Abstract
The safety and efficacy of administering ex vivo expanded peripheral blood progenitor cells (PBPC) to patients with breast cancer who undergo high-dose chemotherapy and PBPC transplantation was investigated. Unselected PBPC were cultured in gas-permeable bags containing 1-L serum-free media, granulocyte colony-stimulating factor, stem cell factor, and pegylated megakaryocyte growth and development factor for 9 days. Cell dose cohorts were assigned to have between 2 and 24 × 109 PBPC cultured at 1, 2, or 3 × 106 cells/mL. Twenty-four patients received high-dose chemotherapy followed by infusion of the cultured PBPC and at least 5 × 106 CD34+ uncultured cryopreserved PBPC per kilogram. No toxicities resulted from infusions of the ex vivo expanded PBPC. The study patients had shorter times to neutrophil (P = .0001) and platelet (P = .01) recovery and fewer red cell transfusions (P = .02) than 48 historical controls who received the same conditioning regimen and posttransplantation care and at least 5 × 106CD34+ PBPC per kilogram. Improvements in all these endpoints were significantly correlated with the expanded cell dose. Nine of 24 (38%) patients recovered neutrophil counts above 500/μL by day 5 or 6 after transplantation, whereas none of the controls had neutrophil recovery before the eighth day. Seven (29%) patients had neutropenia for 3 or fewer days, and 9 (38%) patients did not experience neutropenic fevers or require broad-spectrum antibiotics. Therefore, ex vivo expanded PBPC are capable of ameliorating posttransplantation neutropenia, thrombocytopenia, and anemia in patients receiving high-dose chemotherapy.
Introduction
Patients receiving high-dose chemotherapy are at risk for serious infections during the neutropenic phase induced by their treatment. Various measures have been evaluated to minimize this risk. The substitution of peripheral blood progenitor cells (PBPC) for bone marrow as a source of hematologic reconstitution has been shown to significantly reduce the duration of neutropenia after intensive chemotherapy.1-4 In addition, doses of CD34+cells equal to or exceeding 5 × 106/kg in the PBPC product can ensure neutrophil recovery times within 7 to 10 days of infusion.5,6 Various stem cell mobilization regimens have been developed to optimize CD34+ cell collection, with the objective of reducing neutropenia after transplantation.7-9 Despite this, high doses of CD34+ cells have limited capability to reduce the neutropenic period because of the time required for the PBPC to differentiate into mature neutrophils in vivo.
Ex vivo culture of hematopoietic progenitor cells has been explored as a way of producing large numbers of more mature cells that may be able to accelerate the rate of recovery after myeloablative therapy. The standard approach to ex vivo expansion has involved the culture of purified CD34+ cells in a variety of cytokine combinations. In the current study, we sought to develop a simple expansion methodology using unselected PBPC and a limited number of hematopoietic growth factors. Recent preclinical experiments from our group10 demonstrated that efficient ex vivo expansion of unselected PBPC into mature myeloid cells could be performed using serum-free media containing granulocyte colony-stimulating factor (GCSF), stem cell factor (SCF), and megakaryocyte growth and development factor (MGDF). We now report the translation of these laboratory studies into an effective method of ex vivo expansion for patients with breast cancer undergoing high-dose chemotherapy and stem cell rescue.
Materials and methods
Study patients
Eligible patients included breast cancer patients at high risk who were undergoing PBPC collection, consisting of cyclophosphamide 1875 mg/m2/d and cisplatin 55 mg/m2/d on days -6 through -3, carmustine 600 mg/m2 on day -2, followed by high-dose chemotherapy and stem cell transplantation. Informed consent using an institutional review board-approved protocol was obtained from all patients before PBPC mobilization was initiated. This study was conducted under an Investigational New Drug issued by the Food and Drug Administration.
Peripheral blood progenitor cell mobilization
Stem cells were mobilized with 30 μg/kg per day recombinant methionyl human GCSF (Filgrastim, Amgen, Thousand Oaks, CA) subcutaneously, alone or in combination with 20 μg/kg per day recombinant methionyl human SCF (r-metHuSCF, Amgen, Thousand Oaks, CA). Leukaphereses with 10-L processing were initiated on the fifth day of growth factor administration and continued daily until target cell collections were achieved. Patient eligibility required that PBPC containing at least 5 × 106 CD34+ cells per kilogram be cryopreserved in addition to the PBPC used for ex vivo expansion.
Peripheral blood progenitor cell expansion
Freshly collected PBPC were enumerated and injected into 1-L Teflon-coated gas-permeable bags (American Fluoroseal, Gaithersburg, MD) containing IMDM-based media with 1% human serum albumin and recombinant human transferrin (Amgen, Thousand Oaks, CA), r-metHuSCF, pegylated recombinant human MGDF, and Filgrastim (all at 100 ng/mL; Amgen). The cells were maintained at 37°C in 5% CO2 in humidified incubators. Six cell dose cohorts, with 4 patients per cohort, were enrolled sequentially. The cohorts differed in the total number of cells cultured and the initial cell densities used: cohort 1, 2 × 109 cells at 1 × 106cells/mL; cohort 2, 4 × 109 cells at 1 × 106 cells/mL; cohort 3, 8 × 109 cells at 2 × 106 cells/mL; cohort 4, 16 × 109cells at 2 × 106 cells/mL; cohort 5, 18 × 109 cells at 3 × 106 cells/mL; and cohort 6, 24 × 109 cells at 3 × 106cells/mL. After 9 days of culture, the cells were collected using a Cobe 2991 centrifuge (BioWhittaker, Walkersville, MD), washed with phosphate-buffered saline (PBS) without calcium or magnesium and resuspended in DPBS at 108 to 109 cells/mL. The percentage of viable myeloid cells was determined by measuring the percentage granulocyte and monocyte fractions using a Coulter counter and multiplying the sum by the fraction of viable myeloid cells over total myeloid cells observed using trypan blue exclusion.
Quality control
Cultured cells were sampled for bacterial and fungal contamination on days 0, 6, and 9 of ex vivo incubation, and a Gram stain of a spun sample was performed on day 9. The washed cell product was cultured for Mycoplasma contamination and analyzed for the presence of endotoxin. Acceptability criteria for the cell products included negative cultures for bacteria and fungi and a negative Gram stain. Cell viability exceeded 80% before products were administered to the patients. Mycoplasma cultures and endotoxin assays were performed on the washed cell products on the day of infusion to monitor for quality control.
Flow cytometry
The CD34+ cells in the PBPC product were enumerated using the ProCOUNT 3-color assay (Becton Dickinson, San Jose, CA) according to manufacturer's instructions. Ex vivo expanded cells were subjected to immunofluorescent staining on day 9 of culture using phycoerythrin-conjugated anti-CD11b, fluorescein isothiocyanate (FITC)-conjugated anti-CD15 (both from Becton Dickinson), and FITC-conjugated anti-CD66b (Serotec, Raleigh, NC). Immunofluorescent staining, data acquisition, and analysis were performed as previously described.10
Administration of the ex vivo expanded cells
The expanded cells were administered at least 4 hours after the cryopreserved PBPC. Patients were premedicated with 650 mg acetaminophen and 25 mg diphenhydramine, both by mouth, 30 minutes before the expanded cells were infused through a wide-open central venous catheter.
Safety monitoring
Vital signs, pulse oximetry, and cardiac rhythm were monitored for 24 hours after the ex vivo expanded cell infusions. In addition, urinalysis was performed on all voided specimens, or once every 4 hours, for 24 hours to monitor for hemoglobinuria. Serum chemistry level was measured before and 24 hours after infusion. All antiemetics administered for 24 hours before and after cell infusions were recorded.
Laboratory evaluations
Complete blood count with manual differential and platelet count were performed daily until recovery of the neutrophil count to more than 1000/μL and the platelet count to more than 20 000/μL without transfusion.
Posttransplantation treatment
Patients received Filgrastim 10 μg/kg per day subcutaneously until recovery of the neutrophil count to more than 3000/μL. Single donor platelets were administered for platelet counts lower than 10 000/μL in afebrile inpatients and lower than 20 000/μL in outpatients or febrile inpatients. Packed red blood cells were transfused for hemoglobin levels lower than 9 g/dL.
Statistical analysis
The fold expansion was calculated by dividing the number of viable myeloid cells harvested on day 9 by the number of CD34+ cells at culture initiation. Myeloid cell yield was determined by dividing the number of viable myeloid cells collected on day 9 by the total number of PBPC added to the culture on day 0. The effect of cell density on stem cell expansion and myeloid cell yield was evaluated using analysis of variance.
Linear regression analysis was used to assess the effect of ex vivo expanded cell (EVE) dose on various outcome measures: Yi = α + β1(EVE dose)i + β2(frozen CD34+ dose)i + εi , where i represents subject 1, 2, · · · 24, Yi is outcome measure for subject i, α is the intercept, β1 and β2 are regression coefficients for EVE dose and frozen CD34+ dose, respectively, and εi is the error term N(0,ς).
The historical control patients consisted of 48 breast cancer patients at high risk who had PBPC collections containing at least 5 × 106 CD34+ cells per kilogram and who were treated consecutively between January 1996 and August 1998 at UCLA Medical Center. All patients underwent PBPC collection using a GCSF-based mobilization regimen, then high-dose chemotherapy with the same regimen as the study patients, and then stem cell rescue with infusion of all of the cryopreserved PBPC. After stem cell infusion, all patients received Filgrastim 10 μg/kg per day subcutaneously until recovery of the neutrophil count to more than 3000/μL. Transfusion practices were the same as for the study patients. Comparisons between the treatment and outcome variables were performed using the Wilcoxon rank sum test.
Results
Effect of cell density on cell expansion and cell yield
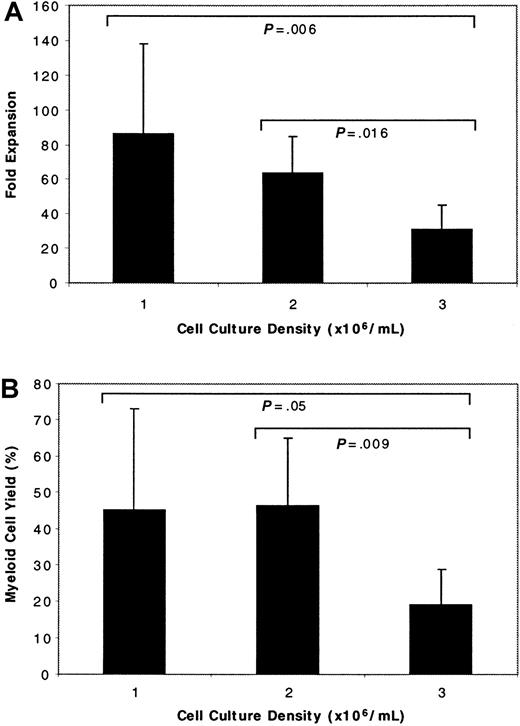
The PBPC were cultured at 1, 2, or 3 × 106cells/mL, using cells from 8 patients at each cell density. The fold expansion was calculated by dividing the number of myeloid cells obtained on day 9 by the number of CD34+ cells initially present in the cultured PBPC. Although the expanded product was derived from unselected PBPC and it was not possible to determine what cell population(s) engendered those present at the time of cell harvest, we previously demonstrated10 that the number of cells obtained using this culture method was proportional to the percentage of CD34+ cells at the start of culture. Therefore, the CD34+ cell population was considered a surrogate for the proliferative pool of cells that generated the myeloid cells present on day 9 of culture. The fold expansion calculated in this way was inversely proportional to the initial PBPC culture density (Figure 1A). There was significantly greater expansion of PBPC when they were seeded at either 1 or 2 × 106 cells/mL than when cultured at 3 × 106 cells/mL (P = .006 for 1 versus 3 × 106 cells/mL; P = .016 for 2 versus 3 × 106 cells/mL). The difference in fold expansion between PBPC cultured at 1 and 2 × 106 cells/mL was not significant (P = .6).
Effect of cell culture density on stem cell expansion and myeloid cell yield.
Unselected PBPC were seeded into cultures at 1 of 3 different cell densities. (A) Fold expansion was calculated by dividing the number of viable myeloid cells obtained after 9 days of culture by the number of CD34+ cells at culture initiation. (B) Myeloid cell yield was determined by dividing the number of viable myeloid cells harvested on day 9 by the total number of PBPC seeded into the cultures. Results are reported as the mean ± SD. Statistics were calculated using analysis of variance.
Effect of cell culture density on stem cell expansion and myeloid cell yield.
Unselected PBPC were seeded into cultures at 1 of 3 different cell densities. (A) Fold expansion was calculated by dividing the number of viable myeloid cells obtained after 9 days of culture by the number of CD34+ cells at culture initiation. (B) Myeloid cell yield was determined by dividing the number of viable myeloid cells harvested on day 9 by the total number of PBPC seeded into the cultures. Results are reported as the mean ± SD. Statistics were calculated using analysis of variance.
Culture outcome also was evaluated by calculating the myeloid cell yield. This was determined by dividing the number of myeloid cells obtained on day 9 of culture by the total number of PBPC added to the culture on day 0. The yield was significantly better when the cells were seeded at 1 or 2 × 106 cells/mL than when they were cultured at 3 × 106 cells/mL (P = .05 andP = .009, respectively). Equivalent yields were obtained for PBPC cultured at either 1 or 2 × 106 cells/mL (Figure 1B). The number of cells harvested on day 9 was always less than the number of PBPC seeded into the cultures because of the dynamics of the culture system. There were many mature cells and cells of limited proliferative capacity in the initial PBPC. By day 9, these cells and their progeny had died, but the cells derived from the more primitive progenitors had not yet increased to the degree that the total cell number in the culture was increased.10 The cells were intentionally harvested at this early time point so that some cell divisions would occur in vivo and refeeding of the cells would not be necessary.
Ex vivo expanded cell immunophenotype
Cells harvested on day 9 contained 32% ± 13% CD15+CD11b− cells and 16% ± 2% CD15+CD11b+ cells by flow cytometry (representative histogram, Figure 2). The initial PBPC culture density did not affect the immunophenotypes of the cell products.
Immunophenotype of ex vivo expanded cells.
PBPC were subjected to immunofluorescent staining on days 0 and 9 of culture using FITC-conjugated anti-CD15 and phycoerythrin-conjugated anti-CD11b, or isotype controls, then analyzed by flow cytometry.
Immunophenotype of ex vivo expanded cells.
PBPC were subjected to immunofluorescent staining on days 0 and 9 of culture using FITC-conjugated anti-CD15 and phycoerythrin-conjugated anti-CD11b, or isotype controls, then analyzed by flow cytometry.
Safety of expanded cell administration
Expanded cells were consistently negative for bacterial and fungal growth, both by culture and Gram stain. Mycoplasma spp were not cultured from any of the cell products. Endotoxin levels were lower than 1 ng/mL in all cell products. Infusion of the expanded cells was not associated with the development of abnormal vital signs, decreased oxygenation, or any symptoms reported by the patients. Serum chemistry monitoring and urinalysis did not detect any untoward effect of the expanded cells.
Effect of ex vivo expanded cells on neutropenia and fevers
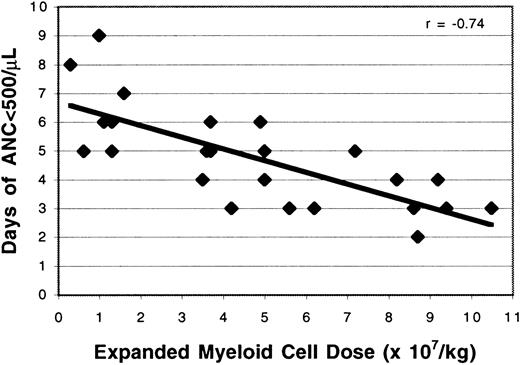
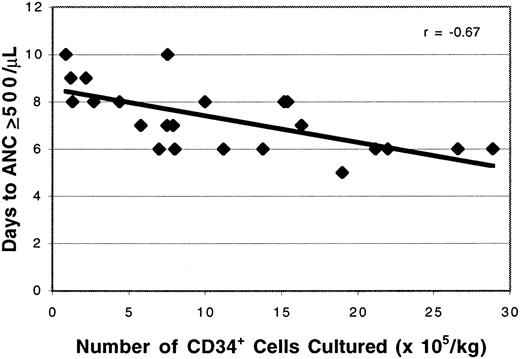
Study patients receiving the cultured PBPC had significantly earlier neutrophil recovery than control patients with breast cancer (7.3 days versus 8.8 days; P = .0001; Table1), even though the control patients received significantly fewer prior courses of chemotherapy (4.0 versus 5.7; P = .01) and significantly higher doses of cryopreserved CD34+ cells than the study patients (11.2 versus 8.4 × 106/kg; P = .01), respectively. There was a strong correlation between the dose of viable myeloid cells administered in the expanded product and the time to neutrophil recovery (r = −0.80; Figure3). A similar relation was observed between the expanded myeloid cell dose and the duration of neutropenia (r = −0.74; Figure 4). After controlling for the dose of cryopreserved CD34+ PBPC, the dose of ex vivo expanded cells had a significant effect on the time to neutrophil recovery (P = .0001) and the number of neutropenic days (P = .0001). The number of CD34+ cells per kilogram added to the cultures also was correlated with the time to neutrophil recovery (r = .67; Figure5) and the number of neutropenic days (r = .62). There was a significant relation between the number of CD34+ cells per kilogram at the start of culture and the time to neutrophil recovery (P = .0003) and the number of neutropenic days (P = .0015). However, linear regression with a stepwise procedure for variable selection revealed that the expanded cell dose, but not the number of CD34+ cells per kilogram cultured on day 0, had a significant effect on neutrophil recovery. In addition, there was no correlation between the number of cryopreserved CD34+ cells infused and the time to neutrophil recovery (r = .0002). The cryopreserved CD34+PBPC dose did not significantly impact the number of neutropenic days or the time to neutrophil recovery (P = .19 for each). Although the maturing myeloid cell populations in the cultured cell products were expected to contribute to early neutrophil recovery, the time to an absolute neutrophil count of more than 500/μL was weakly correlated with the number of CD15+CD11b−cells per kilogram (r = −0.41) or the CD15+CD11b+ cells per kilogram (r = 0.13) infused.
Outcomes of study and control patients
| Variables . | Study . | Control . | P . |
|---|---|---|---|
| No. patients | 24 | 48 | |
| Prior chemotherapy cycles | 5.7 (2.9) | 4.0 (2.1) | .010 |
| CD34+ cells infused | 8.4 (5.3) | 11.2 (7.9) | .010 |
| Days to ANC > 500/μL | 7.3 (1.4) | 8.8 (0.7) | .0001 |
| Days to platelet recovery | 9.3 (1.2) | 10.3 (1.6) | .010 |
| Platelet units transfused | 2.7 (2.1) | 2.8 (1.6) | .25 |
| Red cell units transfused | 3.5 (2.0) | 4.2 (1.4) | .02 |
| Days of hospitalization | 15.4 (2.6) | 16.9 (2.1) | .0005 |
| Variables . | Study . | Control . | P . |
|---|---|---|---|
| No. patients | 24 | 48 | |
| Prior chemotherapy cycles | 5.7 (2.9) | 4.0 (2.1) | .010 |
| CD34+ cells infused | 8.4 (5.3) | 11.2 (7.9) | .010 |
| Days to ANC > 500/μL | 7.3 (1.4) | 8.8 (0.7) | .0001 |
| Days to platelet recovery | 9.3 (1.2) | 10.3 (1.6) | .010 |
| Platelet units transfused | 2.7 (2.1) | 2.8 (1.6) | .25 |
| Red cell units transfused | 3.5 (2.0) | 4.2 (1.4) | .02 |
| Days of hospitalization | 15.4 (2.6) | 16.9 (2.1) | .0005 |
Values are mean (± SD).
Probability determined by the Wilcoxon rank sum test.
ANC, absolute neutrophil count.
Effect of expanded cell dose on the time to neutrophil recovery.
The number of ex vivo expanded cells administered was correlated with the time to neutrophil recovery after cell infusion. A strong linear relationship between these variables is suggested by the correlation coefficient, r = −0.80.
Effect of expanded cell dose on the time to neutrophil recovery.
The number of ex vivo expanded cells administered was correlated with the time to neutrophil recovery after cell infusion. A strong linear relationship between these variables is suggested by the correlation coefficient, r = −0.80.
Effect of expanded cell dose on the duration of neutropenia.
The number of ex vivo expanded cells infused was correlated with the duration of neutropenia (r = −0.74).
Effect of expanded cell dose on the duration of neutropenia.
The number of ex vivo expanded cells infused was correlated with the duration of neutropenia (r = −0.74).
Effect of CD34+ cell number on the time to neutrophil recovery.
The day of neutrophil recovery was correlated with the CD34+ cell content of the PBPC seeded into culture (r = −0.67).
Effect of CD34+ cell number on the time to neutrophil recovery.
The day of neutrophil recovery was correlated with the CD34+ cell content of the PBPC seeded into culture (r = −0.67).
In 9 of the 24 (38%) patients, neutropenic fevers did not develop, and they did not require broad-spectrum antibiotics. However, the requirement for antibiotics was not statistically associated with the dose of expanded cells administered.
Effect of ex vivo expanded cells on peripheral blood progenitor cell and platelet transfusions
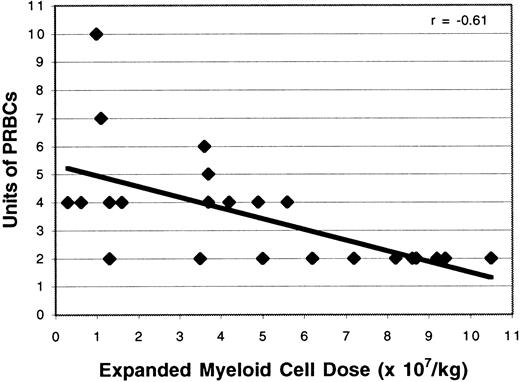
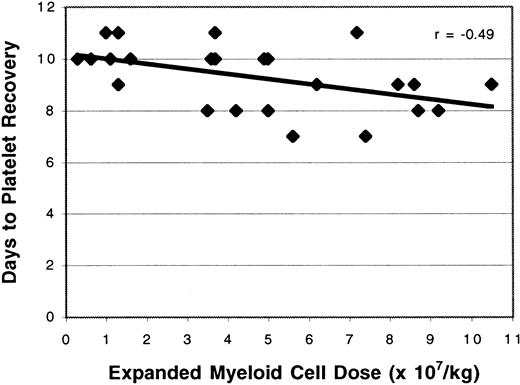
Study patients required significantly fewer PRBC transfusions than the control population (3.5 versus 4.2 units; P = .02; Table 1). The number of PRBC transfusions was correlated with the dose of expanded PBPC administered (r = −0.61; Figure6); this relation was statistically significant (P = .002). Time to platelet recovery was defined as the number of days after transplantation required to achieve a platelet count of at least 20 000/μL for 2 days after the last platelet transfusion. Study patients had a significantly shorter time to platelet recovery than historical controls (9.3 versus 10.3 days,P = .01). The ex vivo expanded cell dose and the duration of thrombocytopenia were related (r = −0.49; Figure7). Although the time to platelet recovery was significantly associated with the expanded cell dose (P = .002), the number of platelet units transfused was not related (P = .3).
Effect of expanded cell dose on the number of red cell transfusions.
The number of ex vivo expanded cells given was correlated with the number of red blood cell units transfused (r = −0.61).
Effect of expanded cell dose on the number of red cell transfusions.
The number of ex vivo expanded cells given was correlated with the number of red blood cell units transfused (r = −0.61).
Effect of expanded cell dose on the time to platelet recovery.
The number of ex vivo expanded cells administered was correlated with the time to platelet recovery after cell infusion (r = −0.49).
Effect of expanded cell dose on the time to platelet recovery.
The number of ex vivo expanded cells administered was correlated with the time to platelet recovery after cell infusion (r = −0.49).
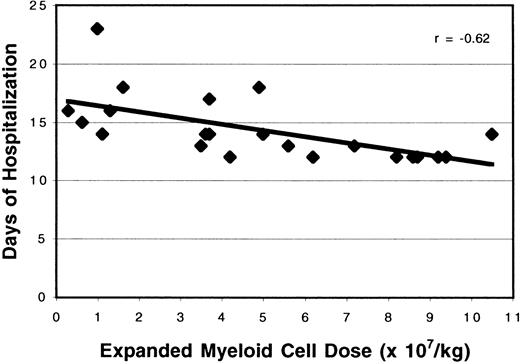
Effect of expanded cells on the duration of hospitalization
Study patients had significantly shorter hospital stays than previously treated patients (15.4 versus 16.9 days;P = .0005). Lengths of hospital stay were correlated with the expanded cell doses infused (Figure8; r = −0.62).
Effect of expanded cell dose on the duration of hospitalization.
The number of ex vivo expanded cells administered was associated with the length of hospital stay (r = −0.62).
Effect of expanded cell dose on the duration of hospitalization.
The number of ex vivo expanded cells administered was associated with the length of hospital stay (r = −0.62).
Discussion
This study demonstrated that ex vivo expanded PBPC reduced posttransplantation neutropenia in a dose-dependent fashion. Eight of 24 patients experienced neutrophil recovery on day 6 after infusion, and one recovered on day 5. This rate of neutrophil recovery has not been previously observed in 418 patients with breast cancer undergoing standard high-dose therapy and PBPC transplantation.7,11 In a recent multicenter study of 203 patients with breast cancer who underwent the same conditioning regimen and the supportive care measures used in this study, the time to neutrophil recovery was 9 or 10 days, and the range was 8 to 15 days.7 A control population of 48 patients with breast cancer who underwent high-dose chemotherapy and infusion of PBPC containing 5 × 106 or more CD34+ cells per kilogram at UCLA between January 1996 and August 1998 had a mean time to neutrophil recovery of 8.8 days, with a range of 8 to 10 days. Because the duration of neutropenia usually dictates the duration of hospitalization, the study patients also had significantly shorter hospital stays than the controls.
Early trials of ex vivo expanded hematopoietic progenitor cells did not demonstrate any measurable clinical effect of the expanded cells on the duration of posttransplantation neutropenia. The failure to achieve an effect on neutrophil recovery in those studies may have been multifactorial. Brugger et al12 gave 4 to 23 × 106 expanded cells per kilogram, which were cell doses below those that had a definite effect in our patient cohort.12 Alcorn et al13 also administered relatively low doses of expanded cells, up to 7.7 × 109total cells, without clinical benefit. Williams et al14administered 0.8 to 157 × 106 expanded cells per kilogram, and 3 patients received more than 40 × 106expanded cells per kilogram, which were cell doses effective in our patients. Nevertheless, no benefit of the expanded cells was apparent. The ex vivo culture duration in that study was 12 days, however, in contrast to our 9-day culture period. We chose to culture the PBPC for 9 days with the objective of optimizing the numbers of cells in the myelocyte and promyelocyte stages (ie, cells capable of additional proliferation after infusion). It is possible that more prolonged culture periods may deplete the expanded products of these cells and necessitate the administration of larger cell doses to have an equivalent effect. Two recent studies have shown that ex vivo expanded cells produced by culturing CD34-selected PBPC are capable of reducing the time to neutrophil recovery in patients with breast cancer or multiple myeloma.15 16 Neither trial reported an effect on transfusions or platelet recovery. Our study is the first to show that post-transplantation cytopenias can be ameliorated by administering PBPC cultured without prior CD34 selection.
The objective of this study was to develop a simple and potentially cost-effective approach of ex vivo expansion. As a result, the PBPC were not subjected to CD34 selection before expansion, and the cultures were performed using a limited number of hematopoietic growth factors. We previously demonstrated that ex vivo expansion of unselected PBPC could be performed efficiently in serum-free media containing GCSF, MGDF, and SCF.10 There are several potential advantages to using unselected PBPC instead of CD34-selected progenitor cells in ex vivo expansion cultures. The process of CD34 selection is costly and time consuming. Because approximately 50% of the CD34+cells are lost during this processing, additional apheresis procedures must be performed to obtain enough cells for expansion. The CD34 selection process also removes many myeloid progenitor cells that could potentially contribute to the yield of the expansion procedure. The removal of lymphoid cells during selection also eliminates a potential source of hematopoietic growth factors not added to the culture system. Therefore, the use of unselected PBPC rather than CD34-selected progenitors in expansion cultures has several theoretical advantages that may make the latter approach more economically viable.
An unexpected finding in this trial was the reduction in red blood cell transfusions associated with increasing doses of ex vivo expanded cells. Preclinical data previously demonstrated that colony-forming units–erythroid increased 1.5-fold during the first 4 to 7 days of expansion culture.10 The reduction in red cell transfusions observed in this trial suggests that modestly increased numbers of erythroid progenitors in ex vivo cultured PBPC may be capable of inducing a clinical benefit. In addition, a dose-dependent reduction in the duration of thrombocytopenia was observed with the infusion of increasing numbers of expanded cells. The mean duration of thrombocytopenia in patients receiving more than 4.5 × 107 expanded cells per kilogram was 8.8 days compared to 9.8 days in patients receiving less than 4.5 × 107 cells per kilogram and 10.3 days in the historical control patients. Therefore, ex vivo expanded cells prepared using SCF, MGDF, and GCSF had a clinical impact on all 3 hematopoietic lineages.
In conclusion, this trial demonstrated that a simple PBPC expansion procedure could significantly reduce neutropenia, thrombocytopenia, red blood cell transfusions, and duration of hospital stay in patients undergoing high-dose chemotherapy and stem cell rescue. This therapy has the potential to decrease morbidity, mortality, and costs associated with high-dose chemotherapy.
Acknowledgments
We thank Robert Briddell for his technical advice and assistance, David Woo for his regulatory help, and Brian Koziol for his review of the manuscript.
Supported by a grant from Amgen Inc and by the Perry Cohan Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ronald L. Paquette, Department of Medicine, Division of Hematology/Oncology, 42-121 Center for the Health Sciences, University of California at Los Angeles, 10833 Le Conte Ave, Los Angeles, CA 90095-1678; e-mail: paquette@ucla.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal