specificity of NF-κB inhibition by Bay 11, the BC1, BC2, BC3, and BJAB cells were treated for 24 hours and evaluated for inhibition of NF-κB and Oct-1/DNA binding. Cells were treated with 5 μmol/L Bay 11, based on the inhibitory effect observed in BC3 cells treated at this concentration after 1 hour (Figure 3). In the PEL cells, NF-κB/DNA binding was inhibited within 1 hour of treatment and maintained throughout the 24 hours assay (Figure4). BJAB cells, which demonstrated no constitutive NF-κB activity in untreated cells, displayed no change in NF-κB DNA binding on treatment with Bay 11 (data not shown). In all cells, treatment with 5 μmol/L Bay 11 did not affect the binding of Oct-1 to DNA in the 24-hour assay, suggesting that at this concentration the inhibitory effects of Bay 11 are specific for NF-κB. Further studies indicated that this specificity was lost on treatment with higher concentrations of Bay 11 (data not shown). Therefore, we used the 5 μmol/L concentration of Bay 11 in subsequent experiments to evaluate the consequences of NF-κB inhibition in PEL cells.
Specific inhibition of NF-κB with Bay 11-7082.
(A), The PEL cells BC1, BC2, BC3, and control BJAB cells were treated with Bay 11 and assessed for NF-κB and Oct-1/DNA binding. Cells were placed in culture at 7.5 × 105 cells/mL and treated with 5 μmol/L Bay 11. After 1 hour and 24 hours, nuclear proteins were extracted and EMSAs performed using NF-κB– or Oct-1–specific radiolabeled oligonucleotide probes. All PEL cell lines demonstrated inhibition of NF-κB/DNA binding within 1 hour of treatment but demonstrated no inhibition of Oct-1/DNA binding. The specificity of the NF-κB inhibition was maintained throughout the 24-hour assay in each cell line. (B), The PEL cells BC1, BC2, and BC3 were treated with 5 μmol/L Bay 11 for 24 hours and assessed by immunoblot for total and phosphorylated p38. All cell lines showed the presence of phosphorylated p38, but the levels of p38 activation were not affected by treatment with Bay 11.
Specific inhibition of NF-κB with Bay 11-7082.
(A), The PEL cells BC1, BC2, BC3, and control BJAB cells were treated with Bay 11 and assessed for NF-κB and Oct-1/DNA binding. Cells were placed in culture at 7.5 × 105 cells/mL and treated with 5 μmol/L Bay 11. After 1 hour and 24 hours, nuclear proteins were extracted and EMSAs performed using NF-κB– or Oct-1–specific radiolabeled oligonucleotide probes. All PEL cell lines demonstrated inhibition of NF-κB/DNA binding within 1 hour of treatment but demonstrated no inhibition of Oct-1/DNA binding. The specificity of the NF-κB inhibition was maintained throughout the 24-hour assay in each cell line. (B), The PEL cells BC1, BC2, and BC3 were treated with 5 μmol/L Bay 11 for 24 hours and assessed by immunoblot for total and phosphorylated p38. All cell lines showed the presence of phosphorylated p38, but the levels of p38 activation were not affected by treatment with Bay 11.
Bay 11-7082 is an irreversible inhibitor of IκB-α phosphorylation, but has also been reported to reversibly stimulate activation of the stress activated protein (SAP) kinases p38 and JNK in human umbilical vein endothelial cells (HUVECs).32 Therefore, to determine whether Bay 11 has an effect on SAP kinases in PEL cells, we performed immunoblot analyses using antibodies to p38 that recognize only its active, phosphorylated form. The data in Figure 4B indicate that p38 is present, and that a proportion of the p38 is phosphorylated in all 3 PEL cell lines. Treatment with Bay 11 for 24 hours did not affect the amount of p38 protein, or the amount of phosphorylated p38 in any of the lines, indicating that the effect of Bay 11 is specific to the NF-κB pathway.
Effect of NF-κB inhibition in KSHV-infected primary effusion lymphoma cells on IL-6 production and survival
We evaluated levels of IL-6, an NF-κB responsive cytokine, in PEL cells. Table 1 indicates the amount of IL-6 detected in the supernatant of each cell line by ELISA. As shown, the IL-6 activity correlated with the levels of NF-κB activity observed in the same PEL cells. To evaluate whether IL-6 production by the PEL cells is NF-κB dependent, we determined the effect of NF-κB inhibition on IL-6 transcription and secretion. PEL cells were treated with Bay 11 for 24 hours, and evaluated by reverse transcriptase-polymerase chain reaction (RT-PCR) and ELISA for IL-6 transcripts and protein (Table 1). After 24 hours, IL-6 produced by the untreated PEL cells was significantly higher than that produced by BJAB cells. Treatment of these cells with Bay 11 resulted in a significant decrease in IL-6 transcription (data not shown) as well as an 81%, 63%, and 95% reduction in IL-6 produced by BC1, BC2, and BC3 cells, respectively.
Secretion of IL-6 after 24-hour treatment with Bay 11
| . | Untreated . | 5 μmol/L Bay 11 . |
|---|---|---|
| BC1 | 754 | 147 |
| BC2 | 38 | 14 |
| BC3 | 297 | 15 |
| BJAB | 9 | 6 |
| . | Untreated . | 5 μmol/L Bay 11 . |
|---|---|---|
| BC1 | 754 | 147 |
| BC2 | 38 | 14 |
| BC3 | 297 | 15 |
| BJAB | 9 | 6 |
Supernatants from 7.5 × 105 cells/mL were examined by enzyme-linked immunosorbent assay after 24-hour treatment with and without 5 μmol/L Bay 11. Values reported are in pg/mL.
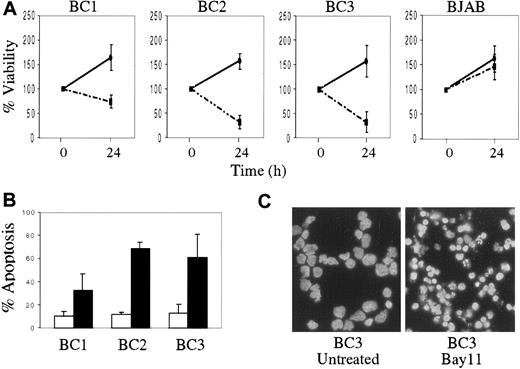
We also evaluated the effect of NF-κB inhibition on PEL cell growth and survival. Compared with BJAB, the KSHV-infected PEL cells demonstrated decreased viability after 24 hours of treatment with Bay 11, as was evident by reduction in cell number and the percentage of viable cells (Figure 5A). In contrast, BJAB cells continued to proliferate after Bay 11 treatment, although their growth rate was slightly decreased compared with that of untreated BJAB cells. To determine whether growth inhibition in the PEL cells treated with Bay 11 was due to induction of apoptosis, cells treated with inhibitor for 24 hours were assessed by annexin V staining and flow cytometry (Figure 5B). PEL cells treated with Bay 11 demonstrated significant apoptosis, compared with untreated cells. Similar results were evident in the treated cells by DAPI staining, which revealed marked nuclear condensation and blebbing (Figure 5C). Taken together, these data suggest that constitutive NF-κB activity is vital for survival of KSHV-infected PEL cells, and that specific inhibition of this activity results in the induction of apoptosis.
Decreased cell viability and the induction of apoptosis in PEL cells treated with Bay 11.
BC1, BC2, BC3, and BJAB cells were placed in culture at 7.5 × 105 cells/mL without Bay 11 or with 5 μmol/L Bay 11 for 24 hours. (A), Cell viability was assessed by hemocytometry and trypan blue exclusion, and is reported as the percentage of viable cells remaining at 24 hours, relative to the initial viable cell number (100%). Data shown are compiled from 5 independent experiments, and are shown for both untreated (continuous line) and treated (dashed line) cells. (B), Induction of apoptosis in PEL cells on inhibition of NF-κB. 2 × 105 cells were labeled with FITC-conjugated annexin-V and analyzed by flow cytometry. The percentage of apoptotic cells from 4 independent experiments are reported for both untreated (white bar) and Bay 11 treated (black bar) cells. (C), DAPI staining of BC3 cells with or without treatment with 5 μmol/L Bay 11 for 24 hours shows significant nuclear condensation and fragmentation in the treated cells.
Decreased cell viability and the induction of apoptosis in PEL cells treated with Bay 11.
BC1, BC2, BC3, and BJAB cells were placed in culture at 7.5 × 105 cells/mL without Bay 11 or with 5 μmol/L Bay 11 for 24 hours. (A), Cell viability was assessed by hemocytometry and trypan blue exclusion, and is reported as the percentage of viable cells remaining at 24 hours, relative to the initial viable cell number (100%). Data shown are compiled from 5 independent experiments, and are shown for both untreated (continuous line) and treated (dashed line) cells. (B), Induction of apoptosis in PEL cells on inhibition of NF-κB. 2 × 105 cells were labeled with FITC-conjugated annexin-V and analyzed by flow cytometry. The percentage of apoptotic cells from 4 independent experiments are reported for both untreated (white bar) and Bay 11 treated (black bar) cells. (C), DAPI staining of BC3 cells with or without treatment with 5 μmol/L Bay 11 for 24 hours shows significant nuclear condensation and fragmentation in the treated cells.
Discussion
In this study, we have found that the transcription factor NF-κB is constitutively activated in KSHV-infected PEL cells. This finding suggests that like EBV and HTLV-1, KSHV infection of lymphocytes can lead to activation of NF-κB. Our data indicate that activation of NF-κB is essential for the survival of KSHV-infected lymphomas, as inhibition of this transcription factor results in apoptosis of PEL cells.
Whereas the transforming proteins of EBV and HTLV-1, LMP-1 and Tax, respectively, are able to activate NF-κB, the molecular source of this activity in PEL remains to be elucidated. Although most PELs are dually infected with KSHV and EBV, the latter virus is unlikely to be involved in the activation of NF-κB in these cells, as PELs have a restricted EBV latency pattern with minimal LMP-1 expression.33,34 To eliminate the possibility of confounding results due to EBV involvement in the activation of NF-κB, we evaluated PEL cells infected only with KSHV (BC3), as well as cells dually infected with KSHV and EBV (BC1 and BC2). It has been reported that the KSHV-encoded proteins K1, a viral signaling receptor, and FLICE inhibitory protein (vFLIP) can activate NF-κB when overexpressed in some mammalian, albeit nonlymphoid, cell types.35,36 Preliminary studies in our laboratory have also suggested that the KSHV-encoded G-protein coupled receptor (GPCR) has NF-κB activating potential (unpublished observation). These findings suggest that in lymphocytes, expression of one or more KSHV-encoded gene products results in the constitutive activity that we have observed. Although this activation may be a direct effect of signaling cascades initiated by viral receptors such as K1 and GPCR,37 38 it could occur indirectly due to modulation of cellular signaling pathways. The KSHV-encoded FLIP, which modulates signaling of the TNF superfamily receptors, could enhance survival of PEL cells via activation of NF-κB and inhibition of apoptotic pathways. As the PEL cells, which have aberrant expression of many surface receptors, express TNFRI (p55) and TNFRII (p75), the possibility of receptor activation and modulation, perhaps through an autocrine or paracrine loop, remains to be explored.
An important observation to emerge from this study is that the NF-κB activity in PEL cells can be inhibited specifically by the compound Bay 11-7082. Inhibition of NF-κB in PEL cells occurs at concentrations that do not affect binding of other transcription factors or activation of the SAP kinase p38. Previous studies have examined the ability of various compounds to inhibit inducible activation of NF-κB, but not constitutive activity, in transformed cells.28-32 Using a variety of inhibitors, we have found that inhibition of NF-κB only occurred in PEL cells when IκB was stabilized by inhibition of phosphorylation, such that degradation of IκB no longer occurs. These findings suggest that in cells in which NF-κB is constitutively activated, inhibition must be upstream of activated dimer release and translocation. The data indicate that activity can be inhibited in cells with constitutive NF-κB activity, even when the source of activation is unknown.
In PEL cells the abundance of activated complexes are p65/p50 heterodimers that have potent transcriptional regulatory capabilities in lymphocytes.39-41 Therefore, it is likely that constitutive activation of these complexes results in up-regulation of genes important for cell growth and survival. Here we show that IL-6, an NF-κB–inducible cytokine, is produced at levels proportional to the NF-κB activity observed in the PEL cells and that inhibition of NF-κB results in a significant decrease of IL-6 secretion by these cells. Because IL-6 enhances B cell proliferation,42-45down-regulation of IL-6 may be a factor in reduced PEL cell growth.
In summary, we have found that NF-κB is a constitutively activated transcription factor that is involved in survival of KSHV-infected PEL cells, as NF-κB inhibition results in induction of apoptosis. This finding suggests that NF-κB is important for PEL survival, and also suggests a new possible target for the treatment of PEL. In our experience with these highly aggressive malignancies, the mean survival of patients is extremely poor even with aggressive chemotherapeutic intervention. Therefore, the possibility of inhibiting NF-κB, which might sensitize the cells to subsequent apoptosic stimuli, is a promising novel therapeutic approach.
Acknowledgments
We thank Dr Daniel Knowles and Dr Amy Chadburn for providing and characterizing the primary patient specimens. We are also grateful to Denise Hernandez-Hopkins and Zahra Asgary for excellent technical assistance.
Supported in part by National Institutes of Health grants CA73531, CA82037, and CA68939 to E.C.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ethel Cesarman, Department of Pathology, Weill Medical College of Cornell University, 1300 York Ave, New York, NY 10021; e-mail: ecesarm@mail.med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal