Abstract
The regulation of CCR6 (chemokine receptor 6) expression during B-cell ontogeny and antigen-driven B-cell differentiation was analyzed. None of the CD34+Lin− hematopoietic stem cell progenitors or the CD34+CD19+ (pro-B) or the CD19+CD10+ (pre-B/immature B cells) B-cell progenitors expressed CCR6. CCR6 is acquired when CD10 is lost and B-cell progeny matures, entering into the surface immunoglobulin D+ (sIgD+) mature B-cell pool. CCR6 is expressed by all bone marrow–, umbilical cord blood–, and peripheral blood–derived naive and/or memory B cells but is absent from germinal center (GC) B cells of secondary lymphoid organs. CCR6 is down-regulated after B-cell antigen receptor triggering and remains absent during differentiation into immunoglobulin-secreting plasma cells, whereas it is reacquired at the stage of post-GC memory B cells. Thus, within the B-cell compartment, CCR6 expression is restricted to functionally mature cells capable of responding to antigen challenge. In transmigration chemotactic assays, macrophage inflammatory protein (MIP)-3α/CC chemokine ligand 20 (CCL20) induced vigorous migration of B cells with differential chemotactic preference toward sIgD− memory B cells. These data suggest that restricted patterns of CCR6 expression and MIP-3α/CCL20 responsiveness are integral parts of the process of B-lineage maturation and antigen-driven B-cell differentiation.
Introduction
Accumulating data implicate chemokines and chemokine receptors in B-lineage maturation,1-4 B-cell zone architecture, and the antigen (Ag)-driven B-cell response within peripheral lymphoid tissue.5 6 However, the physiologic role of CCR6 (chemokine receptor 6) and its natural ligand, macrophage inflammatory protein (MIP)-3α/CC chemokine ligand 20 (CCL20), in these phenomena is currently unknown. Furthermore, their expression patterns have not been completely elucidated. In this study, we addressed these issues and analyzed the regulation of CCR6 expression and MIP-3α/CCL20 responsiveness during B-lineage maturation in bone marrow (BM) and Ag-driven differentiation into effector plasma cells and memory B cells in the periphery.
Sequence similarities suggest that CCR6, CCR7, CCR9, and the orphan receptor Bonzo/STRL33/TYMSTR form a separate branch of the CC chemokine receptor family. They are coupled to the Gαi class of pertussis toxin-sensitive α subunits of G proteins, share a restricted pattern of expression in vivo, and display no ligand-binding promiscuity.7 CCR6 messenger RNA (mRNA) is limited to lymphoid tissues, fetal liver, testis, small intestine, and peripheral blood mononuclear cells (PBMCs).8 Within PBMCs, CCR6 expression has been found in B and T lymphocytes, but granulocytes, monocytes, eosinophils, and natural killer cells are negative. In the T-cell population, CCR6 is restricted mainly to memory CD4+subsets expressing α4β7 integrin, the intestinal lymphocyte homing receptor.9 CCR6 is also expressed on immature dendritic cells but is lost during their maturation.10 CCR6 appears to be highly selective for a single chemokine, MIP-3α/CCL20/Exodus-1/LARC. However, other natural ligands of CCR6, particularly antimicrobial peptides secreted by intestinal epithelial cells, β-defensins, have been recently described.11 The MIP-3α/CCL20 gene (SCYA20) has been identified by computer search using expressed sequence tags from different complementary DNA libraries available in GenBank, the European Molecular Biology Laboratory, and DNA Databank of Japan public sequence databases.12-14 The N-terminal amino acid sequence of MIP-3α/CCL20 is similar to those of the Exodus family: SLC/Exodus-2/C6-kine/TCA4 and MIP-3β/Exodus-3/CKβ11. Like its receptor, MIP-3α/CCL20 has a restricted pattern of expression in vivo. As assessed by Northern blot analysis, MIP-3α/CCL20 mRNA is present mostly in the appendix, thymus, lymph nodes, tonsils, PBMCs, fetal liver, and lung but is absent from spleen and BM.8,12,15 MIP-3α/CCL20 is constitutively expressed by keratinocytes in the basal and suprabasal layers of the epidermis and venular endothelial cells in skin.16 It is likely that the MIP-3α/CCL20-CCR6 pair is involved in transendothelial migration and constitutive trafficking of Langerhans' cell precursors into the epidermis. MIP-3α/CCL20 gene expression in PBMCs is strongly induced by inflammatory stimuli such as tumor necrosis factor (TNF)-α, lipopolysaccharide, and phorbol 12-myristate 13-acetate. MIP-3α/CCL20 is a selective chemotactic factor for lymphocytes and a negative regulator of normal and chronic myelogenous leukemia myeloid progenitors in colony formation assays.12,17 Poorly expressed in the absence of inflammatory stimuli, MIP-3α/CCL20 mRNA was found to be abundant in inflamed epithelial crypts of palatine tonsils and intestinal epithelial cells, especially those lying immediately over Peyer's patches and in other mucosal lymphoid structures, including the follicle-associated epithelium.18
Here, we report that CCR6 expression is restricted to naive and memory B cells and is down-regulated mainly by engagement of the B-cell Ag receptor. We also demonstrate that MIP-3α/CCL20 is an efficient B-cell chemoattractant with a differential preference toward memory B cells.
Materials and methods
Flow cytometric analysis
Cell surface Ags were analyzed by single-parameter or multiparameter fluorescence-activated cell sorter (FACS) analysis using the following monoclonal antibodies (MoAbs): anti-CD14-fluorescein isothiocyanate (FITC), anti-CD44-FITC (both from Diaclone, Besançon, France), anti-IgD-phycoerythrin (PE; PharMingen, San Diego, CA), anti-CD10-FITC, anti-CD34-FITC, anti-CD38-PE, anti-CD19-PE-cyanin 5 (PECy5) (all from Becton Dickinson, Mountain View, CA), and anti-CD20-FITC (Immunotech, Marseille, France). CXCR4 expression was detected by indirect immunofluorescence using anti-CXCR4 MoAb (NIBSC, Potters Bar, England), and DTAF-conjugated goat antimouse immunoglobulin (Ig) G (H+L) F(ab′)2 (Immunotech). Anti-CCR6-PE and anti-CXCR5-PE MoAbs were purchased from R & D Systems (Abingdon, England). Mouse isotype-matched FITC-, PE-, or PECy5-conjugated control IgG1 and IgG2a were purchased from Becton Dickinson. Uncoupled control mouse Igs were purchased from ICN (Costa Mesa, CA). A FACScan flow cytometer with CellQuest software (Becton Dickinson) was used for data acquisition and analysis. After gating on viable cells, 5000 events per sample were analyzed. For each marker, the threshold of positivity was defined beyond the nonspecific binding observed in the presence of relevant control IgG.
Cell preparation
B-cell–enriched populations were obtained from palatine tonsils as previously described.19 Briefly, after one cycle of rosette formation and depletion of residual T cells with CD2 magnetic beads (Dynabeads M-450, Dynal AS, Oslo, Norway), the resulting B-cell populations consistently contained 95% or more CD19+ B cells, 1% or fewer CD14+ monocytes, and 1% or fewer CD3+ T cells and DRC1+ dendritic cells. For some experiments, total tonsillar B cells were separated into surface (s)IgD+ and sIgD− populations by incubating for 30 minutes with saturating amounts of anti-IgD MoAb (TA4-1) and subsequent removal of IgDhigh cells from the cell suspension using goat antimouse IgG-conjugated magnetic beads (Dynal). Surface IgD− B cells were further separated into CD44high and CD44low/− B cells using a similar protocol and saturating amounts of anti-CD44 MoAb (BF24, Diaclone). All of the purification procedures were carried out at 4°C to prevent spontaneous apoptosis. As assessed by flow cytometry, naive B cells were 96% ± 4% CD19+, 83% ± 4% sIgD+, 97% ± 2% CD44+, and 7% ± 3% CD38+; memory B cells were 92% ± 6% CD19+, 20% ± 6% sIgD+, 73% ± 17% CD44+, and 10% ± 10% CD38+; and germinal center (GC) B cells were 94% ± 3% CD19+, 11% ± 4% sIgD+, 11% ± 10% CD44+, and 90% ± 2% CD38+ (n = 5). These results are consistent with previously published data on B-cell subpopulations and separation.20 21 The viability of these cell fractions was consistently higher than 90%.
BM cells, obtained from normal adult donors after informed consent, were collected in heparin-containing medium. Umbilical cord blood samples from normal full-term newborn infants were obtained from a cord blood bank. Low-density mononuclear cells (MNCs) were prepared by centrifugation on Ficoll density gradient (Nyegaard, Oslo, Norway). BM- and umbilical cord blood–derived CD34+hematopoietic stem cell progenitors were isolated as previously described using a magnetic cell sorting system (miniMACS; Miltenyi Biotech GmbH, Bergisch Gladbach, Germany).22 The purity of CD34+ cells recovered was more than 90% as determined by flow cytometry using anti-CD34 (PE-HPCA2, Becton Dickinson) MoAb staining. PBMCs were isolated from heparinized blood of voluntary donors by Ficoll density centrifugation. The viability of these cell fractions was consistently higher than 90%.
Cell cultures
For in vitro culture assays, cells were cultured in RPMI 1640 medium (Gibco BRL, Paisley, Scotland) containing 10 mmol/L HEPES, 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L sodium pyruvate, and 10% heat-inactivated fetal calf serum (complete medium [CM]). B cells (1 × 106 cells/mL) were activated by incubation in CM for 2 days, unless otherwise indicated, with polyclonal anti-IgM Ab coupled to beads (5 μg/mL; Irvine Scientific, Santa Anna, CA), anti-CD40 MoAb (G28.5, 1 μg/mL), interleukin (IL)-4 (20 ng/mL; Schering Plough, Kenilworth, NJ), or a combination of these. The concentration of endotoxin in the culture medium and in the reagents used was consistently below 1 ng/mL.
In vitro plasma cell differentiation of GC B cells
sIgD−CD44− GC B cells (> 90% CD38+CD20+) were purified by immunomagnetic bead cell sorting. Freshly isolated GC B cells were seeded in 6-well plates (Costar, Cambridge, MA) at 106cells/mL in CM supplemented with IL-10 (50 ng/mL), IL-2 (20 UI/mL), IL-4 (20 ng/mL), and anti-CD40 MoAb (1 μg/mL) and were incubated for 9 days. Cultures were fed every 3 days with fresh CM supplemented with cytokines and anti-CD40 MoAb. On days 3, 6, and 9, cells were harvested, washed twice, and stained with anti-CD20-FITC and anti-CD38-PE or anti-CCR6-PE MoAbs. Double-color analysis of the cell surface phenotype was then performed by flow cytometry. As previously described,23 in vitro differentiated plasmablasts were CD38 highCD20−.
Filamentous-actin polymerization assay
Intracellular filamentous (F)-actin polymerization was tested as previously described.22 Briefly, B cells (8 × 106/mL) were incubated in HEPES-buffered RPMI 1640 medium at 37°C with or without MIP-3α/CCL20 (500 ng/mL). After the indicated times, cells (100 μL) were added to 400 μL of the assay buffer containing 4 × 10−7 mol/L FITC-labeled phalloidin, 0.5 mg/mL L-α-lysophosphatidylcholine (both from Sigma, St Louis, MO), and 4.5% formaldehyde in phosphate-buffered saline. Fixed cells were then analyzed by FACS, and mean fluorescence intensity (MFI) was determined for each sample. The percentage MFI modulation was calculated for each sample at each time point as follows: [1 − (MFI before addition of chemokine/MFI after addition of chemokine)] × 100. For double-staining experiments, cells were incubated for 20 minutes at 4°C with PE-labeled anti-IgD MoAb prior to F-actin polymerization assay.
In vitro chemotaxis assay
MIP-3α/CCL20-dependent chemotaxis of B cells was measured by an in vitro 2-chamber migration assay followed by flow cytometry. Assays were performed in serum-free conditions using Iscove's modified Dulbecco's medium supplemented with 1.5% bovine serum albumin (Cohn's fraction V, Sigma), sonicated lipids, and iron-saturated human transferrin (migration buffer).22MIP-3α/CCL20 (500 ng/mL) in migration buffer or buffer alone was added to the lower chamber, and 100 μL of cells suspended in migration buffer was added to the upper chamber of Costar Transwells (6.5-mm diameter, 5-μm pore size, polycarbonate membrane, Costar). A total of 2 × 105 B cells was added to the upper chamber of the Transwell system and allowed to transmigrate for 3 hours at 37°C. Input cells and transmigrated cells in the lower chamber were stained with FITC-labeled anti-CD19 and PE-labeled anti-IgD MoAbs and counted by FACScan (Becton Dickinson) for 60 seconds. Events were analyzed separately within gated sIgDhigh and sIgD− populations of B cells. The results are expressed as the percentage of the input B cells that migrated to the lower chamber.
Statistical analysis
Data are expressed as the mean ± SEM. Differences between groups were assessed using the unpaired Student t test, andP values <.05 were considered significant.
Results
During B-cell ontogeny, CCR6 is acquired at the stage of mature CD19+CD10−sIgD+ B cells
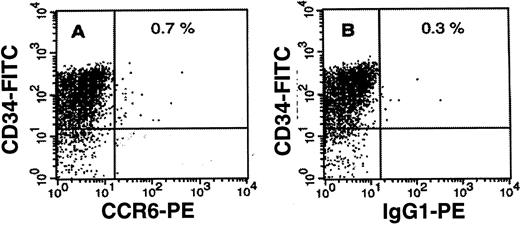
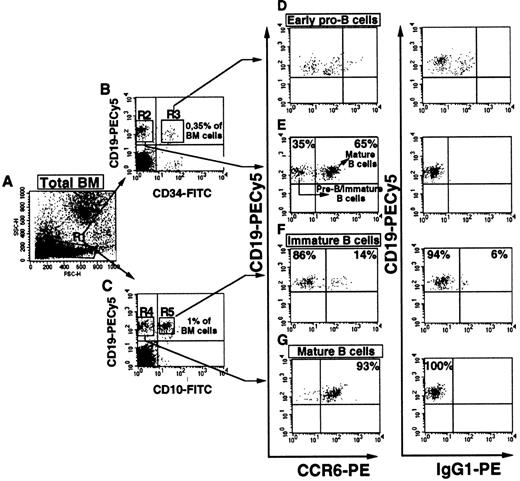
We first analyzed CCR6 expression on CD34+hematopoietic stem cell progenitors. Enriched (≥ 90% purity) BM CD34+ progenitors did not express CCR6 at the cell surface (Figure 1). We used 3-parameter FACS analysis to assess CCR6 expression at the different stages of B lymphopoiesis in BM defined by the coexpression of CD34, CD19, and CD10. Dot plots of 1 representative BM sample of 5 analyzed (Figure2) showed that the CD34+CD19+ pro–B-cell fraction was 0.35% of the BM MNCs (Figure 2B, R3 gate). This subset of B-cell progenitors completely lacked CCR6 expression (Figure 2D), whereas most of the cells did express CXCR4 (not shown). In contrast, CD19+CD34− B-lineage progeny (Figure 2B, R2 gate) contained 2 distinct populations in respect to CCR6 expression: two thirds of the cells were CCR6+, and the others were CCR6− (Figure 2E). We next analyzed CCR6 expression on CD19+CD10+ cells, the population that included most of the pre-B subsets and immature B cells. This B-cell fraction constituted about 1% of the BM MNCs in this donor (Figure 2C, R5 gate). Flow cytometry detected no CCR6+ cells in the CD10-bearing fraction of BM B cells (Figure 2F), whereas most (> 93% in this example) CD19+CD10− BM B cells were CCR6+ (Figure 2G). Most of these CD19+CD10− cells coexpressed sIgD, arguing for their mature B-cell status (not shown). In 3 independent experiments, an average of 1.52% ± 0.16% BM cells were CD19+CCR6+, which represented 63.7% ± 24.7% of all CD19+ BM cells. A similar pattern of CCR6 expression was observed in cord blood–derived populations (not shown). Therefore, CCR6 acquisition during B-cell lymphopoiesis appears to be synchronized with the entry into the mature B-cell pool (CD34−, CD19+, CD10−, sIgDhigh). In agreement with these results, none of the CD10-expressing pro-B (REH, 207) and pre-B (BV173, OB5, Nalm-6) cell lines tested expressed CCR6 (data not shown).
CCR6 expression on CD34+ BM-derived hematopoietic stem cells.
Enriched (> 90% purity) CD34+ BM-derived hematopoietic stem cells were obtained as described in “Materials and methods.” Cells expressing CCR6 and/or CD34 were identified by 2-parameter immunofluorescence analysis using PE-labeled anti-CCR6 (A) or control IgG1 (B) MoAbs and FITC-labeled CD34 MoAb. Percentages of double-positive cells are indicated. One representative donor of the 5 tested is shown.
CCR6 expression on CD34+ BM-derived hematopoietic stem cells.
Enriched (> 90% purity) CD34+ BM-derived hematopoietic stem cells were obtained as described in “Materials and methods.” Cells expressing CCR6 and/or CD34 were identified by 2-parameter immunofluorescence analysis using PE-labeled anti-CCR6 (A) or control IgG1 (B) MoAbs and FITC-labeled CD34 MoAb. Percentages of double-positive cells are indicated. One representative donor of the 5 tested is shown.
CCR6 expression is acquired during the transition to mature CD19+CD10− sIgD+ B-cell status and is absent during early and late stages of B lymphopoiesis in BM.
(A) Lymphoid gating of normal adult BM cells (> 90% cell viability) was based on forward and side scatter characteristics of the lymphocytes (R1). Three-parameter immunofluorescence analysis was used to detect CCR6 expression within CD19+ populations in this gate. (B) Plots of CD19 versus CD34 were used to gate dual-positive pro-B (R3) and CD34−CD19+ cells (R2), a population that includes immature and mature B cells. (C) Plots of CD19 versus CD10 were used to discriminate between dual-positive immature B cells (R5) and CD19+CD10− mature B cells (R4). These gated cells were then plotted for CD19 versus CCR6 (D-G). Data with PE-labeled anti-CCR6 and isotype-matched IgG1 control MoAbs are shown. For each population, the threshold of positivity was placed according to the nonspecific binding of control IgG. Populations corresponding to early pro-B and immature B cells and their respective percentage values within total BM cells are given in panels B and C, respectively. One representative example of 5 samples from different BM donors is shown.
CCR6 expression is acquired during the transition to mature CD19+CD10− sIgD+ B-cell status and is absent during early and late stages of B lymphopoiesis in BM.
(A) Lymphoid gating of normal adult BM cells (> 90% cell viability) was based on forward and side scatter characteristics of the lymphocytes (R1). Three-parameter immunofluorescence analysis was used to detect CCR6 expression within CD19+ populations in this gate. (B) Plots of CD19 versus CD34 were used to gate dual-positive pro-B (R3) and CD34−CD19+ cells (R2), a population that includes immature and mature B cells. (C) Plots of CD19 versus CD10 were used to discriminate between dual-positive immature B cells (R5) and CD19+CD10− mature B cells (R4). These gated cells were then plotted for CD19 versus CCR6 (D-G). Data with PE-labeled anti-CCR6 and isotype-matched IgG1 control MoAbs are shown. For each population, the threshold of positivity was placed according to the nonspecific binding of control IgG. Populations corresponding to early pro-B and immature B cells and their respective percentage values within total BM cells are given in panels B and C, respectively. One representative example of 5 samples from different BM donors is shown.
CCR6 is expressed by all peripheral blood–derived B cells and B-cell subpopulations within peripheral lymphoid organs
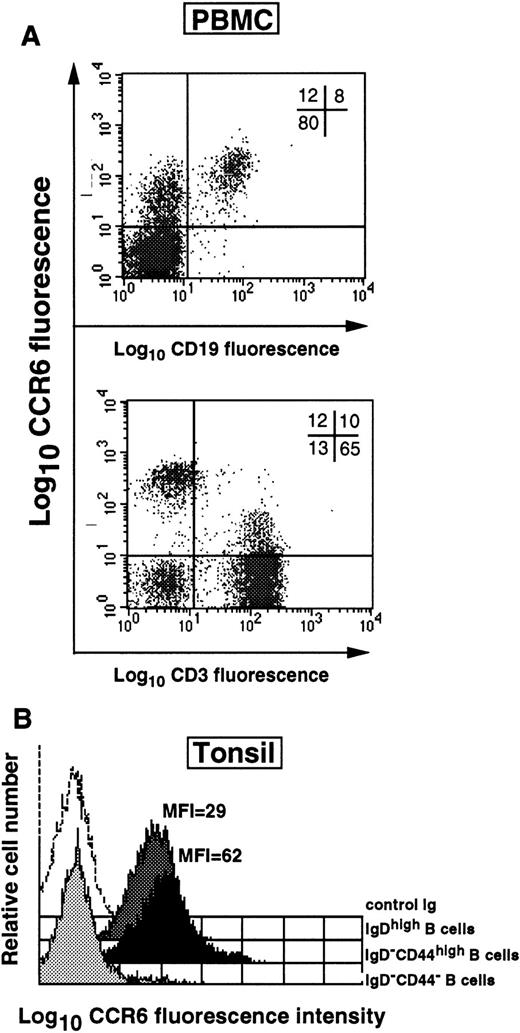
As in the case of BM-derived mature B cells, all CD19+peripheral blood–derived B cells (8% of all the MNCs, Figure3A) coexpressed CCR6. In addition, a subpopulation of CD3+CCR6+ T cells was detected in PBMCs (about 10% of all of the MNCs and 13% of CD3+ T cells, Figure 3A). Interestingly, within peripheral lymphoid organs such as spleen and palatine tonsil, there were 2 distinct B-cell populations: CCR6+ (67% ± 11%, n = 5) and CCR6− (33% ± 11%, n = 5) (data not shown). CCR6 was clearly detected in naive (sIgDhigh) and memory (sIgD−CD44high) B cells but was totally absent from GC (sIgD−CD44−) B cells, which result from oligoclonal expansion of Ag-specific B cells in vivo (Figure 3B). Furthermore, the MFI values for CCR6 expression by memory B cells was 2-fold higher than that for naive cells (94% CCR6-expressing cells, MFI = 62, vs 97%, MFI = 29, respectively). All subsets of tonsillar B cells, including GC B cells, contained CCR6 mRNA with the largest amounts in memory B cells (not shown). These data show that CCR6 was constitutively present in naive B cells, upregulated in memory B cells, but lost during Ag-driven oligoclonal B-cell expansion in GC. In agreement with the lack of CCR6 expression on primary GC B cells, none of the 6 GC-origin, Burkitt lymphoma cell lines tested were CCR6+ (not shown).
Naive, memory, and GC B cells within secondary lymphoid tissue differ in their CCR6 expression.
(A) CCR6 expression was studied within PBMC populations by 2-parameter immunofluorescence analysis using PE-labeled CCR6 MoAb and FITC-labeled CD19 or CD3 MoAbs. (B) Freshly isolated tonsillar B cells were separated according to the expression of distinctive immunophenotypic markers into sIgDhigh (naive), sIgD−CD44high (memory), and sIgD−CD44− GC B cells, as described in “Material and methods.” CCR6 expression was then assessed by FACS analysis using anti-CCR6-PE MoAb. MFI values for CCR6 staining for individual samples are indicated. Open histograms represent staining with IgG1 control MoAb. One representative donor of 4 is shown.
Naive, memory, and GC B cells within secondary lymphoid tissue differ in their CCR6 expression.
(A) CCR6 expression was studied within PBMC populations by 2-parameter immunofluorescence analysis using PE-labeled CCR6 MoAb and FITC-labeled CD19 or CD3 MoAbs. (B) Freshly isolated tonsillar B cells were separated according to the expression of distinctive immunophenotypic markers into sIgDhigh (naive), sIgD−CD44high (memory), and sIgD−CD44− GC B cells, as described in “Material and methods.” CCR6 expression was then assessed by FACS analysis using anti-CCR6-PE MoAb. MFI values for CCR6 staining for individual samples are indicated. Open histograms represent staining with IgG1 control MoAb. One representative donor of 4 is shown.
sIgD− memory B-cell subset but not sIgDhigh naive B cells respond in vitro to MIP-3α/CCL20 by actin cytoskeleton reorganization
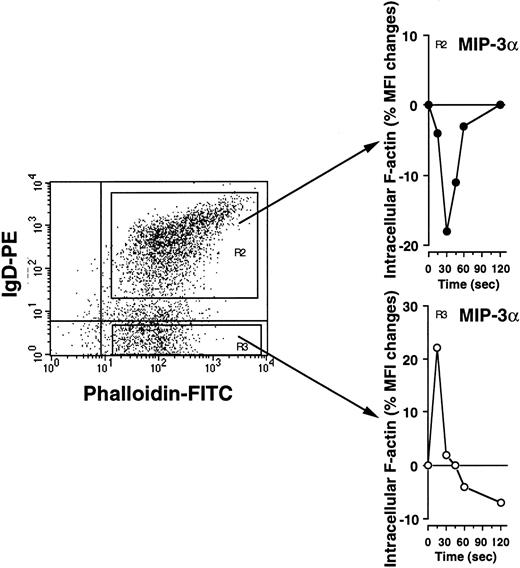
Chemokine-dependent cell movement is thought to be driven mostly by the rapid—within seconds of receptor triggering—polymerization of actin monomers (G-actin) into filaments (F-actin) near the plasma cell membrane. Intracellular F-actin filaments can be easily quantified by flow cytometry using FITC-labeled phalloidin as a probe. To determine whether CCR6 is functional in naive and memory B cells, we quantified the change in the intracellular F-actin content induced by MIP-3α/CCL20 in cells stained with anti-IgD MoAb. Surface IgD expression was chosen as a marker to discriminate between sIgDhigh naive and sIgD− memory B cells. Surface IgD− cells responded to MIP-3α/CCL20 (500 ng/mL) by F-actin assembly and cytoskeleton reorganization (Figure4). The MIP-3α/CCL20–induced response of sIgD− B cells was rapid (a 24% peak increase in F-actin content in 15 seconds) but short-lived, probably reflecting rapid receptor desensitization (Figure 4, R3 gate). In contrast, sIgDhigh B cells did not respond to MIP-3α/CCL20 during the 120 seconds of observation, and the F-actin content decreased in these cells (Figure 4, R2 gate). Thus, in the F-actin polymerization assay, unstimulated sIgD−CD44high memory but not sIgDhigh naive B cells express functional CCR6.
sIgD− memory but not sIgDhigh naive B cells respond rapidly to MIP-3α/CCL20 by actin cytoskeleton reorganization.
The ability of CCR6 to signal in unstimulated tonsillar B cells stained with anti-IgD-PE MoAb was assessed by quantifying changes in intracellular F-actin after MIP-3α/ CCL20 stimulation (500 ng/mL). sIgD− (R3) responding and sIgDhigh (R2) nonresponding populations are shown. Data from one representative experiment of 3 are shown.
sIgD− memory but not sIgDhigh naive B cells respond rapidly to MIP-3α/CCL20 by actin cytoskeleton reorganization.
The ability of CCR6 to signal in unstimulated tonsillar B cells stained with anti-IgD-PE MoAb was assessed by quantifying changes in intracellular F-actin after MIP-3α/ CCL20 stimulation (500 ng/mL). sIgD− (R3) responding and sIgDhigh (R2) nonresponding populations are shown. Data from one representative experiment of 3 are shown.
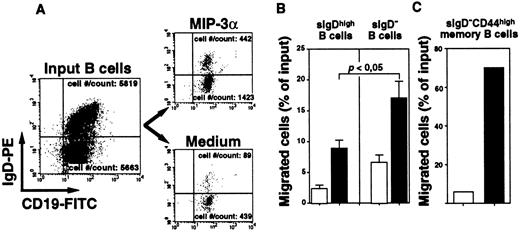
MIP-3α/CCL20 preferentially attracts sIgD− memory B cells in transmigration chemotactic assays
We performed in vitro chemotactic assays using the Transwell system to examine more precisely the chemotactic activity of MIP-3α/CCL20 for different subsets of B cells. Cells were stained with FITC-labeled anti-CD19 and PE-labeled anti-IgD MoAbs to compare the phenotypes of the input B cells and migrated B cell populations. The responding sIgD− B cells were mostly CD44high memory B cells, because sIgD−CD44− GC B cells do not express CCR6 and do not respond to MIP-3α/CCL20 in migration assays (data not shown). The percentage of unfractionated B cells migrating toward MIP-3α/CCL20 above the chemokinetic background level in 6 independent experiments using different donors was 8.4% ± 1.6%. Although both sIgDhigh and sIgD− B cells migrated toward MIP-3α/CCL20 gradient, the MIP-3α/ CCL20–responding cell population was enriched in sIgD− memory B cells (Figure5A). Surface IgD− memory B cells were attracted twice as efficiently as sIgDhigh naive B cells by MIP-3α/CCL20 (17% ± 2.8% vs 8.9% ± 1.4%, respectively; n = 6; P < .05) (Figure 5B). Using purified IgD−CD44high memory B cells, we confirmed that most of these cells (70%) migrated toward MIP-3α/CCL20 (Figure 5C), whereas 12.5% ± 7.5% purified sIgDhigh naive B cells migrated in similar conditions (not shown). These observations strongly suggest that MIP-3α/CCL20 preferentially attracts sIgD− memory B cells.
MIP-3α/CCL20 preferentially attracts sIgD− memory B cells in transmigration chemotaxis assays.
Chemotactic response of B cells to MIP-3α/CCL20 in Transwell chemotaxis assay. MIP-3α/CCL20 (500 ng/mL) or migration buffer was placed in individual lower wells of 24-well Transwell plates, and unfractionated B cells or purified sIgD−CD44high memory B cells were layered into upper wells. The phenotype of medium- and MIP-3α-migrating B cells harvested after a 3-hour incubation at 37°C was compared to that of input B cells using anti–CD19-FITC and anti–IgD-PE MoAbs. The representative dot plots of input cells and transmigrated sIgDhigh naive and sIgD− memory subsets of B cells are shown in panel A. Absolute cell numbers (no./count) of naive and memory B cells that transmigrated are given. The percentage of naive and memory B cells that transmigrated toward MIP-3α (■) or migration buffer (□) are shown in panel B. Data represent mean ± SEM values obtained in 6 independent experiments using cells from different donors. Statistical difference between migration of naive and memory B-cell groups was analyzed by unpaired Student t test. The percentage of purified sIgD−CD44high memory B cells toward MIP-3α/CCL20 versus migration buffer is shown in panel C. Data from one representative experiment is shown.
MIP-3α/CCL20 preferentially attracts sIgD− memory B cells in transmigration chemotaxis assays.
Chemotactic response of B cells to MIP-3α/CCL20 in Transwell chemotaxis assay. MIP-3α/CCL20 (500 ng/mL) or migration buffer was placed in individual lower wells of 24-well Transwell plates, and unfractionated B cells or purified sIgD−CD44high memory B cells were layered into upper wells. The phenotype of medium- and MIP-3α-migrating B cells harvested after a 3-hour incubation at 37°C was compared to that of input B cells using anti–CD19-FITC and anti–IgD-PE MoAbs. The representative dot plots of input cells and transmigrated sIgDhigh naive and sIgD− memory subsets of B cells are shown in panel A. Absolute cell numbers (no./count) of naive and memory B cells that transmigrated are given. The percentage of naive and memory B cells that transmigrated toward MIP-3α (■) or migration buffer (□) are shown in panel B. Data represent mean ± SEM values obtained in 6 independent experiments using cells from different donors. Statistical difference between migration of naive and memory B-cell groups was analyzed by unpaired Student t test. The percentage of purified sIgD−CD44high memory B cells toward MIP-3α/CCL20 versus migration buffer is shown in panel C. Data from one representative experiment is shown.
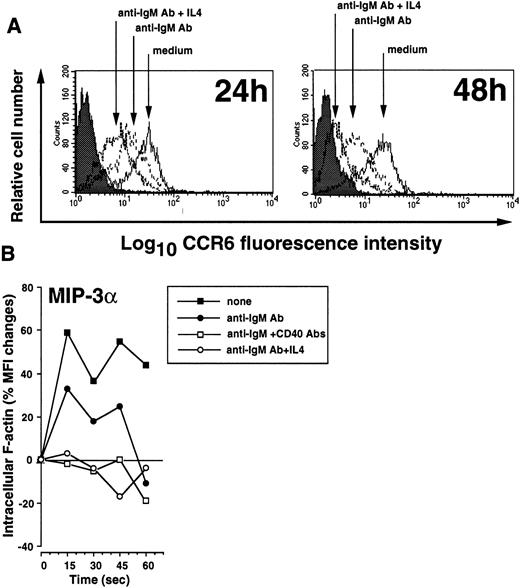
B cells rapidly down-regulate CCR6 in response to B-cell Ag receptor cross-linking
We tested whether signals essential for the B-cell response, such as B-cell Ag receptor (BCR) engagement, CD40 triggering, or cytokines, regulate CCR6 expression. BCR cross-linking of resting B cells by incubation with anti-IgM beads for 2 days decreased the relative number of CCR6-expressing cells and the density of CCR6 on the cell surface (85% CCR6-expressing cells, MFI = 23, vs 69% CCR6-expressing cells, MFI = 13, in untreated and anti-IgM Ab-treated cells, respectively) (Figure 6A). This effect reached a maximum after 48 hours and was strongly enhanced by IL-4 (20 ng/mL) (28% CCR6+ cells, MFI = 9, after 48 hours poststimulation) but not by IL-13 or IL-2 (not shown). Consistent with these observations, the peak response to MIP-3α/CCL20 as assessed by intracellular–F-actin polymerization was decreased by 44% after IgM BCR cross-linking and totally abolished after stimulation with anti-IgM Ab plus IL-4 or with anti-IgM Ab plus anti-CD40 MoAb (Figure 6B). In this latter case, the expression of CCR6 was comparable to that observed with anti-IgM Ab alone (not shown). In contrast to the effect of BCR cross-linking, stimulation with anti-CD40 MoAb alone or with several cytokines (IL-2, IL-4, IL-7, IL-10, IL-12, TNF-α, lymphotoxin (LT)-α, transforming growth factor (TGF)-β) had no effect on CCR6 expression in B cells (not shown). Thus, BCR engagement, but not CD40 triggering or the presence of cytokines, induces rapid down-regulation of CCR6 expression and MIP-3α/CCL20 responsiveness in human B cells.
Down-regulation of CCR6 expression and MIP-3α/CCL20 responsiveness following B-cell Ag receptor cross-linking.
(A) Tonsillar B cells were cultured for 24 or 48 hours in CM alone or in the presence of anti-IgM Ab (5 μg/mL) with or without IL-4 (20 ng/mL). Cells were then analyzed by FACS for cell surface CCR6 expression. Data from 1 representative experiment of 5 are shown. (B) The response to MIP-3α/CCL20 of B cells stimulated for 48 hours with anti-IgM Ab (5 μg/mL), alone or in combination with anti-CD40 MoAb (1 μg/mL) or IL-4 (20 ng/mL), was assessed by F-actin polymerization assay, as described in “Materials and methods.” Representative data from 4 independent experiments are shown.
Down-regulation of CCR6 expression and MIP-3α/CCL20 responsiveness following B-cell Ag receptor cross-linking.
(A) Tonsillar B cells were cultured for 24 or 48 hours in CM alone or in the presence of anti-IgM Ab (5 μg/mL) with or without IL-4 (20 ng/mL). Cells were then analyzed by FACS for cell surface CCR6 expression. Data from 1 representative experiment of 5 are shown. (B) The response to MIP-3α/CCL20 of B cells stimulated for 48 hours with anti-IgM Ab (5 μg/mL), alone or in combination with anti-CD40 MoAb (1 μg/mL) or IL-4 (20 ng/mL), was assessed by F-actin polymerization assay, as described in “Materials and methods.” Representative data from 4 independent experiments are shown.
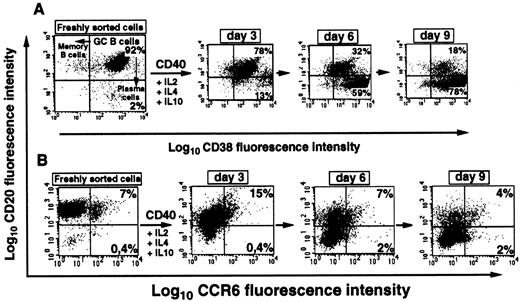
Lack of CCR6 expression during in vitro plasma cell differentiation of GC B cells and on myeloma cell lines
To determine CCR6 expression in an in vitro model of plasma cell differentiation, GC B cells were stimulated for 9 days with the combination of anti-CD40 MoAb (1 μg/mL), IL-2 (10 U/mL), IL-4 (20 ng/mL), and IL-10 (50 ng/mL). Two markers modulated during plasma cell differentiation (CD20 and CD38) and CCR6 expression were simultaneously assessed on days 3, 6, and 9 using 2-parameter FACS analysis. Plasmablast differentiation is associated with strong CD38 up-regulation and loss of CD20 expression.23 As expected, CD20+CD38+ GC B cells rapidly down-regulated CD20 expression, whereas both the percentage and fluorescence intensity of CD38+ cells progressively increased during culture (Figure 7A). On day 9 of culture, the relative frequency of CD20−CD38bright cells was 78% with a 2-fold increase in CD38 MFI between days 0 and 9. These cells exhibited typical plasmacytoid morphology after May-Grünwald-Giemsa staining and were plasma cell progenitors (plasmablasts) (data not shown). CCR6, not expressed on GC B cells, was not reacquired during the plasma cell differentiation process in vitro (Figure 7B) and was also absent from the 6 myeloma cell lines tested (OPM2, NCI, RMPI 8226, U 266, MDN, BCN). This contrasts with CXCR4, which was expressed during this plasma-cell differentiation process and was also present on most myeloma cell lines (not shown). Thus, in contrast to its re-expression on post-GC memory B cells, CCR6 was not re-expressed during plasma cell differentiation.
CCR6 is not expressed during in vitro plasmablast differentiation of GC B cells.
(A) Phenotypic changes during the differentiation of GC B cells into plasmablasts in vitro. GC B cells purified by immunomagnetic cell sorting (> 90% cell viability) were cultured in the presence of IL-10, IL-2, IL-4, and anti-CD40 MoAb, as described in “Materials and methods.” At the indicated time points, CD38 and CD20 expression was determined by double staining with anti-CD38-PE and anti-CD20-FITC MoAbs. Note the progressive increase in the CD38highCD20− population corresponding to the plasmacytoid differentiation stage. Percentages of cells within each quadrant are indicated. (B) Analysis of CCR6 expression by double staining with anti-CD20-FITC and anti-CCR6-PE MoAbs during plasmablast differentiation is presented in panel A. One representative experiments of 3 using cells from different donors is shown.
CCR6 is not expressed during in vitro plasmablast differentiation of GC B cells.
(A) Phenotypic changes during the differentiation of GC B cells into plasmablasts in vitro. GC B cells purified by immunomagnetic cell sorting (> 90% cell viability) were cultured in the presence of IL-10, IL-2, IL-4, and anti-CD40 MoAb, as described in “Materials and methods.” At the indicated time points, CD38 and CD20 expression was determined by double staining with anti-CD38-PE and anti-CD20-FITC MoAbs. Note the progressive increase in the CD38highCD20− population corresponding to the plasmacytoid differentiation stage. Percentages of cells within each quadrant are indicated. (B) Analysis of CCR6 expression by double staining with anti-CD20-FITC and anti-CCR6-PE MoAbs during plasmablast differentiation is presented in panel A. One representative experiments of 3 using cells from different donors is shown.
Discussion
Serpentine receptors of the Gαi-linked chemoattractant receptor subfamily trigger tissue-specific homing of lymphocytes, modulate lymphocyte activation and cell proliferation, and have several critical functions during early lymphopoiesis. They are also important mediators controlling lymphoid tissue architecture and morphogenesis.24-26 The essential role of CXCR4 and its ligand, stromal cell–derived factor (SDF)-1α, in B-lineage maturation in vivo has been confirmed insdf-1−/− and cxcr4−/−mice, which display strongly impaired B lymphopoiesis and abnormally low numbers of B-lymphoid precursors in fetal liver and BM.1,2 In this study we examined the expression of CCR6 chemokine receptor and responsiveness to its ligand MIP-3α/CCL20 during B-cell–lineage maturation in BM and during the B-cell response in the periphery. We show that CCR6 is absent from multipotent primitive and committed CD34+ subsets of hematopoietic stem cell progenitors. Multiparameter FACS analysis did not find CCR6+ cells among the early committed CD34+CD19+ B cell progenitors or within most CD19+CD10+ cells, which include pre–B- and immature B-cell fractions of BM MNCs. This argues against the CCR6/MIP-3α/CCL20 pair being involved during the early stages of B lymphopoiesis. The continuum of human B-lineage maturation in BM ends at the stage of CD19+CD10−sIgM+sIgD+ B cells. We found that virtually all mature BM B cells are CCR6+. These cells constitute the pool of newly formed B cells that emigrate to the periphery and rapidly differentiate into a recirculating sIgDhigh follicular B cell pool. Although the appropriate developmental signals required for CCR6 acquisition and maturation of sIgM+ B cells in BM remain to be determined, this process seems to be initiated in parallel with down-regulation of CD10, a marker lost as the maturity of B-lineage progeny increases, and sIgD is acquired. In agreement with this, we did not detect CCR6 on human pro-B/pre-B cell lines. That CCR6 expression is a marker of the mature B cell pool was further confirmed by the study of umbilical cord blood– and peripheral blood–derived B cells and B cells populating peripheral lymphoid organs. Moreover, only resting (naive and memory) B cells, but not GC B cells representing in vivo Ag-activated B-cell clones, expressed CCR6. In agreement with this, no CCR6 expression was detected on any of the 6 cell lines of GC origin like Burkitt lymphoma cell lines. This suggests that the responsiveness to MIP3α is lost after the encounter with Ag in the peripheral lymphoid tissue and Ag-driven B cell differentiation. Indeed, in vitro BCR triggering down-regulated CCR6 and responsiveness to MIP-3α/CCL20. Interestingly, T-cell Ag receptor-triggering by anti-CD3 MoAb also results in down-regulation of CCR6 expression, which was more pronounced in Th1 polarized cell lines.27 In B cells, CCR6 is reacquired at the postselection stage of sIgD− memory B cells. Furthermore, in contrast to memory B cells, naive B cells did not respond to MIP-3α/CCL20 as assessed by F-actin polymerization, suggesting that the functional response via chemokine receptors is also controlled by cytoplasmic events. This is in agreement with previous findings demonstrating that freshly isolated B cells do not respond to MIP-3α/CCL20 by Ca++ flux.10 Such dissociation between chemokine receptor expression and responsiveness to chemokines by Ca++ mobilization or cyclic adenosine monophosphate production has also been reported in other studies.28 Differential chemotactic responses of B-cell subsets was also observed in in vitro transmigration assays. Indeed, although MIP-3α/CCL20 induced consistent migration of unfractionated B cells, it preferentially attracted sIgD−CD44high memory B cells. The efficiency of migration of purified memory B cells was 70%, and thus MIP-3α/CCL20 is one of the most efficient known chemoattractants for these cells. The difference in MIP-3α/CCL20 responsiveness between naive and memory B cells we observed may reflect the cells' different migratory behavior in vivo. The higher CCR6 expression and responsiveness to MIP-3α on memory B cells is consistent with CCR6 being important in the local and systemic trafficking of effector/memory B cells. Indeed, in situ hybridization in human tissues revealed abundant MIP-3α/CCL20 mRNA expression in the anatomic sites where memory/effector B cells are preferentially recruited.9,29 They represent the sites of continuous antigenic challenge and chronic inflammation, such as the epithelial crypts of palatine tonsils and adenoids9 or subepithelial regions of intestinal and lung mucosa.15 At these sites, MIP-3α/CCL20 is probably released from activated epithelial cells and from inflammatory cells, such as macrophages, eosinophils, and dendritic cells,14 that are indeed abundant at mucosal and submucosal sites. The chemotactic gradient of MIP-3α/CCL20 present at these sites may bring together the critical cellular elements of innate (dendritic cells) and adaptive (effector/memory T and B cells) immune responses. The integrated mucosal immune system is completely dependent upon selective homing of effector cells of diverse phenotype and function to the sites exposed to previously encountered Ags.30 Consistent with this, α4β7+sIgD−memory B cells within intestinal mucosa lamina propria maintain long-term memory to various pathogens, eg, rotaviruses, and this site contains mainly memory cell–derived sIgD−L-selectin−CD20−CD38highIgA-secreting plasma cells.31,32 The organized gut-associated lymphoid tissues, ie, Peyer's patches and appendix, are considered to be inductive sites for mucosa-associated local immune response, and 50% of MNCs at these sites are L-selectinhigh sIgDhigh naive B cells.32 Because the MIP-3α/CCL20 gene is strongly induced in endothelial cells by TNF-α, the cytokine expressed early during immune/inflammatory response, MIP-3α/CCL20 would be expected to recruit CCR6-expressing cells from the circulation as well.9,11 Our data support the notion that CCR6 might be a putative “tissue-specific” homing receptor mediating the positioning of effector/memory cells in mucosa-associated effector sites. The MIP-3α/CCL20-CCR6 pair may thus contribute to the dichotomy between the mucosal/effector and the systemic immune compartments. Recent data show that CCR4 expression is essentially restricted to CLA+ and not α4β7+ memory T cells.33 Indeed, CCR4+ T cells are abundant within chronically inflamed skin lesions, whereas α4β7+ intestinal lamina propria T cells are mostly CCR4−. Thus, within the T-cell compartment, CCR4 and CCR6 may function as mutually exclusive tissue-specific homing receptors to skin and mucosa-associated inductive sites, respectively. Constitutive expression of MIP-3α/CCL20 was recently reported in keratinocytes and venular endothelial cells in normal skin and might be involved in the constitutive trafficking of CLA+ precursors of Langerhans' cells.16 It is, however, unlikely that MIP-3α/CCL20 attracts to this site CCR6-expressing B cells that do not express skin homing receptors. Whether CCR6 is also a marker of a selective functional subset of memory B cells, as was recently reported for CCR7 expression within the pool of memory T cells, remains an open and intriguing question.34
MIP-3α/CCL20 may be a link between innate and adaptive humoral immune responses at host-environment interfaces such as mucosal surfaces. This notion is further supported by the recent findings that β-defensins (hBD-1 and hBD-2), the small antimicrobial peptides secreted in high concentrations by specialized epithelial cells upon microbial invasion, are selectively chemotactic for CCR6+cells.35,11 Defensins have bactericidal, fungicidal, and antiviral properties and are up-regulated in the mucosal epithelium after microbial invasion. Interestingly, they significantly increase Ag-specific IgG (IgG1, IgG2b, and IgG2a) and IgM, but not IgA, Ab production. This response is associated with the proliferative response and IFN-γ, IL-5, IL-6, and IL-10 secretion of Ag-specific CD4+ T cells.36 Through attraction of CCR6-expressing immature dendritic cells, memory T and B cells, β-defensins may thus participate in the initiation of both primary and recall immune responses at the mucosal sites.
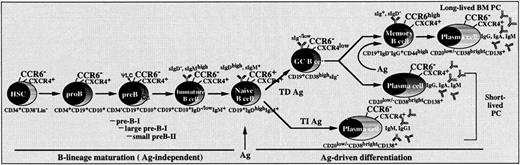
Our study demonstrates that MIP-3α/CCL20 is an efficient B-cell chemoattractant with a differential preference toward memory B cells. During B-cell ontogeny in BM and Ag-driven B-cell differentiation in the periphery, expression of the MIP-3α/CCL20 receptor, CCR6, is restricted to mature naive and memory B cells. Its absence from GC cells may be a consequence of recent BCR triggering or of their entry into cell cycle. Because CCR6 was absent from GC B cells, in vitro differentiated plasmablasts, and myeloma cell lines, it seems likely that CCR6 loss accompanies Ag-triggered clonal expansion of B cells in vivo (Figure 8). The role of CCR6 within the B-cell compartment seems to be restricted to B cells capable of responding to antigenic challenge. This also shows that expression of a selective set of chemokine receptors is an integral part of the B-lineage development and Ag-driven differentiation.
Schematic representation of CCR6 expression during human B-cell ontogeny and Ag-driven differentiation.
CCR6 surface expression is acquired when B cells reach the mature stage. Mature, naive B cells lose CCR6 after BCR triggering by Ag during both T-dependent TD and T-independent TI humoral immune responses and during plasma cell differentiation. CCR6 is re-expressed at the post-GC memory B-cell stage. Transient expression of CCR6 at each defined stage of B-cell maturation is compared with that of CXCR4, constitutively expressed from CD34+Lin−multipotent hematopoietic stem cell progenitors to terminally differentiated Ig-secreting plasma cells (PC).
Schematic representation of CCR6 expression during human B-cell ontogeny and Ag-driven differentiation.
CCR6 surface expression is acquired when B cells reach the mature stage. Mature, naive B cells lose CCR6 after BCR triggering by Ag during both T-dependent TD and T-independent TI humoral immune responses and during plasma cell differentiation. CCR6 is re-expressed at the post-GC memory B-cell stage. Transient expression of CCR6 at each defined stage of B-cell maturation is compared with that of CXCR4, constitutively expressed from CD34+Lin−multipotent hematopoietic stem cell progenitors to terminally differentiated Ig-secreting plasma cells (PC).
Acknowledgments
The authors thank D. Treton for excellent technical assistance and Drs A. Lange and J. Silber (K. Dluski Hospital, Wroclaw, Poland) for their encouragement and constant support. We also acknowledge Drs F. Audiat (Hôpital Necker, Paris, France) and E. Joussenet (Centre de Transfusion Sanguine des Armées, Hôpital Percy, Clamart, France) for providing us with BM samples and buffy coats, respectively.
Supported by grants from the Agence Nationale de Recherche sur le SIDA (ANRS), INSERM, the Association Claude Bernard, and the Université Paris-Sud (Paris XI). Supported by fellowships from the Association pour la Recherche sur le Cancer (R.K. and J.B.) and ANRS (E.A.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
During revision of this manuscript, a recent publication from the E. C. Butcher group37 reported that in murine B cells, CCR6 expression and MIP-3α/CCL20 responsiveness is restricted to a subset of peripheral mature B cells. These results are consistent with our findings in human B cells and confirm the important role of selective chemokine receptor expression pattern during B cell ontogeny and B cell response in the periphery.
Author notes
Yolande Richard, INSERM U131, 32 rue des Carnets, 92 140 Clamart, France; e-mail:yolande.richard@inserm.ipsc.u-psud.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal