Abstract
High-dose chemoradiotherapy (HDT) with autologous stem cell transplantation (ASCT) is the treatment of choice for patients with relapsed aggressive non-Hodgkin lymphoma (NHL). However, its role in the treatment of patients with primary refractory disease is not well defined. The outcomes of 85 patients with primary refractory aggressive NHL who underwent second-line chemotherapy with ICE with the intent of administering HDT/ASCT to those patients with chemosensitive disease were reviewed. Patients were retrospectively classified as induction partial responders (IPR) if they attained a partial response to doxorubicin-based front-line therapy or as induction failures (IF) if they had less than partial response. Forty-three patients (50.6%) had ICE-chemosensitive disease; there was no difference in the response rate between the IPR and the IF groups. Intention-to-treat analysis revealed that 25% of the patients were alive and 21.9% were event-free at a median follow-up of 35 months. Among 42 patients who underwent transplantation, the 3-year overall and event-free survival rates were 52.5% and 44.2%, respectively, similar to the outcomes for patients with chemosensitive relapsed disease. No differences were observed between the IPR and IF groups, and there were no transplantation-related deaths. More than one extranodal site of disease and a second-line age-adjusted International Prognostic Index of 3 or 4 before ICE chemotherapy were predictive of poor survival. These results suggest that patients with primary refractory aggressive NHL should receive second-line chemotherapy, with the intent of administering HDT/ASCT to those with chemosensitive disease. Newer therapies are needed to improve the outcomes of patients with poor-risk primary refractory disease.
Introduction
CHOP chemotherapy is the standard front-line therapy for patients with aggressive non-Hodgkin lymphoma (NHL), based on the results of the United States High Priority Lymphoma Study.1 However, the 5-year failure-free survival rate is only 40% to 45%; nearly 50% of patients fail to achieve complete remission (CR), and approximately 10% to 20% of patients achieving CR eventually have relapses.1 For patients with relapsed disease that is sensitive to second-line chemotherapy, the Parma trial2 demonstrated that high-dose chemoradiotherapy (HDT) with autologous stem cell transplantation (ASCT) is the treatment of choice, resulting in 5-year event-free survival (EFS) rates of 40% to 45%. However, patients with relapsed disease that is resistant to standard-dose second-line chemotherapy have poor outcomes with HDT/ASCT. The long-term EFS rate is only 10% to 15%, similar to that observed with standard-dose salvage regimens.3-7
The role of HDT/ASCT for patients with primary refractory aggressive NHL is controversial.8,9 One of the earliest studies of HDT/ASCT that separately evaluated patients with primary refractory disease from those with relapsed disease, by Philip et al,3 reported that no patients with primary refractory disease were disease-free beyond 1 year. However, none of those patients was sensitive to second-line chemotherapy. In several subsequent trials10-14 of HDT/ASCT for patients with primary refractory disease, chemosensitivity was not routinely assessed. The importance of chemosensitivity in determining the outcome of HDT/ASCT for this group of patients was demonstrated by recent reports from the American Bone Marrow Transplant Registry (ABMTR) and the Southwest Oncology Group (SWOG). Data from both groups suggest that among patients with primary refractory disease, it is the subgroup sensitive to second-line chemotherapy that derives benefit from HDT/ASCT; in both studies, patients with primary refractory disease unresponsive to second-line therapy had poor outcomes.15 16
We reviewed our data for 90 consecutive patients with primary refractory aggressive NHL who were eligible to receive HDT and ASCT if they had chemosensitive disease, assessing their outcomes with respect to the EFS and overall survival (OS) after HDT/ASCT, the response rate to second-line chemotherapy, the ability to mobilize peripheral blood progenitor cells (PBPC), and the ability of the second-line International Prognostic Index (IPI) to predict survival.
Patients and methods
Patients
Between February 1993 and March 1999, we treated 90 potentially transplantation-eligible patients with primary refractory aggressive NHL with ICE (ifosfamide, carboplatin, and etoposide) second-line chemotherapy, with the intent of subsequent consolidative HDT/ASCT for all chemosensitive patients. Approval for these studies was obtained from the institutional review board. Informed consent was provided according to the Declaration of Helsinki. Primary refractory disease was defined as failure to achieve CR with a doxorubicin-containing front-line (induction) regimen. Patients were retrospectively classified into 2 groups, those who had partial response (PR) to induction therapy (IPR) and those in whom induction therapy failed (IF)—ie, they had progressive disease or less than PR with front-line therapy. Five patients were excluded from this analysis because they underwent allogeneic bone marrow transplantation after ICE chemotherapy. The remaining 85 patients were reviewed for this report.
Eligibility for second-line chemotherapy (ICE)
All patients were staged according to the Cotswolds modification of the Ann Arbor staging system,17 and all representative histologic specimens were reviewed by 1 of 3 hematopathologists. Of the patients who had achieved PR to front-line therapy, 87.5% had tissue biopsies confirming the presence of persistent lymphoma. Histologic diagnoses were retrospectively converted from the International Working Formulation (IWF)18 to the World Health Organization classification19 in consultation with the hematopathologists. Patients with lymphoma that had transformed from low-grade to intermediate-grade, as per the IWF classification, were eligible. All patients had normal baseline cardiac function (left ventricular ejection fraction more than 50%) and renal function (serum creatinine level 1.5 mg/dL or lower or creatinine clearance 60 mL/min or greater) before ICE chemotherapy was initiated.
ICE chemotherapy
Three cycles of ICE chemotherapy, administered as previously described,20 were planned as cytoreduction for all patients (Table 1). There were no dose reductions; instead treatment was delayed until the absolute neutrophil count was greater than 1000/μL and the platelet count was greater than 50 000/μL. Computed tomography of the chest, abdomen, and pelvis was performed before the initiation of ICE and 2 to 4 weeks after the third cycle of ICE, to evaluate the extent of disease and the response to chemotherapy. Gallium imaging and bone marrow biopsy were performed before the initiation of ICE and repeated after the third cycle of ICE if results of the initial studies were positive.
ICE chemotherapy
| . | Day 1 . | Day 2 . | Day 3 . | Days 5-12 . |
|---|---|---|---|---|
| Ifosfamide | x | |||
| Carboplatin | x | |||
| Etoposide | x | x | x | |
| G-CSF | x |
| . | Day 1 . | Day 2 . | Day 3 . | Days 5-12 . |
|---|---|---|---|---|
| Ifosfamide | x | |||
| Carboplatin | x | |||
| Etoposide | x | x | x | |
| G-CSF | x |
Ifosfamide: 5 g/m2 mixed with an equal amount of mesna and administered over 24 hours. Carboplatin: dosed at an area under the curve of 5 (5× [creatinine clearance + 25]) and capped at 800 mg, administered as a bolus. Etoposide: 100 mg/m2, administered as a bolus. G-CSF: 5 μg/kg for the first two cycles, then 10 μg/kg beginning on day 5 of the third cycle and continuing through leukapheresis. Cycles were to be administered every 14 days, for a total of 3 cycles.
Complete remission was defined as no evidence of disease on restaging studies performed 2 to 4 weeks after the completion of the third cycle of ICE. Conditional CR was defined as the presence of residual radiographic abnormalities smaller than 2 cm but with no clinical signs or symptoms of lymphoma. Restaging gallium scans had to be normal for patients to be considered to have achieved CR. Partial response was defined as a 50% or greater reduction in the sum of the products of the diameters of all measurable lesions. Patients achieving CR or PR were deemed chemosensitive and were allowed to proceed with HDT/ASCT. Patients who did not respond to ICE were offered alternative chemotherapy regimens or best supportive care, depending on their clinical status.
High-dose therapy and autologous stem cell transplantation
Patients with chemosensitive disease were eligible for HDT/ASCT even if small, cleaved lymphocytes were still detectable in their bone marrow. ICE/G-CSF–mobilized PBPC were collected after the third cycle of ICE, usually before restaging, when the white blood cell count exceeded 3000/μL. Patients from whom less than 2 × 106CD34+ cells/kg were collected were considered to have failed mobilization. If they were eligible to receive HDT, they underwent bone marrow harvest and were reinfused with bone marrow cells in addition to PBPC. All patients undergoing HDT/ASCT were required to have adequate pulmonary function (diffusion capacity greater than 50% of predicted) and liver function (serum bilirubin level less than 2 mg/dL).
All patients received 1 of 5 transplantation-conditioning regimens: CBV (cyclophosphamide, carmustine, etoposide), BEAM (carmustine, etoposide, cytarabine, melphalan), high-dose ICE, ifosfamide/etoposide/total body irradiation (TBI), or cyclophosphamide/etoposide/TBI. The choice of the conditioning regimen depended on the patient's age, the extent of prior therapy, and the clinical trials active at the time of transplantation.
After restaging, patients eligible for HDT/ASCT were evaluated for radiotherapy. Considerations for radiotherapy were the following: (1) TBI was given only to previously unirradiated patients and was administered in an accelerated fractionation schedule, with a total dose of 12 Gy delivered as 1.5-Gy fractions twice daily for 4 days. (2) Involved-field radiotherapy (IFRT) was added to sites of disease that measured 5 cm or more before ICE was started or to sites with residual nodal masses before HDT, if the patient had not received dose-limiting radiotherapy to those sites. IFRT was delivered as 1.5-Gy fractions twice daily. (3). If a patient was to receive TBI, IFRT was limited to 18 Gy, and thus the total dose to that site was 30 Gy. (4) Patients who did not receive TBI but met the criteria for IFRT received IFRT in the fractionation schema described above, to a total dose of 30 Gy. IFRT was administered as outpatient treatment, and the minimal interval between the 2 daily fractions was 7 hours; all planned fields were delivered at each treatment session.
Determination of the second-line IPI and age-adjusted IPI
The IPI and age-adjusted IPI (AAIPI) were determined before ICE therapy was started and were calculated as previously described.21 22 In this report we refer to these indices as the second-line IPI (sIPI) and the second-line age-adjusted IPI (sAAIPI), respectively, reflecting the fact that they are determined at the time of diagnosis of refractory disease instead of at initial diagnosis. Adverse factors for the IPI include age greater than 60, Karnofsky performance status (KPS) less than 80, lactate dehydrogenase (LDH) greater than normal, more than 1 extranodal site, and stage III/IV disease. The IPI is calculated as follows: 0 to 1 factor, IPI 1; 2 factors, IPI 2; 3 factors, IPI 3; and 4 to 5 factors, IPI 4. The AAIPI incorporates KPS, LDH, and stage and is determined as follows: 0 factors, AAIPI 1; 1 factor, AAIPI 2; 2 factors, AAIPI 3; and 3 factors, AAIPI 4.
Statistical analysis
EFS and OS were assessed from the first day of ICE chemotherapy. An event was defined as treatment failure (ICE failure, ICE toxicity precluding ASCT, or residual disease after ASCT), relapse after ASCT, or death from any cause. If relapse or determination of treatment failure occurred before a patient's death, the former date was used for the calculation of EFS. Survival curves were generated using the method of Kaplan and Meier23 and were compared using the log-rank test.24
The following factors, determined before the initiation of ICE therapy, were evaluated as possible prognostic indicators for overall survival: age (younger than 60 vs 60 or older), sex, immunophenotype (B vs T cell), status of refractory disease (IPR vs IF), stage (I/II vs III/IV), LDH (normal vs elevated), KPS (less than 80 vs 80 or greater), number of ENS involved (1 or none vs more than 1), bone marrow involvement, sIPI (1 vs 2 vs 3 vs 4), and sAAIPI (1 vs 2 vs 3 vs 4). Univariate analyses were performed using the log-rank test.24 Factors that were potentially predictive of OS (P < .05) were entered into a multivariate analysis using the Cox proportional hazards model.25
The log-rank test was used to compare the OS and EFS survival rates of patients who received IFRT with the outcomes of patients who did not receive IFRT.24 The associations between categorical variables were assessed using the Fisher exact test.26 All statistical calculations were performed using S-PLUS 2000 (Mathsoft, Seattle, WA).
Results
Patient characteristics
Pre-ICE chemotherapy treatment characteristics of the 85 patients are listed in Table 2. All patients received doxorubicin as part of their prior chemotherapy, and 87% of the patients received only one prior regimen. Forty patients had PR to front-line therapy (IPR), whereas induction therapy failed in 45 patients (IF). Thirty-one percent of the patients had bone marrow involvement before they received ICE chemotherapy, and 79% of the patients had advanced (stage III or IV) disease. Seventy-one (83.5%) of the 85 patients had a B-cell phenotype, and 64.7% of the patients had “poor-risk” disease, defined by an sAAIPI of 3 or 4. None had transformed lymphoma.
Patient characteristics before ICE chemotherapy
| Patient characteristic . | No. patients . |
|---|---|
| Age (y) | |
| Median (range) | 46 (20-66) |
| Younger than 60 | 75 |
| 60 or older | 10 |
| Response to primary therapy | |
| Induction partial response (IPR) | 40 |
| Induction failure (IF) | 45 |
| Histologic diagnosis (WHO classification) | |
| Diffuse large B-cell | 71 |
| Peripheral T-cell lymphoma, unspecified | 12 |
| Anaplastic large cell: T-cell | 2 |
| Stage | |
| I | 1 |
| II | 17 |
| III | 7 |
| IV | 60 |
| Karnofsky performance status | |
| ≥80 | 45 |
| <80 | 40 |
| Extranodal sites involved | |
| ≤1 | 38 |
| >1 | 47 |
| Bone marrow involvement | |
| Yes | 20 |
| No | 65 |
| Prior chemotherapy | |
| 1 prior regimen | 74 |
| CHOP | 34 |
| CHOP-like | 9 |
| NHL-15* | 31 |
| ≥2 regimens | 11 |
| including CHOP | 10 |
| including CHOP-like chemotherapy | 1 |
| Second-line IPI | |
| 1 | 19 |
| 2 | 22 |
| 3 | 18 |
| 4 | 26 |
| Age-adjusted second-line IPI | |
| 1 | 8 |
| 2 | 22 |
| 3 | 28 |
| 4 | 27 |
| Patient characteristic . | No. patients . |
|---|---|
| Age (y) | |
| Median (range) | 46 (20-66) |
| Younger than 60 | 75 |
| 60 or older | 10 |
| Response to primary therapy | |
| Induction partial response (IPR) | 40 |
| Induction failure (IF) | 45 |
| Histologic diagnosis (WHO classification) | |
| Diffuse large B-cell | 71 |
| Peripheral T-cell lymphoma, unspecified | 12 |
| Anaplastic large cell: T-cell | 2 |
| Stage | |
| I | 1 |
| II | 17 |
| III | 7 |
| IV | 60 |
| Karnofsky performance status | |
| ≥80 | 45 |
| <80 | 40 |
| Extranodal sites involved | |
| ≤1 | 38 |
| >1 | 47 |
| Bone marrow involvement | |
| Yes | 20 |
| No | 65 |
| Prior chemotherapy | |
| 1 prior regimen | 74 |
| CHOP | 34 |
| CHOP-like | 9 |
| NHL-15* | 31 |
| ≥2 regimens | 11 |
| including CHOP | 10 |
| including CHOP-like chemotherapy | 1 |
| Second-line IPI | |
| 1 | 19 |
| 2 | 22 |
| 3 | 18 |
| 4 | 26 |
| Age-adjusted second-line IPI | |
| 1 | 8 |
| 2 | 22 |
| 3 | 28 |
| 4 | 27 |
The details of the NHL-15 regimen have been described elsewhere.27
Response to ICE chemotherapy
Fifty-nine (69.4%) of the 85 patients completed all 3 cycles of ICE. Of the 26 patients who did not complete all 3 cycles, 21 had disease progression during ICE chemotherapy. Three patients did not complete treatment because of ICE-related toxicity and were analyzed as treatment failures: one patient had prolonged pancytopenia after the first cycle of ICE and was treated with modified CytaBOM; one patient had ifosfamide-induced encephalopathy; and one patient had fungal pneumonia complicated by multiorgan failure. Two patients declined further treatment before completing all 3 cycles and were considered ICE failures. Forty-three patients achieved either CR or PR, for an overall response rate of 50.6% (Table3). There was no difference between the IPR and IF groups with regard to the response rate to ICE (P = .517).
Response to ICE chemotherapy
| Status of refractory disease . | No. patients . | Response to ICE . | ||
|---|---|---|---|---|
| CR . | PR . | Overall response rate (%) . | ||
| IPR | 40 | 10 | 12 | 55.0 |
| IF | 45 | 4 | 17 | 46.7 |
| All patients | 85 | 14 | 29 | 50.6 |
| Status of refractory disease . | No. patients . | Response to ICE . | ||
|---|---|---|---|---|
| CR . | PR . | Overall response rate (%) . | ||
| IPR | 40 | 10 | 12 | 55.0 |
| IF | 45 | 4 | 17 | 46.7 |
| All patients | 85 | 14 | 29 | 50.6 |
The median number of CD34+ cells harvested was 7.0 × 106/kg (range, 0.2-36.4) in a median of 3 apheresis procedures (range, 1-9). Thirty-six (76.6%) of the 47 patients in whom PBPC were harvested mobilized more than 5 × 106 CD34+ cells/kg, and 9 patients (19.1%) mobilized less than 2 × 106 CD34+cells/kg.
HDT and ASCT
Among 43 patients with chemosensitive disease, 4 patients in the IF group and one patient in the IPR group subsequently had progressive disease before receiving the conditioning regimen and therefore did not undergo HDT/ASCT. In addition, 4 patients in whom ICE treatment failed underwent HDT/ASCT: one patient underwent transplantation at another institution, one patient had a marked response to IFRT to the mediastinum and subsequently achieved a minimal disease state, one patient had a mixed response to ICE, and one patient had prolonged pancytopenia after cycle 1 of ICE but then achieved PR after treatment with 3 cycles of modified CytaBOM.
The conditioning regimens for the 38 ICE-chemosensitive patients (IPR 21, IF 17) and the 4 patients in whom ICE failed (IPR 4, IF 0) but who underwent HDT/ASCT are listed in Table 4. Twenty-six (61.9%) of the 42 patients who underwent transplantation received hyperfractionated IFRT before HDT/ASCT.
Characteristics and conditioning regimens for patients who underwent HDT/ASCT
| Characteristic . | No. patients (%) . |
|---|---|
| Response to ICE | |
| ICE-chemosensitive | 38/43 (88.4) |
| ICE-failure | 4/42 (9.5) |
| Conditioning regimen | |
| BEAM | 11 |
| Cyclophosphamide/etoposide/TBI | 11 |
| Ifosfamide/etoposide/TBI | 11 |
| CBV | 7 |
| High-dose ICE | 2 |
| Hyperfractionated involved-field radiotherapy | 26/42 (61.9) |
| Characteristic . | No. patients (%) . |
|---|---|
| Response to ICE | |
| ICE-chemosensitive | 38/43 (88.4) |
| ICE-failure | 4/42 (9.5) |
| Conditioning regimen | |
| BEAM | 11 |
| Cyclophosphamide/etoposide/TBI | 11 |
| Ifosfamide/etoposide/TBI | 11 |
| CBV | 7 |
| High-dose ICE | 2 |
| Hyperfractionated involved-field radiotherapy | 26/42 (61.9) |
Nine patients failed mobilization. Four of these 9 patients did not undergo HDT/ASCT because of progressive disease. Four of the remaining 5 patients who did undergo HDT/ASCT received bone marrow cells in addition to PBPC. One patient mobilized 0.8 × 106CD34+ cells/kg after ICE but had 1.4 × 106CD34+ cells/kg harvested before treatment at our institution. This patient received only the PBPC as support for HDT.
There were no transplantation-related deaths; however, 4 (9.5%) of 42 patients died within 100 days of stem cell reinfusion. Three of these patients had disease unresponsive to HDT/ASCT and died of complications related to disease progression. One patient returned 80 days after ASCT with hepatic failure and adrenal insufficiency; he was found to have recurrent disease in the liver and died on day 90.
Overall and event-free survival
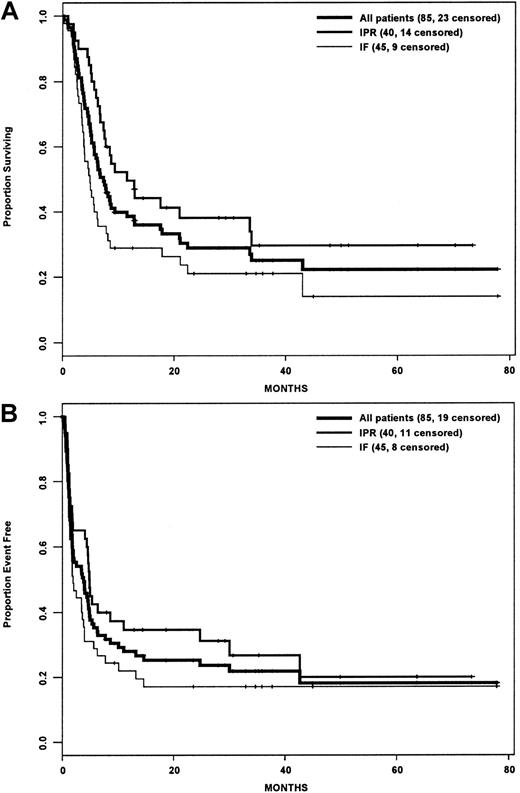
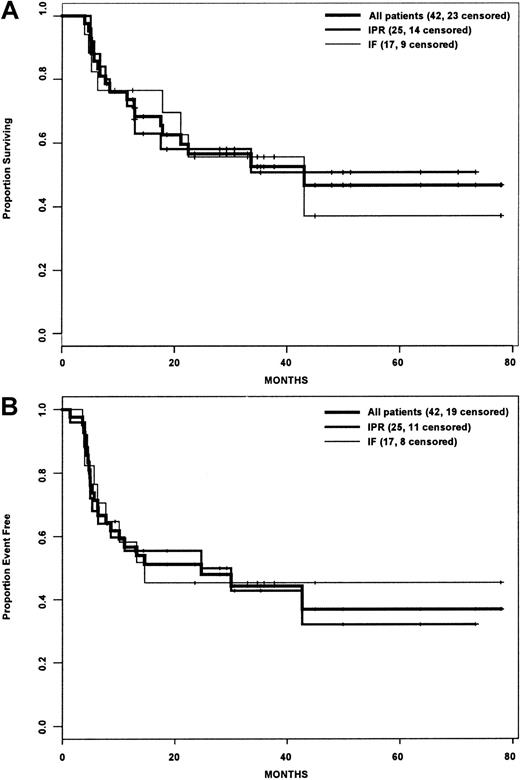
Actuarial estimates of the 3-year OS and EFS rates for the entire cohort were 25% and 22%, respectively (Figure1A,B). Although the IPR group had a statistically significant improved OS compared with the IF group (P = .015), there was no difference in EFS between the 2 groups (P = .081). For the subset of patients who underwent HDT/ASCT, the OS and EFS rates were 52.5% and 44.2% (Figure2A,B), and there was no difference between the IPR and IF groups with regard to OS (P = .895) or EFS (P = .868) rates.
Survival analysis by intention-to-treat.
(A) Overall survival. (B) Event-free survival. IPR indicates the group of patients who achieved PR with front-line therapy; IF indicates the group of patients who achieved less than PR with front-line therapy.
Survival analysis by intention-to-treat.
(A) Overall survival. (B) Event-free survival. IPR indicates the group of patients who achieved PR with front-line therapy; IF indicates the group of patients who achieved less than PR with front-line therapy.
Survival analysis of patients who underwent transplantation.
(A) Overall survival. (B) Event-free survival. IPR indicates the group of patients who achieved PR with front-line therapy; IF indicates the group of patients who achieved less than PR with front-line therapy.
Survival analysis of patients who underwent transplantation.
(A) Overall survival. (B) Event-free survival. IPR indicates the group of patients who achieved PR with front-line therapy; IF indicates the group of patients who achieved less than PR with front-line therapy.
Only 1 of the 4 patients who underwent HDT/ASCT after ICE chemotherapy failed is alive. This patient, who achieved a minimal disease state with IFRT to the mediastinum before HDT, remains event-free 4 years and 1 month after the initiation of ICE chemotherapy. Two patients died of progressive disease within 100 days of transplantation. One patient, who underwent transplantation at another institution, had a relapse 9 months after ASCT and died 3 months later.
The median survival time of the patients who did not undergo HDT/ASCT was 3.7 months (range, 0.2-33.3). Among these patients, the IPR group had a statistically significant longer survival time than did the IF group (P = .006); their median survival times were 6.3 and 3.4 months, respectively.
Prognostic factors
In univariate analyses, immunophenotype, status of refractory disease, number of ENS involved, stage, LDH, KPS, sIPI, and sAAIPI were all predictive of OS. Age and bone marrow involvement did not predict outcome. The sAAIPI, number of ENS, immunophenotype, and status of refractory disease were entered into a multivariate regression analysis. Immunophenotype was no longer predictive of OS (P = .53). Table 5 lists the hazard ratios for OS using a 3-variable model (status of refractory disease, sAAIPI, and number of ENS). There were no differences between the IPR and IF groups with regard to the sAAIPI (P = .26) or the number of ENS (P = .20). The administration of IFRT did not have an impact on either OS (P = .336) or EFS (P = .217).
Variables predictive of overall survival in multivariate analysis
| Factor . | Hazard ratio5-150 . |
|---|---|
| sAAIPI 3 or 4 (vs 1 or 2) | 4.96 (2.46-10.0; P < .001) |
| No. ENS > 1 (vs ≤ 1) | 1.81 (1.02-3.23; P = .044) |
| IF (vs IPR) | 2.22 (1.31-3.76; P = .003) |
| Factor . | Hazard ratio5-150 . |
|---|---|
| sAAIPI 3 or 4 (vs 1 or 2) | 4.96 (2.46-10.0; P < .001) |
| No. ENS > 1 (vs ≤ 1) | 1.81 (1.02-3.23; P = .044) |
| IF (vs IPR) | 2.22 (1.31-3.76; P = .003) |
Confidence intervals and P values are included in parentheses.
Eight (29.6%) of the 27 patients with sAAIPI of 4 had disease that was sensitive to ICE chemotherapy, but progressive disease developed in 2 of these 8 patients before ASCT. Of the 6 remaining patients who underwent ASCT, only 2 are alive (and event-free), for a survival rate of 7.4% among the patients with sAAIPI 4. In contrast, 7 (87.5%) of the 8 patients with sAAIPI of 1 are alive.
Discussion
Patients with primary refractory aggressive NHL, especially those whose disease progresses during front-line therapy, have poor prognoses. The role of HDT/ASCT for these patients has not been defined because there have been no randomized trials evaluating HDT/ASCT in this group of patients. Results from the ABMTR and SWOG suggest that HDT/ASCT may be effective therapy for these patients provided they have chemosensitive disease.15 16 The only variable that correlated with outcome in the ABMTR analysis was sensitivity to second-line therapy; patients with chemoresistant disease had a 3-year survival rate of 19% compared with 48% for patients with chemosensitive disease (P = .0002).
The interpretation of the results of various HDT/ASCT trials, which have included patients with primary refractory aggressive NHL, has been confounded by many factors, such as the lack of chemosensitivity determination, inconsistent definitions of “primary refractory,” inclusion of patients with various histologic diagnoses,10,11,13,14,28-31 the use of nonuniform second-line chemotherapy,13,29,30 and the grouping together of patients with relapsed and primary refractory disease.10,14 28-30
To address the role of HDT/ASCT for patients with primary refractory aggressive NHL, we have reviewed the characteristics and outcomes of a large series of patients who were treated with uniform cytoreduction. Extrapolating from the experience of HDT/ASCT for patients with relapsed disease, since 1993 we have offered HDT/ASCT to patients with primary refractory aggressive NHL only if they have had chemosensitive disease.
The reported response rates to commonly used second-line regimens for patients with relapsed or refractory intermediate grade lymphoma vary between 35% and 80%.5-7,32 In a series reported by Prince et al,33 in which most patients received DHAP or miniBEAM as second-line therapy, 45.6% of patients with primary refractory intermediate- or high-grade lymphoma had chemosensitive disease, lower than the 58% response rate to DHAP observed among patients with relapsed disease in the Parma trial.2Josting et al34 recently reported on the outcomes of patients with “primary progressive” aggressive NHL, which included patients who had relapses within 90 days of achieving CR with front-line therapy. Patients received one of several second-line regimens, with an overall response rate of only 15%. Only a small proportion of patients had extranodal disease (14%), and the reasons for the poor response to second-line therapy are not clear. The response rate to ICE chemotherapy was 50.6%, which is substantially lower than the 81% response rate observed in patients who had relapsed disease.20 However, most (65%) of the patients in this report also had poor-risk disease, as defined by an sAAIPI of 3 or 4. Importantly, patients in whom induction therapy failed responded similarly to patients who had partial responses to front-line therapy, which suggests that these 2 groups of patients are equally susceptible to ICE cytoreductive chemotherapy.
The response rate to ICE does not appear to be different than that of other salvage/second-line regimens.5-7,32 Our observation of a lower response rate in this subset of patients compared to those with relapsed disease has been an inconsistent finding among patients treated with other regimens, with some reports suggesting an inferior response rate for patients with primary refractory disease6,7 and others indicating equivalent responses.5,31 32 The reasons for this discrepancy are not readily apparent and may be related to patient selection, histology, and number of patients involved.
Approximately 25% of patients with primary refractory disease are alive, and 22% are event-free 3 years from the initiation of ICE chemotherapy. The 3-year overall and event-free survival rates for patients undergoing HDT/ASCT were 52.5% and 44.2%, respectively, with no difference observed between the IPR and IF groups. Similar results have been observed for patients with chemosensitive primary refractory disease by the ABMTR10 and by Prince et al.33These outcomes compare favorably with the 5-year overall and event-free survival rates of 53% and 46%, respectively, observed among the patients with relapsed disease who underwent transplantation in the Parma trial.2 Therefore, if chemosensitivity is established, there appears to be little difference in outcome after HDT/ASCT between patients with relapsed disease and patients with primary refractory disease.
There were no transplantation-related deaths, and the 100-day peri-transplantation mortality rate was only 9.5% (4 patients died of progressive disease within this period). Therefore, in addition to being efficacious for primary refractory aggressive NHL, HDT/ASCT is feasible and safe.
PBPC yields in patients with relapsed or refractory aggressive NHL range from 1.6 to 8.6 × 106 CD34+ cells/kg, depending on the mobilization regimen used.35-39 Given that 77% of patients in this series mobilized more than 5 × 106 CD34+ cells/kg, we conclude that PBPC mobilization in patients with primary refractory disease is comparable to that obtained in patients treated with ICE for relapsed aggressive lymphoma.20
We and others22,40 have demonstrated the prognostic significance of the sIPI for patients with relapsed or refractory aggressive NHL. When the sAAIPI was applied to the patients randomized to receive HDT/ASCT in the Parma trial, however, it was not predictive of OS.41 Only 16 patients in the latter analysis had 2 or 3 adverse factors for the AAIPI, and the lack of correlation of the sAAIPI with OS may have resulted from the small number of patients with high sAAIPI scores. Consistent with our prior observations,20 22 the number of ENS and the sAAIPI were predictive of OS in this series of patients with primary refractory aggressive lymphoma. Patients with sAAIPI scores of 4 did extremely poorly, with only one third of patients responsive to ICE and only a minority event-free after transplantation. Given these data, it appears that patients with primary refractory aggressive NHL and sAAIPI scores of 4 derive limited benefit from current HDT/ASCT approaches; newer transplantation strategies tailored for these patients with poor prognoses are needed.
There have been no comparative trials addressing the role of IFRT either before or after HDT/ASCT for aggressive NHL. In both arms of the Parma trial, there were no differences in the proportions of relapse or the sites of relapse between the groups who received IFRT and the groups who did not.2 Phillips et al12 observed a slight trend in improved survival among patients who received IFRT, but Cox analysis demonstrated that IFRT had no prognostic significance. In this report, IFRT did not impact either OS or EFS. The benefit, or lack thereof, of IFRT in the setting of HDT/ASCT for aggressive NHL cannot be determined from any of these trials, including our own, because IFRT was administered to all patients who met predefined criteria. The potential added benefit of IFRT is best assessed in prospective randomized trials.
Our data strongly suggest that patients with primary refractory aggressive NHL should be treated with second-line chemotherapy with the intent of performing HDT/ASCT for those patients with chemosensitive disease. The critical feature separating patients with primary refractory disease from those with relapsed disease appears to be the sensitivity to second-line chemotherapy; nevertheless, approximately 50% of patients have ICE-chemosensitive disease, and the outcome of these patients after HDT/ASCT is comparable to the outcome of patients with relapsed disease.
Supported in part by the Lymphoma Foundation, the Dewitt Wallace Fund, the Priovolos Family Lymphoma Research Fund, and the National Institutes of Health (grant 5P01CA5826-34). T.K. is a Mortimer J. Lacher fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Craig H. Moskowitz, Memorial Sloan-Kettering Cancer Center, Box 350, 1275 New York Ave, New York, NY 10021; e-mail:moskowic@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal