Abstract
The role of corticosteroids in the prophylaxis of graft-versus-host disease (GVHD) is not well established. We have conducted a prospective, randomized, open-label, single-center study about the effect of adding methylprednisolone (MP) to the widely used prophylactic regimen consisting of cyclosporine A and methotrexate. A total of 108 consecutive patients treated with allogeneic bone marrow transplantation from an HLA-identical sibling donor for malignant blood disease were entered into the study; 53 patients were randomized to receive and 55 were randomized not to receive prophylactic MP. The dose of MP was 0.5 mg/kg on days 14 to 20, 1 mg/kg on days 21 to 34, 0.5 mg/kg on days 35 to 48, and thereafter the dose was slowly tapered and the administration discontinued on day 110. In the group given prophylactic MP, the incidence of acute GVHD was lower (19% vs 56%,P = .0001), there was a trend toward a lower incidence of chronic GVHD among low-risk patients (P = .06), and during the first 4 months the time spent at hospital was shorter and there were fewer infections. The total amount of MP given was similar in the study groups because of a higher incidence of acute GVHD and its treatment in the group of patients not given prophylactic MP. There were no significant differences between the study groups in relapse rate or survival. In conclusion, the addition of MP to the combination of cyclosporine and methotrexate markedly reduced the incidence of acute GVHD without causing untoward effects. The timing of corticosteroid administration is probably important for the efficacy.
Introduction
Graft-versus-host disease (GVHD) with its consequences is the most important complication of allogeneic bone marrow transplantation (BMT), and its prevention and treatment are crucial for the success of this form of treatment. Cyclosporine A and methotrexate have been most commonly used to prevent GVHD, and the combination of cyclosporine and a short course of methotrexate is the most widely used form of prophylaxis.1 Corticosteroids are the first-line treatment of acute GVHD, but their role in prophylaxis is not well established. Corticosteroids have been used for the prophylaxis of GVHD together with cyclosporine,2,3methotrexate,4 cyclophosphamide,5tacrolimus,6 antilymphocyte globulin,7 and monoclonal ricin-combined antibodies8,9 in various combinations. Studies of the effect of the addition of corticosteroid to the combination of cyclosporine and a short course of methotrexate have generally shown no benefit.10-12 In a randomized study, Storb and coworkers10 found that the addition of methylprednisolone (MP) to the combination of cyclosporine and methotrexate increased the incidence of acute GVHD. In that study, the administration of MP was started on the day of transplantation and continued until day 35. If corticosteroid treatment was postponed until day 15, no increase of acute GVHD was seen. In another randomized study using a similar schedule, the addition of MP to the combination of cyclosporine and methotrexate had no significant effect on GVHD.11 We report here a randomized prospective study about the effect of adding MP to the combination of cyclosporine and methotrexate for the prophylaxis of GVHD in 108 recipients of an allograft from an HLA-identical sibling donor using a different administration schedule. In this study, the addition of MP to the prophylactic regimen resulted in a marked decrease in the incidence of acute GVHD.
Patients and methods
A total of 108 consecutive adult patients treated for malignant hematologic disorder with allogeneic BMT from an HLA-identical sibling donor at Helsinki University Central Hospital were entered into the study from 1989 to 1994. The characteristics of the patients and donors are shown in Table 1. There were no significant differences between the study groups. Histocompatibility typing of the donors and recipients was performed by serology.
Characteristics of patients and donors
| . | Treatment group . | |
|---|---|---|
| MP+ . | MP− . | |
| Patients | ||
| Total no. | 53 | 55 |
| Female | 24 | 30 |
| Male | 29 | 25 |
| Age, median (range) | 42 (18-54) | 41 (17-52) |
| Disease | ||
| AML | 22 | 21 |
| CML | 15 | 13 |
| ALL | 5 | 10 |
| MDS | 3 | 6 |
| MM | 4 | 4 |
| NHL | 2 | 1 |
| CLL | 2 | 0 |
| Low risk* | 33 | 37 |
| High risk* | 20 | 18 |
| Conditioning | ||
| TBI | 34 | 34 |
| Busulfan | 19 | 21 |
| Donors | ||
| Female | 16 | 19 |
| Male | 37 | 36 |
| Donor/patient sex | ||
| Different | 26 | 25 |
| Same | 27 | 30 |
| Female to male | 9 | 7 |
| . | Treatment group . | |
|---|---|---|
| MP+ . | MP− . | |
| Patients | ||
| Total no. | 53 | 55 |
| Female | 24 | 30 |
| Male | 29 | 25 |
| Age, median (range) | 42 (18-54) | 41 (17-52) |
| Disease | ||
| AML | 22 | 21 |
| CML | 15 | 13 |
| ALL | 5 | 10 |
| MDS | 3 | 6 |
| MM | 4 | 4 |
| NHL | 2 | 1 |
| CLL | 2 | 0 |
| Low risk* | 33 | 37 |
| High risk* | 20 | 18 |
| Conditioning | ||
| TBI | 34 | 34 |
| Busulfan | 19 | 21 |
| Donors | ||
| Female | 16 | 19 |
| Male | 37 | 36 |
| Donor/patient sex | ||
| Different | 26 | 25 |
| Same | 27 | 30 |
| Female to male | 9 | 7 |
AML indicates acute myeloid leukemia; CML, chronic myeloid leukemia; ALL, acute lymphatic leukemia; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin's lymphoma; CLL, chronic lymphatic leukemia.
Low risk: acute leukemia in first remission or CML in first chronic phase; high risk: all others.
As conditioning treatment for transplantation, the patients received cyclophosphamide in a dose of 60 mg/kg of body weight on 2 successive days and either total body irradiation (TBI) or busulfan. Fifty-three patients participated in a multicenter study of the Nordic BMT Group comparing TBI and busulfan in the conditioning for BMT.13 The low-risk patients not participating in the study were given TBI with one exception, in which busulfan was given because radiotherapy was not available within a reasonable time. The high-risk patients were given TBI or busulfan according to individual judgment, depending, for example, on previous radiotherapy and the availability of TBI. TBI was given in 6 2-Gy doses during 5 days, days −4 to 0 in relation to the transplant; 1 dose was given per day with the exception of 2 doses given on 1 day. The total dose was 12 Gy (lungs shielded to 10 Gy), and the dose rate was 4 cGy per minute. Busulfan was given in the dose of 1 mg/kg 4 times daily for 4 days, for a total dose 16 mg/kg, on days −8 to −5, followed by cyclophosphamide in the above-mentioned doses on days −4 and −3.
All patients received a bone marrow graft. The mean number of nucleated cells in the graft was 3.0 × 108/kg of the recipient's body weight (median 3.0, range 1.9-4.4) in the group given MP and 3.0 × 108/kg (median 3.0, range 1.6-4.4) in the group not given MP for prophylaxis. The grafts were nonmanipulated with the exception of the removal of red cells and plasma in case of ABO incompatibility.
Postgrafting immunosuppression consisted of cyclosporine and methotrexate with or without MP according to randomization. Cyclosporine treatment was initiated on the day before transplantation and given in the dose of 3 mg/kg per day as continuous intravenous infusion. This was continued until approximately 2 weeks post-transplantation, when the patient was able to take the drug by mouth. The oral dose was 3 mg/kg per day taken in 2 parts at 12-hour intervals. This dose was modified in case of adverse effects and to keep the whole-blood cyclosporine concentration under the level of 200 μg/L (CYCLO-Trac RIA, Incstar Corp, Stillwater, MN). Cyclosporine administration was continued until 1 year post-transplantation and then tapered off in approximately 6 weeks.
Methotrexate was administrated at the dose of 15 mg/m2 of the body surface area intravenously on day 1 and 10 mg/m2on days 3, 6, and 11 after grafting. Six hours after each dose, the same-milligram dose of calcium folinate was given intravenously.
Fifty-three patients were randomized to receive and 55 not to receive MP. The randomization was carried out with closed envelopes in sets of 4, separately for patients over and under the age of 35 years. The schedule of MP administration is shown in Table2. The drug was given orally. Acute14,15 and chronic16 17 GVHD were graded according to previously published criteria. All cases of gastrointestinal GVHD were biopsy-proven. Liver GVHD was documented either by liver biopsy or by biopsy-proven gastrointestinal GVHD and simultaneous clinical and laboratory findings compatible with liver GVHD. Because our previous experience had shown that skin biopsies taken early at the onset of acute GVHD yielded too inconclusive results to be used as the basis of treatment decisions and because our policy to treat GVHD early and intensely hampered the use of later biopsies, skin GVHD was in most present cases diagnosed on clinical grounds. Acute GVHD was treated at its appearance, independent of the grade, with 10 mg/kg per day of MP divided into 4 doses intravenously. The dose was halved every 3 days thrice and thereafter tapered according to the clinical situation. In corticosteroid-resistant cases, antilymphocyte globulin (Atgam, Upjohn, Kalamazoo, MI) was used as second-line treatment. The treatment of chronic GVHD consisted of MP and, according to individual judgment, cyclosporine, thalidomide, psoralen plus ultraviolet A, and low-dose irradiation of lymph nodes.
Schedule of methylprednisolone administration
| Days post-transplantation . | Dose, mg/kg/day . |
|---|---|
| 14-20 | 0.5 |
| 21-34 | 1.0 |
| 35-48 | 0.5 |
| 49-69 | 0.25 |
| 70-89 | 0.12 |
| 90-99 | 0.12 every other day |
| 100-110 | 0.06 every other day |
| Days post-transplantation . | Dose, mg/kg/day . |
|---|---|
| 14-20 | 0.5 |
| 21-34 | 1.0 |
| 35-48 | 0.5 |
| 49-69 | 0.25 |
| 70-89 | 0.12 |
| 90-99 | 0.12 every other day |
| 100-110 | 0.06 every other day |
Doses of 0.25 mg/kg/day or more were divided in 2 parts.
Cotrimoxazole was given for 1 year for the prophylaxis ofPneumocystis carinii infections. In case of sulfa allergy, pentamidine inhalations were given. Acyclovir was administered for 5 weeks post-transplantation to prevent herpes simplex infections.
The documentation of engraftment was based on blood counts and routine marrow aspirates. Cytogenetic analysis was carried out routinely 2, 4, and 12 months post-transplantation if there was sex mismatch or a chromosome marker in the malignant cells.
The study was approved by the ethics committee of the Department of Medicine, Helsinki University Central Hospital. The date of the present analysis was July 8, 1998. The median follow-up of living patients was 77 months (range 50-109 months). One patient was lost to follow-up 37 months after transplantation.
Statistics
The cumulative risks of acute and chronic GVHD, probability of neutrophil and platelet recovery, risk of relapse, and survival were analyzed using Kaplan-Meier curves and log-rank statistics with SPSS software for Windows 95. Only patients who survived at least 100 days post-transplantation were included in the analysis of chronic GVHD, though manifestations of chronic GVHD were recorded in some patients at an earlier time point. No patient with signs of chronic GVHD died before day 100. Survival and relapse-free survival were calculated from transplantation to death from any cause and to relapse or death, respectively. Relapse of acute leukemia was defined as more than 5% blasts in an essentially normocellular marrow. The relapses of chronic myeloid leukemia (CML) included both hematologic and cytogenetic relapses. Disease progression after the achievement of minimal disease state of myeloma, lymphoma, or chronic lymphatic leukemia after transplantation was also included in the analysis of the risk of relapse. The differences between the groups in the total dose of MP given, cyclosporine concentrations, infections, hospitalization days, and transfused red cell and platelet (pooled random donor) units were tested with the Mann-Whitney U test.
Results
Engraftment
All patients showed engraftment, with the minimum requirement of reaching 1.0 × 109 neutrophils/L. Table3 shows the recovery of the neutrophil and platelet counts in the study groups. One patient in each group received granulocyte colony-stimulating factor. Neutrophil recovery was significantly faster in the group given MP, and there was a trend toward faster platelet recovery.
Recovery of neutrophil and platelet counts, transfusions, and hospitalization after transplantation
| . | Treatment group . | Pvalue . | |||
|---|---|---|---|---|---|
| MP+ (n = 53) . | MP− (n = 55) . | ||||
| Median . | Range . | Median . | Range . | ||
| Time to recovery, day post-transplantation | |||||
| Neutrophils > 0.5 × 109/L | 17 | 12-30 | 20 | 12-32 | <.0001 |
| Neutrophils > 1.0 × 109/L | 19 | 13-36 | 24 | 15-52 | <.0001 |
| Platelets > 20 × 109/L | 17 | 13-29 | 18 | 13-31 | .08 |
| Platelets > 50 × 109/L | 20 | 14-341 | 21 | 16-45 | .35 |
| Transfusions, units | |||||
| First 4 months | |||||
| Platelets | 24 | 8-368 | 40 | 12-372 | .06 |
| Red blood cells | 4 | 0-55 | 6 | 0-46 | .07 |
| First year | |||||
| Platelets | 28 | 8-368 | 48 | 16-533 | .09 |
| Red blood cells | 4 | 0-56 | 8 | 0-96 | .15 |
| Hospitalization, days | |||||
| First 4 months | 39 | 20-115 | 45 | 22-99 | .03 |
| First year | 53 | 20-174 | 64 | 22-240 | .13 |
| . | Treatment group . | Pvalue . | |||
|---|---|---|---|---|---|
| MP+ (n = 53) . | MP− (n = 55) . | ||||
| Median . | Range . | Median . | Range . | ||
| Time to recovery, day post-transplantation | |||||
| Neutrophils > 0.5 × 109/L | 17 | 12-30 | 20 | 12-32 | <.0001 |
| Neutrophils > 1.0 × 109/L | 19 | 13-36 | 24 | 15-52 | <.0001 |
| Platelets > 20 × 109/L | 17 | 13-29 | 18 | 13-31 | .08 |
| Platelets > 50 × 109/L | 20 | 14-341 | 21 | 16-45 | .35 |
| Transfusions, units | |||||
| First 4 months | |||||
| Platelets | 24 | 8-368 | 40 | 12-372 | .06 |
| Red blood cells | 4 | 0-55 | 6 | 0-46 | .07 |
| First year | |||||
| Platelets | 28 | 8-368 | 48 | 16-533 | .09 |
| Red blood cells | 4 | 0-56 | 8 | 0-96 | .15 |
| Hospitalization, days | |||||
| First 4 months | 39 | 20-115 | 45 | 22-99 | .03 |
| First year | 53 | 20-174 | 64 | 22-240 | .13 |
Treatment
MP was given according to the prophylaxis schedule to all patients randomized to receive this drug. The day +11 dose of methotrexate was not given to 7 patients randomized to get MP or to 17 patients randomized not to receive MP because of severe mucositis or liver toxicity. The cyclosporine blood concentrations in the 2 study groups were compared at 1, 2, 3, and 4 months. There were no significant differences between the groups at any time (figures not shown).
Acute GVHD
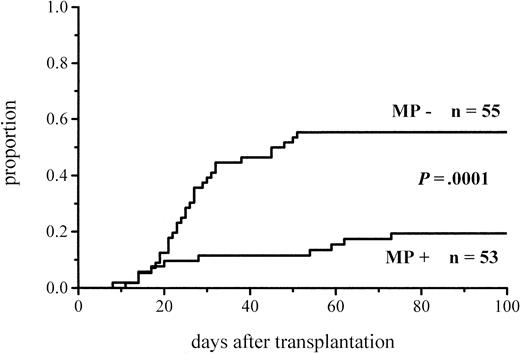
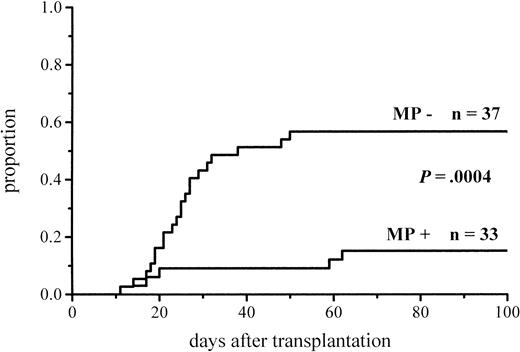
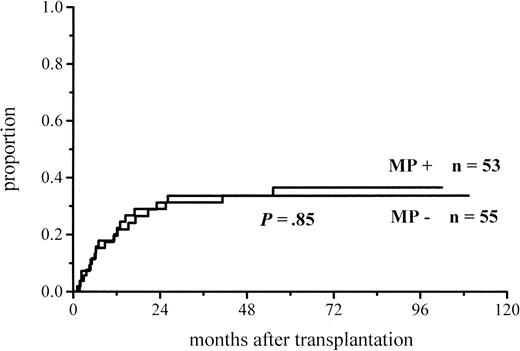
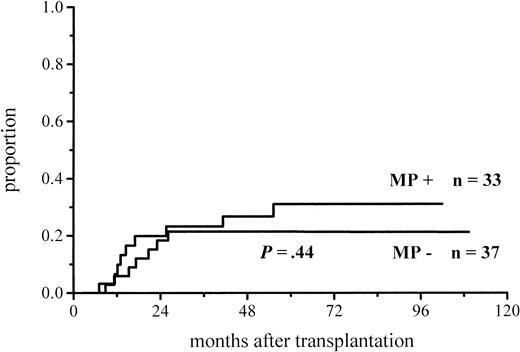
The cumulative incidence of acute GVHD and the maximum grades are shown in Figure 1 and Table4. A total of 19% of the patients given MP for prophylaxis had acute GVHD compared with 56% of those not given MP. The difference in the cumulative incidence of acute GVHD is highly significant, P = .0001. In the groups given and not given MP, the proportions of patients with grade II-IV acute GVHD were 7 of 53 (13%) and 20 of 55 (36%), respectively (P = .005), and those of patients with grade III-IV acute GVHD were 3 of 53 (6%) and 9 of 55 (16%), respectively (P = .08). Among the patients given prophylactic MP, there was a marginally significantly lower incidence of corticosteroid-resistant acute GVHD defined as needing antilymphocyte globulin for second-line treatment because of nonresponsiveness to MP (2 vs 8 patients, P = .05). Four of the 10 cases of acute GVHD in the group given MP became manifest late, between days 54 and 73, later than any case of GVHD in the control group. Because patients given more cytotoxic therapy might show more tissue toxicity and possibly have a higher risk of GVHD, low-risk patients (acute leukemia in first remission and CML in first chronic phase) were studied separately. The difference in the cumulative incidence of acute GVHD was similar to that between the entire randomization groups and was highly significant (Figure2; Table 4). Fifteen percent of the low-risk patients randomized to receive and 57% of those randomized not to receive MP developed acute GVHD of any grade, and the respective proportions of patients with grade II-IV acute GVHD were 2 of 33 (6%) and 13 of 37 (35%).
Cumulative incidence of grade I-IV acute GVHD in patients given or not given MP.
Graft-versus-host disease in patients given or not given methylprednisolone
| . | Treatment group . | Pvalue4-150 . | |
|---|---|---|---|
| MP+ . | MP− . | ||
| Acute GVHD, all patients | 10/53 (19%) | 31/55 (56%) | .0001 |
| Grade I | 3 | 11 | |
| Grade II | 4 | 11 | |
| Grade III | 1 | 5 | |
| Grade IV | 2 | 4 | |
| Grade II-IV | 7 (13%) | 20 (36%) | .005 |
| Acute GVHD, low-risk4-151patients | 5/33 (15%) | 21/37 (57%) | .0004 |
| Grade I | 3 | 8 | |
| Grade II | 1 | 9 | |
| Grade III | 0 | 3 | |
| Grade IV | 1 | 1 | |
| Grade II-IV | 2 (6%) | 13 (35%) | .003 |
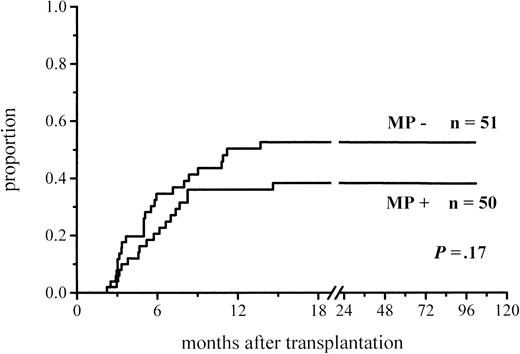
| Chronic GVHD, all patients‡ | 18/50 (36%) | 25/52 (48%) | .17 |
| Limited | 12 | 16 | |
| Extensive | 6 | 9 | |
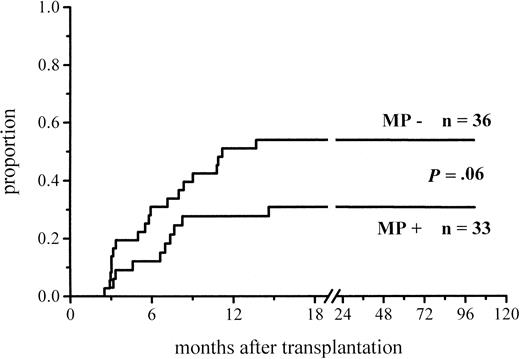
| Chronic GVHD, low-risk patients‡ | 10/33 (30%) | 19/37 (51%) | .06 |
| Limited | 8 | 13 | |
| Extensive | 2 | 6 | |
| . | Treatment group . | Pvalue4-150 . | |
|---|---|---|---|
| MP+ . | MP− . | ||
| Acute GVHD, all patients | 10/53 (19%) | 31/55 (56%) | .0001 |
| Grade I | 3 | 11 | |
| Grade II | 4 | 11 | |
| Grade III | 1 | 5 | |
| Grade IV | 2 | 4 | |
| Grade II-IV | 7 (13%) | 20 (36%) | .005 |
| Acute GVHD, low-risk4-151patients | 5/33 (15%) | 21/37 (57%) | .0004 |
| Grade I | 3 | 8 | |
| Grade II | 1 | 9 | |
| Grade III | 0 | 3 | |
| Grade IV | 1 | 1 | |
| Grade II-IV | 2 (6%) | 13 (35%) | .003 |
| Chronic GVHD, all patients‡ | 18/50 (36%) | 25/52 (48%) | .17 |
| Limited | 12 | 16 | |
| Extensive | 6 | 9 | |
| Chronic GVHD, low-risk patients‡ | 10/33 (30%) | 19/37 (51%) | .06 |
| Limited | 8 | 13 | |
| Extensive | 2 | 6 | |
Significance of the difference in cumulative incidence.
See Table 1.
At risk for at least 100 days.
Cumulative incidence of grade I-IV acute GVHD in low-risk patients given or not given MP.
Cumulative incidence of grade I-IV acute GVHD in low-risk patients given or not given MP.
Chronic GVHD
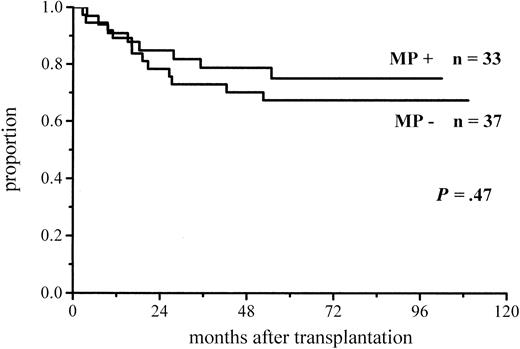
A total of 102 patients survived more than 100 days and hence were at risk for chronic GVHD. Thirty-six percent of the patients given MP and 48% of those not given MP for prophylaxis developed chronic GVHD (Figure 3; Table 4). The difference did not reach significance, but among the low-risk patients the difference in the incidence of chronic GVHD was almost significant (30% vs 51%,P = .06) (Figure 4; Table4). There was no difference between the study groups in the distribution of the limited and extensive forms of chronic GVHD.
Cumulative incidence of chronic GVHD in patients at risk for at least 100 days and given or not given MP.
Cumulative incidence of chronic GVHD in patients at risk for at least 100 days and given or not given MP.
Cumulative incidence of chronic GVHD in low-risk patients at risk for at least 100 days and given or not given MP.
Cumulative incidence of chronic GVHD in low-risk patients at risk for at least 100 days and given or not given MP.
The amount of MP administered
Because there was considerably more acute GVHD in the group randomized not to be given prophylactic MP, more MP was used for the treatment in this group. The mean total dose of MP administered during the first 4 months post-transplantation for prophylaxis and treatment was 55 mg/kg (median 35 mg/kg, range 20-260 mg/kg) in the group given MP for prophylaxis and 64 mg/kg (median 74 mg/kg, range 0-224 mg/kg) in the group with no MP in the prophylactic regimen. The difference is not significant, but there was a trend toward more MP being given to the patients randomized not to receive prophylactic MP.
Relapse
Figures 5 and6 show the cumulative incidence of relapse for all and low-risk patients. The relapses in the total patient material include disease progression after the achievement of minimal disease state of myeloma, lymphoma, or chronic lymphatic leukemia after transplantation. Of the patients given and of those not given prophylactic MP, 17 of 53 (32%) and 16 of 55 (29%) relapsed, respectively. Among the low-risk patients, the relapse rates were 9 of 33 (27%) in the group given and 7 of 37 (19%) in the group not given MP (nonsignificant). Three of the relapses were cytogenetic relapses of CML: 1 in the group with MP and 2 in the group without MP. Thus, there were no significant differences in the relapse rates between the study groups.
Cumulative incidence of relapse in low-risk patients given or not given MP.
Infections
Table 5 shows the infections observed in the study groups during the first 4 months and the first year after transplantation. Cytomegalovirus (CMV) infection was diagnosed in case of CMV viremia (early antigen–positive) and symptoms or signs likely to be caused by this infection. In deep fungal infection there was histologic or microbiologic documentation of fungus from a deep organ or fungemia. Septicemias were microbiologically documented. Pneumonias included all lung infiltrates, regardless of etiology, except lung abnormalities seen simultaneously with documented sepsis, deep fungal infection, or CMV infection as defined above. Most viral infections other than CMV were herpes simplex infections.
Infections in patients given or not given methylprednisolone
| Type of infection . | First 4 months (patients/episodes) . | First year (patients/episodes) . | ||||
|---|---|---|---|---|---|---|
| MP+ (n = 53) . | MPÜn = 55) . | P value5-150 . | MP+ (n = 53) . | MPÜn = 55) . | P value5-150 . | |
| Sepsis | 13/15 | 12/12 | .46 | 17/19 | 14/15 | .29 |
| Pneumonia | 4/4 | 13/15 | .02 | 12/15 | 27/38 | .004 |
| Other bacterial | 10/12 | 20/24 | .03 | 15/19 | 23/38 | .10 |
| Deep fungal | 0/0 | 5/5 | .03 | 3/4 | 5/7 | .38 |
| CMV | 9/9 | 18/20 | .05 | 12/14 | 20/27 | .09 |
| Other virus | 12/13 | 14/23 | .45 | 26/37 | 23/49 | .23 |
| All infection episodes | 53 | 99 | 108 | 174 | ||
| Type of infection . | First 4 months (patients/episodes) . | First year (patients/episodes) . | ||||
|---|---|---|---|---|---|---|
| MP+ (n = 53) . | MPÜn = 55) . | P value5-150 . | MP+ (n = 53) . | MPÜn = 55) . | P value5-150 . | |
| Sepsis | 13/15 | 12/12 | .46 | 17/19 | 14/15 | .29 |
| Pneumonia | 4/4 | 13/15 | .02 | 12/15 | 27/38 | .004 |
| Other bacterial | 10/12 | 20/24 | .03 | 15/19 | 23/38 | .10 |
| Deep fungal | 0/0 | 5/5 | .03 | 3/4 | 5/7 | .38 |
| CMV | 9/9 | 18/20 | .05 | 12/14 | 20/27 | .09 |
| Other virus | 12/13 | 14/23 | .45 | 26/37 | 23/49 | .23 |
| All infection episodes | 53 | 99 | 108 | 174 | ||
Difference between the numbers of patients with infection.
During the first 4 months, there were significantly fewer pneumonias, other bacterial infections, and deep fungal infections among the patients given MP for prophylaxis. The difference in pneumonias remained highly significant for the first year after transplantation. There was an almost significant trend toward fewer CMV infections in the patients randomized to receive MP. There was no difference in the incidence of sepsis. The total number of infection episodes was markedly lower in the group given MP.
Avascular bone necrosis
Magnetic resonance imaging was carried out in patients with symptoms suggestive of avascular bone necrosis. This complication was diagnosed in 6 patients in both groups. There was no difference in the time of appearance of the complication, the median time post-transplantation being 13 months (range 6-54 months) among the patients given and 21 months (range 2-110 months) among those not given prophylactic MP.
Blood transfusions and hospitalization
Post-transplantation blood cell transfusions during the first 4 months and the first year are shown in Table 3. There was an almost significant trend toward fewer blood cell units given in the MP+ randomization group.
The time spent at the hospital during the first 4 months was significantly shorter among the patients given MP for prophylaxis (Table 3).
Survival
Figures 7 and8 show the survivals of all and low-risk patients according to the study arm. At the time of the analysis, 32 of the 53 patients (60%) given MP and 28 of the 55 (51%) not given MP were surviving. Among the low-risk patients, the proportions of surviving patients were 25 of 33 (76%) for the patients given and 25 of 37 (68%) for those not given MP. The survivals did not differ significantly.
Causes of death
The principal causes of death are shown in Table6. Relapse was the most common cause of death, followed by GVHD. There was no difference between the study groups; neither was there any difference in the causes of death among the low-risk patients. Five low-risk patients died of relapse in the group given and 6 in the group not given MP. Two low-risk patients in the MP+ group and 4 in the MP− group died of GVHD.
Principal causes of death
| . | Treatment group . | |
|---|---|---|
| MP+(n = 53) . | MP−(n = 55) . | |
| Relapse | 13 | 15 |
| GVHD | 6 | 7 |
| Pneumonitis | 1 | 2 |
| Infection | 2 | |
| Intracerebral hemorrhage | 1 | |
| ARDS, DIC | 1 | |
| Total | 21 | 27 |
| . | Treatment group . | |
|---|---|---|
| MP+(n = 53) . | MP−(n = 55) . | |
| Relapse | 13 | 15 |
| GVHD | 6 | 7 |
| Pneumonitis | 1 | 2 |
| Infection | 2 | |
| Intracerebral hemorrhage | 1 | |
| ARDS, DIC | 1 | |
| Total | 21 | 27 |
ARDS indicates acute respiratory distress syndrome; DIC, disseminated intravascular coagulation.
Discussion
Corticosteroids are effective in the treatment of most patients with acute GVHD and should therefore also be useful in the prophylaxis of GVHD. However, their role in the prevention of GVHD is not well established. Including the present study, the addition of corticosteroid to the most widely used prophylactic regimen, the combination of cyclosporine and methotrexate, has been evaluated in 3 fully published randomized trials with contradictory results. In the study of Storb and coworkers,10 the addition of MP to cyclosporine and methotrexate increased the incidence of acute and chronic GVHD in sibling transplantations, whereas in the study of Atkinson et al11 with small numbers of patients, no difference in GVHD was seen between the patients given and those not given prednisolone. In the present study, the addition of MP to cyclosporine and methotrexate highly significantly reduced the incidence of acute GVHD. In the study group given MP, 4 of the 10 cases of acute GVHD became manifest late, during the tapering of the MP dose. Late occurrence of acute GVHD among patients given corticosteroid was also observed in the studies by Storb et al10 and Atkinson et al.11
Another study showing no benefit of the addition of prednisone to the combination of cyclosporine and methotrexate has recently been published in the abstract form,18 but because details of the treatment, such as drug dosages and timing of administration were not given, this study cannot be further discussed here.
There were differences in the prophylactic scheme between the 2 previous studies and the present one, which are likely to explain the differences in the results. The most obvious difference is the timing of corticosteroid administration. In the previous studies, the corticosteroid treatment was initiated on the day of transplantation at a dose of 1 mg/kg per day; the dose was halved at 3 weeks and discontinued 30 to 35 days post-transplantation. In the present study, MP was started on day 14 after transplantation at 0.5 mg/kg per day, the maximum dose of 1 mg/kg per day was given on days 21 to 35, and thereafter the dose was slowly tapered and the administration discontinued on day 110. The reasoning behind this schedule was 2-fold: MP was initiated only after methotrexate administration was completed to avoid any interference with the effect of this drug, and the highest dose was scheduled to be given at the time of the highest risk of the appearance of symptomatic GVHD. In our previous experience, the median time of the appearance of acute GVHD in patients given cyclosporine and methotrexate prophylaxis was day 26 post-transplantation. The importance of the timing of corticosteroid administration is supported by the observation in the Seattle study10 that if the initiation of MP treatment was postponed to day 15, the increasing effect on the incidence of acute GVHD disappeared.
Another difference between the present study and the 2 previous ones is the dosing of cyclosporine. The intravenous dose at the early stage was the same, but after the switch to oral route we gave a much lower dose: 3 mg/kg per day compared with 12.5 mg/kg per day. We chose this low dose because preliminary information indicated that patients on low-dose cyclosporine may do at least as well as those on a higher dose and that increasing the intensity of GVHD prophylaxis may increase the relapse rate.19 We also had significant problems with thrombotic microangiopathy at the time of designing the present study. Lower cyclosporine doses might have increased the incidence of acute GVHD among the patients not given MP compared with the other studies. This does not, however, seem to be the case. Because we also treated grade I GVHD, which apparently differs from the policy applied in the other studies, the comparison is not perfectly valid, but the incidence and severity of acute GVHD do not seem to be essentially different in the 3 studies. The incidence of grade II-IV acute GVHD among patients not given corticosteroid prophylaxis was 36% in both the Seattle study10 and the present one. There was less grade II-IV acute GVHD in the noncorticosteroid group of Atkinson et al11 than in the present study (15% vs 36%) but more grade I-IV GVHD (75% vs 56%). These differences may be partly explained by the often vague separation of grade I from grade II in clinical practice. Thus, the beneficial effect of the addition of MP in the present study does not appear to have been caused by an unduly high GVHD incidence in the control arm.
A further detail where the 3 studies may have differed is the use of folinic acid rescue after methotrexate treatment, as in the present study. This detail is not usually reported but, according to a recent survey among European centers, approximately one third of the centers gave folinic acid subsequent to methotrexate administration.1
More patients in the group not given MP had day +11 methotrexate omitted because of toxicity—in most cases severe mucositis—compared with the triple prophylaxis group. There were no obvious differences in the baseline characteristics of the study groups to explain the difference in toxicity, which might have been a mere chance occurrence. The difference in the methotrexate administration could have been one reason for the difference in the incidence of acute GVHD. However, there was a trend toward less acute GVHD among the patients not given the fourth dose of methotrexate compared with those given the full course; the incidence was 14% versus 21% in the group given MP and 41% versus 65% in the group not given MP, respectively. Therefore, the fact that fewer patients in the group not given prophylactic MP received the fourth dose of methotrexate does not explain the higher incidence of acute GVHD in this group.
A trend was observed toward less chronic GVHD in the group given prophylactic MP. Although this effect did not reach significance, the trend may indicate that a low dose of corticosteroids during the first 3 to 4 months after transplantation may have some prophylactic effect on chronic GVHD. This is logical because corticosteroid is the first-line treatment of chronic GVHD. Storb and coworkers10observed an increase in chronic GVHD among patients administered prophylaxis with MP in addition to cyclosporine and methotrexate compared with those not given MP. Likewise, Deeg et al3found that the addition of MP to cyclosporine prophylaxis increased the incidence of chronic GVHD. The causes of these unexpected findings and the difference from the results of the present study are not clear, but an obvious factor is the duration of MP prophylaxis. We gave MP until day 110 post-transplantation, whereas the administration was discontinued on day 35 in the study by Storb et al10 and on day 72 in the study by Deeg et al.3
In some studies, the intensification of GVHD prophylaxis has been associated with an increased risk of relapse.20-22 In the present study, the addition of MP to the prophylactic regimen had no effect on the relapse rate. It may be noteworthy that we used relatively low cyclosporine doses.
There were fewer infections among the patients given MP for prophylaxis than in the control group. Because GVHD, especially chronic GVHD, is associated with immunosuppression, the low incidence of GVHD among the patients given prophylactic MP may at least partly explain the lower infection rate. Because pneumonia after BMT is often associated with immunologic processes,23 the reduction of GVHD may explain the lower rate of pneumonias. High doses of MP, more often used for the treatment of acute GVHD in the study group not given prophylactic corticosteroid, probably contributed to the higher incidence of infections.
The recovery of the neutrophil counts after transplantation was significantly faster among the patients who received MP prophylaxis, as also reported previously by Storb et al.10 This was seen despite the fact that more patients not given MP for prophylaxis had the last methotrexate dose omitted because of toxicity. The faster neutrophil recovery was probably caused by demargination because there was no significant difference in the platelet recovery. The nonsignificant trend toward fewer blood cell transfusions given to patients with MP prophylaxis may reflect the lower incidence of GVHD in this study group. GVHD is associated with cytopenias in a significant proportion of patients; the mechanisms may be manifold and complex.24 25 The significantly shorter hospitalization during the first 4 months in the group randomized to receive prophylactic MP was due to the lower incidence of acute GVHD and fewer infections.
The adverse effects of corticosteroids, particularly infections, hypertension, hyperglycemia, and avascular bone necrosis are well known, and they have to be taken into account when weighing the advantages and disadvantages of corticosteroid administration. In the present study, the prophylactic use of MP did not result in greater exposure to corticosteroids because there was markedly more acute GVHD in the control group and the doses used for treatment were higher than the prophylactic doses. As shown above, there were fewer infections in the group given MP prophylaxis. Blood pressure and glucose balance were not prospectively recorded. There were no differences in the incidence or time of the onset of avascular bone necrosis.
The treatment policy of acute GVHD applied in this study was aggressive. We treated even early (grade I) acute GVHD with high-dose corticosteroids to stop effectively the GVHD process. While this policy may be more efficient in the treatment of acute GVHD than a more conservative approach, adverse effects may outweigh the benefits. In a registry study of the European Group for Blood and Marrow Transplantation (EBMT), it was found that patients treated for acute GVHD at centers applying a very intense treatment policy had a worse outcome than those treated at centers applying a more conservative approach.26 The treatment of all grades, including grade I, at first signs with initially at least 10 mg/kg per day of MP resulted in poorer survival and higher mortality in infections and poor graft function than the treatment of only grade II+ acute GVHD with 2 mg/kg per day or less. Although these results were only suggestive due to the nature of the study, the findings indicated that a very intense treatment policy may not be optimal for the outcome. In the present study, the patients randomized not to receive MP did, in fact, receive as much MP on the average as those randomized to receive MP for prophylaxis because of markedly more GVHD and intense treatment.
The randomization was not stratified according to risk groups. Therefore, the analysis of the outcome of low-risk patients is a retrospective subgroup analysis. However, the distribution of low-risk patients in the randomization groups was balanced, and there were no significant differences between the low-risk groups. The analysis of the outcome parameters of these more homogenous patient groups showed findings in line with the results obtained in the analysis of the entire treatment groups. It was especially useful to observe that there was no difference in the relapse rate among the low-risk patients given or not given MP for prophylaxis.
In conclusion, the present study showed that the addition of MP to the combination of cyclosporine and methotrexate markedly reduced the incidence of acute GVHD without causing untoward effects. Based on the results of 2 previous studies and the present one, it appears that the timing of corticosteroid administration is important for the effect. There was a trend toward less chronic GVHD among the patients administered prophylactic MP. The addition of MP had no effect on the relapse rate or survival.
Supported by the Sigrid Jusélius Foundation and the Blood Disease Research Foundation, Helsinki, Finland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tapani Ruutu, Department of Medicine, Helsinki University Central Hospital, PO Box 340, FIN-00029 HUS, Helsinki, Finland.