Introduction
Life-threatening complications from bacterial infections are a major and growing clinical problem, aggravated by the emergence and spread of antibiotic resistance in bacterial pathogens and by an increase in the number of immunocompromised patients.1-3 During bacterial infection, the host responds to invading microbes with a number of different defense mechanisms. Some bacteria or bacterial products, however, can modulate the host response and cause serious complications from the disease. In severe infections, such as sepsis and septic shock, the coagulation and fibrinolytic systems are targets for such modulation. These cascades are normally activated upon tissue injury and/or blood vessel damage. During infection, inflammatory mediators of the parasite and/or host can manipulate the procoagulant/anticoagulant equilibrium and cause severe bleeding disorders.4 5 Although such effects induced by pathogenic bacteria often lead to similar clinical pictures, different microbes seem to interfere at different stages of the coagulation and fibrinolytic systems. This review aims to provide an overview of how pathogenic bacteria can manipulate the coagulation and fibrinolytic cascades in infectious diseases.
Current view on hemostasis
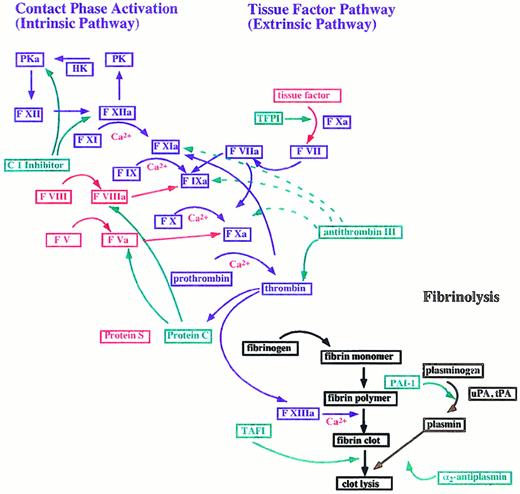
The coagulation cascade can be divided into an extrinsic and an intrinsic pathway (Figure 1). The extrinsic pathway is triggered upon complex formation of exposed tissue factor (TF) with coagulation factor VII (F VII). This is followed by an activation of factor IX (F IX) and/or factor X (F X) by the TF–F VII complex and subsequent conversion of prothrombin to thrombin, which finally induces the formation of fibrin from fibrinogen.6The intrinsic pathway is initiated after activation of the contact system, a process involving F XI, factor XII (F XII), plasma kallikrein (PK), and high molecular weight kininogen (HK).7,8 In addition to its procoagulative functions, activation of the contact system also leads to the release of bradykinin, which is generated by cleavage of HK by PK. Kinins are considered to be important pro-inflammatory mediators.9
The blood coagulation cascade.
The coagulation cascade is divided into an intrinsic and an extrinsic pathway. Coagulation factors are represented in blue and are designated by an “a” upon activation. Arrows symbolize the proteolytic conversion. Cofactors are marked in red and coagulation inhibitors in green. Proteins that participate in fibrinolysis have a brown color. The following abbreviations are used: F, factor; HK, high molecular weight kininogen; PAI-1, plasminogen activator inhibitor; PK, plasma kallikrein; TAFI, thrombin activatable fibrinolysis inhibitor; TFPI, tissue factor pathway inhibitor; tPA, tissue plasminogen activator; and uPA, urokinase-type plasminogen activator. Strong and weak interactions are indicated by solid and dotted lines.
The blood coagulation cascade.
The coagulation cascade is divided into an intrinsic and an extrinsic pathway. Coagulation factors are represented in blue and are designated by an “a” upon activation. Arrows symbolize the proteolytic conversion. Cofactors are marked in red and coagulation inhibitors in green. Proteins that participate in fibrinolysis have a brown color. The following abbreviations are used: F, factor; HK, high molecular weight kininogen; PAI-1, plasminogen activator inhibitor; PK, plasma kallikrein; TAFI, thrombin activatable fibrinolysis inhibitor; TFPI, tissue factor pathway inhibitor; tPA, tissue plasminogen activator; and uPA, urokinase-type plasminogen activator. Strong and weak interactions are indicated by solid and dotted lines.
At present, it is generally accepted that coagulation is driven mainly by the extrinsic pathway.6 This notion is based on the observation that patients with deficiencies in the contact factors F XII, HK, and PK do not suffer from any bleeding disorders.8 It therefore seems likely that these proteins play only a secondary role in hemostasis. However, F XI deficiency leads to minor bleeding abnormalities, a dysfunction explained by the ability of thrombin to activate F XI by a mechanism that is independent of the contact system.10 Thrombin-activated F XI triggers an augmentation stage of coagulation and allows continuation of clotting after down-regulation of TF activity by TF-pathway inhibitor (TFPI).11,12 Work published to date indicates that activation of F XI by thrombin also attenuates fibrinolysis inside a fibrin clot.13 14
Coagulation is controlled at 3 levels. First, thrombin changes to an anticoagulant factor upon binding to thrombomodulin (TM), a glycoprotein bound to the endothelium.15 The formation of the thrombin/TM complex allows a rapid cleavage and activation of the anticoagulant protein C. Activated protein C then down-regulates further formation of thrombin by proteolytically inactivating the coagulation cofactors factor V (F V) and factor VIII (F VIII). Additionally, thrombin, in complex with TM, loses its ability to clot fibrinogen. Second, in order to prevent an expansion of clotting in the periphery of the damaged tissue, coagulation factors are inactivated by circulating proteinase inhibitors such as TFPI, C1 inhibitor (C1 INH), and antithrombin III (AT III).16-18 Finally, fibrinolysis is hindered inside the clot by a thrombin-dependent activation of the plasma protein thrombin-activatable fibrinolysis inhibitor (TAFI). TAFI exhibits carboxypeptidase-B–like activity and suppresses fibrinolysis, most likely by removing carboxy-terminal lysine residues. Since plasmin binds to carboxy-terminal lysines of progressively degraded fibrin, this mechanism might protect the clot from degradation.19
At the site of tissue injury, fibrinolysis is initiated by the conversion of plasminogen to plasmin.20 Plasmin has multiple functions, such as degradation of fibrin, inactivation of the cofactors F V and F VIII, and activation of metalloproteinases, which play an important role in wound healing and tissue-remodeling processes.21-23 Plasminogen is activated by tissue plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA),24 proteins released from endothelial cells in response to thrombin and upon cell damage.25 Plasminogen activity is regulated by plasmin inhibitors such as α2-antiplasmin and α2-macroglobulin and also attenuated by plasminogen activator inhibitor 1 (PAI-1), the primary physiological inhibitor of tPA and uPA.26 27
Lately, the connection between hemostasis and inflammation has attracted much interest.28 This link was established by recent work showing that the coagulation factors thrombin, F X, and F VII trigger intracellular signal cascades by binding to specific cell-membrane–spanning receptors, which have no known role in hemostasis.29-32 The receptors for thrombin (protease-activated receptors [PARs]) are the best characterized. PARs belong to the family of G-protein–coupled receptors, which are activated upon binding to a protease that cleaves the exodomain of the receptor. This manipulation unmasks a tethered peptide ligand that then stimulates its own receptor.33 So far, 4 receptors that are triggered by this mechanism have been identified. Thrombin activates PAR-1, PAR-3, and PAR-4, whereas PAR-2 is activated by trypsin, mast cell tryptase, and a cysteine proteinase fromPorphyromonas gingivalis.34-37 The receptors for F X (effector cell protease receptor–1 [EPR-1]) and F VII (TF) do not belong to the family of G-protein–coupled receptors and are activated by different mechanisms.38,39 Additionally, a receptor for protein C (endothelial cell protein C [EPCR]) has been described.40 Whether this receptor triggers intracellular signaling is currently under investigation.41-43 Patients with sepsis have significantly increased levels of a soluble form of EPCR in plasma, indicating that EPCR can be used as a marker protein in infectious diseases.44 The binding of coagulation factors to these receptors and their subsequent activation trigger inflammatory reactions such as leukocyte recruitment, release of cytokines and nitric oxide, and the production of reactive oxygen species.30,39 45-47
Bacteria and the extrinsic pathway
As mentioned above, the coagulation cascade is initiated mainly by binding of F VII to cell-surface–bound TF. The physiological importance of TF was demonstrated by experiments in transgenic mice, which show that interruption of the gene for TF is associated with lethal embryonic bleeding and impaired vascular development.48,49 TF is expressed in many tissues, including brain, lung, placenta, and kidney.50 Cells that constitutively produce TF are usually not in contact with blood, but are found in perivascular tissues and stroma.11 Peripheral blood cells and endothelium do not normally produce TF. However, TF activity in these cells is increased after stimulation with such substances as endotoxin, tumor necrosis factor α (TNF-α), phorbol esters, serum, or vascular endothelial growth factor (VEGF). Inflammatory mediators such as endotoxin and TNF-α regulate TF-gene induction by using a promoter region containing DNA-binding motifs for nuclear factor κB and activator protein 1, whereas TF expression induced by serum, phorbol esters, and VEGF is controlled by a promoter region containing overlapping Sp1/endothelial growth factor 1 sites.6,51-53 Recently, Giesen et al54provided evidence that small amounts of thrombogenic TF are also found in plasma.
Recently, Gando et al55 reported that TF values from sepsis patients are significantly higher than from trauma patients, indicating an important role for TF-triggered coagulation complications during septic conditions. The observation that TF activity is enhanced during infection is in accordance with the finding that several bacterial species, such as Mycobacterium leprae,Neisseria meningitidis, Rickettsia conorii,Rickettsia rickettsii, Staphylococcus aureus, andStreptococcus sanguis, are able to trigger TF expression in monocytes and endothelial cells.56-64 It seems likely, therefore, that the onset of coagulation in sepsis results partially from induction of TF expression in endothelial cells and monocytes by bacterial products. The finding that Gram-positive microorganisms also trigger TF activity indicates that bacterial products other than endotoxin could be involved in the up-regulation of procoagulant activity. Gram-positive bacteria may induce this indirectly, as various exotoxins and peptidoglycans have been shown to trigger the induction of pro-inflammatory cytokines.6,65-69 Notably, cytokines such as interferon γ, interleukin 1β (IL-1β), and TNF-α, are strong inducers of TF expression.70 These observations indicate that TF activity is increased in response to products from both Gram-positive and Gram-negative bacteria and that this can be one of the initial steps for inducing coagulation disorders in infectious diseases.
Bacteria and the contact system
Numerous studies provide accumulating evidence that the contact system is activated during sepsis.71 It was demonstrated as early as 1970 that patients with hypotensive septicemia have significantly decreased levels of contact factors.72 More recently, it has been reported that activation of the contact system occurs in children with meningococcal septic shock and that low levels of F XII and HK in systemic inflammatory response syndrome (SIRS) patients correlate with a fatal outcome of the disease.73-75 In one of the more recent studies, it was also shown that levels of PK-α2–macroglobulin complexes are increased in SIRS patients, indicating an activation of the contact system.74 The α2-macroglobulin binds to activated PK followed by a removal of the complex by a receptor-mediated mechanism in the reticuloendothelial system. These findings are in accordance with experiments in baboons demonstrating that irreversible hypotension induced by infusion ofEscherichia coli correlates with a decline in levels of HK and an increase of PK-α2–macroglobulin complexes.76 The administration of an inhibitory monoclonal antibody to F XII, which blocks contact activation, prevented the development of irreversible hypotension and led to an extension of the survival time in infected animals.77These observations suggest an important role for the contact system in the hemodynamic derangements of septic patients.78
In 1996, Ben Nasr et al79 reported that contact factors bind to the surface of E coli and Salmonella spp bacteria that express curli organelles or thin aggregative fimbriae. Subsequent studies demonstrated that the contact system is activated as a consequence of the assembly of contact factors on the bacterial surface. This is followed by the release of bradykinin, a potent inducer of fever, pain, and hypotension.80 Moreover, the absorption of contact system proteins and fibrinogen by these surface organelles led to a depletion of relevant coagulation factors and caused a hypocoagulatory state in mice.80 Apart from Gram-negative bacteria, cell-wall peptidoglycan products fromStreptococcus pyogenes have been shown to trigger the contact system.81 In fact, most streptococcal serotypes bind kininogens by their M proteins,82 which are considered to be important virulence factors on the outer membrane of the microorganism.83 Once HK is absorbed on the bacterial surface, it is susceptible to proteolytic cleavage by PK, leading to the formation of bradykinin.84
In addition to bacterial-surface molecules, secreted microbial products have been demonstrated to activate the contact system. Early observations identified bacterial endotoxin as a potent activator of F XII.85 Recently, with the use of a low-grade endotoxemia model in humans, it has become apparent that F XI is also activated by a contact-system–independent mechanism.86 Apart from endotoxin, many bacterial proteinases that are able to trigger the release of kinins have been isolated (Table1).87-93 In order to generate bioactive kinins from HK, bacterial proteinases act on kininogens directly92,93 or activate the contact factors F XII and PK, which in turn cleave HK, causing the release of bradykinin.87 91 As kinins increase vascular permeability, the generation of these pro-inflammatory peptides in infectious foci might be a bacterial strategy that facilitates penetration and spreading of the pathogen into the tissue.
Generation of kinins by bacterial proteinases
| Bacterial species . | Gram reaction . | Target . | Reference . |
|---|---|---|---|
| Bacillus stearothermophilus | Gram-positive | F XII/PK | 87 |
| Bacillus subtilis | Gram-positive | F XII/PK | 87 |
| Porphyromonas gingivalis | Gram-negative | HK | 92 |
| Porphyromonas gingivalis | Gram-negative | PK | 91 |
| Porphyromonas gingivalis | Gram-negative | PK/HK | 176 |
| Pseudomonas aeruginosa | Gram-negative | F XII | 87 |
| Pseudomonas aeruginosa | Gram-negative | not known | 88 |
| Serratia marcescens | Gram-negative | F XII | 87 |
| Staphylococcus aureus | Gram-positive | HK | 87 |
| Streptococcus pyogenes | Gram-positive | HK | 93 |
| Streptomyces caespitosus | Gram-positive | HK | 87 |
| Vibrio cholerae | Gram-negative | not known | 177 |
| Vibrio vulnificus | Gram-negative | PK | 178 |
| Vibrio vulnificus | Gram-negative | F XII/PK | 87 |
| Bacterial species . | Gram reaction . | Target . | Reference . |
|---|---|---|---|
| Bacillus stearothermophilus | Gram-positive | F XII/PK | 87 |
| Bacillus subtilis | Gram-positive | F XII/PK | 87 |
| Porphyromonas gingivalis | Gram-negative | HK | 92 |
| Porphyromonas gingivalis | Gram-negative | PK | 91 |
| Porphyromonas gingivalis | Gram-negative | PK/HK | 176 |
| Pseudomonas aeruginosa | Gram-negative | F XII | 87 |
| Pseudomonas aeruginosa | Gram-negative | not known | 88 |
| Serratia marcescens | Gram-negative | F XII | 87 |
| Staphylococcus aureus | Gram-positive | HK | 87 |
| Streptococcus pyogenes | Gram-positive | HK | 93 |
| Streptomyces caespitosus | Gram-positive | HK | 87 |
| Vibrio cholerae | Gram-negative | not known | 177 |
| Vibrio vulnificus | Gram-negative | PK | 178 |
| Vibrio vulnificus | Gram-negative | F XII/PK | 87 |
F XII indicates factor XII; PK, plasma kallikrein; HK, high molecular weight kininogen.
Bacteria and fibrinogen/fibrin
The systemic activation of coagulation during infection results in the cleavage of fibrinogen by thrombin. The fibrin monomers generated can then become deposited in various organs, leading to microvascular and macrovascular thrombosis. The concomitant stimulation and aggregation of platelets, either by thrombin or by bacterial substances, may also cause thrombocytopenia.94 As a result of widespread thrombus formation, tissue ischemia and organ failure may occur. At the same time, fibrinogen degradation products can trigger the release of monocyte/macrophage–derived IL-1, IL-6, and PAI-1. Whereas IL-1 and IL-6 induce additional vascular endothelial damage, PAI-1 inhibits fibrinolysis, which accelerates further thrombus formation.95 Apart from these functions, fibrinogen is an important factor that regulates cellular interactions in the vasculature, such as the binding of leukocytes to the endothelial cells.96
As shown for intra-abdominal infections, fibrinous exudates can incorporate large numbers of bacteria, viz E coli andBacteroides fragilis. Once bacteria are sequestered within the fibrin deposit, they are protected from phagocytosis, which might be a microbial strategy for avoiding the host defense machinery. Inside the clot, the microorganism can continue to proliferate, leading to the formation of abscesses. However, the host can also benefit from the encapsulation of the microorganism, since the entrapment of bacteria in a fibrin deposit might act to diminish the magnitude of bacterial dissemination, thus preventing host mortality.97
Fibrinogen-binding proteins (FgBPs) have been studied mostly in staphylococci and streptococci. To date, 5 different FgBPs have been characterized in S aureus.98-102 Three of them are secreted: (1) an 87-kd coagulase, which binds to prothrombin and forms a complex that has thrombinlike activity and converts fibrinogen to fibrin,103 (2) a 60-kd FgBP, which binds to fibrinogen and prothrombin,98 and (3) a 16-kd FgBP, which binds to the α-chain of fibrinogen.99 The last-named protein has been shown to contribute to bacterial virulence in wound infections owing to its ability to delay healing processes.104 Additionally, 2 surface-associated clumping factors (ClfA and ClfB) of S aureus have been found to adhere to fibrinogen-containing substrates.102 ClfA was shown to play an important role in the pathogenesis of S aureus endocarditis,105 whereas the function of ClfB is not completely understood.106 The ability of staphylococcal surface proteins to bind to fibrinogen is believed to be important in promoting bacterial adherence to host tissues during an infection and may serve as a mechanism for colonization of the cardiac tissue in infective endocarditis. A membrane-bound FgBP inStaphylococcus epidermidis, designated Fbe, which shares sequence homology with ClfA, has been identified recently.107 However, unlike ClfA, Fbe seems to mediate the binding of the microorganism to fibrinogen-coated surfaces.108 A recent serological study showed that antibody levels to the FgBPs, ClfA and Fbe, were significantly risen in convalescent-phase sera from patients with S aureussepticemia, indicating that these 2 proteins are expressed during infection.109
Most group A streptococcal serotypes (S pyogenes) express FgBPs on their surface.82 Apart from fibrinogen-mediated adherence of these bacteria to endothelial and epithelial cells, streptococcal FgBPs seem to have an important antiphagocytic function owing to their ability to impair deposition of complement.110-113
Bacteria and fibrinolysis
Plasmin(ogen) plays a central role in fibrinolysis as it dissolves insoluble fibrin matrices.114 In addition to its fibrinolytic properties, plasmin degrades extracellular matrix proteins such as laminin and fibronectin and activates metalloproteinases.23,115 Studies with plasminogen-deficient mice have shown an effect of the enzyme on cell migration and tissue remodeling.116 In addition to plasmin(ogen), plasminogen activators have been demonstrated to be crucial factors in fibrinolysis. Transgenic mice deficient in tPA and uPA showed impaired clot lysis and suffered extensive spontaneous fibrin deposition.117 Also, these mice developed venous thrombosis upon endotoxin administration to a higher extent than wild-type animals.
In sepsis, progressive failure of multiple organs is often accompanied by fibrin deposition and formation of microthrombi. Clinical studies have provided evidence that plasminogen concentrations in septic patients are significantly decreased.118 Moreover, plasminogen levels or the ratio of plasminogen to α2-antiplasmin have been demonstrated to be good markers for survival from septicemia.118 Recently, Aiuto et al119 reported that infusion of recombinant tPA in a patient suffering from meningococcal purpura fulminans resulted in a dramatic improvement in hemodynamics and an increase in skin perfusion. This effect might be explained by the observation that PAI-1 levels in sepsis patients are significantly increased, leading to the consumption of plasminogen activators, which in turn down-regulates plasmin activity.118,120 121 Taken together, these studies indicate that disturbance of the fibrinolytic process in infectious diseases, either by low plasmin levels in blood or by the lack of plasminogen activation, can lead to enhanced fibrin deposition, causing life-threatening sequelae.
As shown in Table 2, many bacterial species have been demonstrated to interact with human plasmin(ogen).122 Interestingly, the mode of interaction varies for different species.123 For example, most group A streptococci secrete the nonenzymatic plasminogen activator streptokinase. Binding of streptokinase to plasminogen converts the zymogen into an active enzyme by inducing a conformational change without hydrolyzing a peptide bond, which is normally required to activate plasminogen.124 Also, staphylococci secrete a nonenzymatic plasminogen activator, staphylokinase. The mechanism of plasminogen activation by staphylokinase differs from its activation by streptokinase, however, in that small amounts of active plasmin are required for an efficient activation.125 The crystal structures of the catalytic domain of human plasmin in complex with streptokinase or staphylokinase have been solved recently.126,127 In contrast to nonenzymatic activators, a surface-bound plasminogen-binding protein (Pla) that exhibits protease activity has been found in Yersinia pestis.128Bacterially bound plasmin is thought to trigger dissemination ofY pestis from a peripheral site and/or to facilitate the escape of the microorganism from fibrin-mediated entrapment.129 In addition to microbial plasminogen activators, many pathogenic bacteria express nonactivating plasminogen-binding molecules on their surface (Table 2). Once plasminogen is bound to the bacterial surface, the zymogen can be activated by different mechanisms. Whereas streptococci and staphylococci produce their own activators, Borrelia burgdorferi, Salmonella enteritidis, and E coli can activate bound plasminogen by recruited tPA or uPA.130,131 Studies with B burgdorferi, S aureus, and S pyogenes have demonstrated that plasmin, mobilized to the bacterial surface, is protected from inhibition by host proteinase inhibitors, in particular α2-antiplasmin, resulting in a long-lasting surface-associated enzymatic activity.132-134 An important advantage for bacteria, which bind and activate plasminogen, was demonstrated by Coleman et al,135 who used plasminogen-deficient mice to show that the active enzyme gives rise to enhanced dissemination of B burgdorferi. Recently, Svensson et al136 reported that plasminogen-binding serotypes of S pyogenes have a strong tendency to cause impetigo lesions, suggesting a central role for bacterially activated plasmin during localized infection in the epidermis. These studies indicate once more that the interaction of bacteria with plasmin(ogen) might have an important role in bacterial pathogenesis.
Interaction of bacteria or bacterial products with plasmin(ogen)
| Bacterial species . | Gram reaction . | Interactions investigated . | Reference . |
|---|---|---|---|
| Borrelia burgdorferi | Gram-negative | Activation of bound plasminogen in the presence of host-derived plasminogen activators | 130, 132, 135 |
| Escherichia coli | Gram-negative | Binding of plasminogen to G fimbria and curli organelles followed by activation by tPA | 131, 179 |
| Fusobacterium nucleatum | Gram-negative | Activation of bound plasminogen by streptokinase, uPA, or Porphyromonas gingivalis culture supernatant | 180 |
| Helicobacter pylori | Gram-negative | Determination of binding constants and mapping of bacterial-binding site in plasminogen | 122, 181 |
| Mycoplasma fermentans | Gram-negative | Activation of bound plasminogen by tPA | 182 |
| Neisseria gonorrhoeae | Gram-negative | Determination of the number of bacterial binding sites for plasminogen, activation by tPA, and measurements of plasmin activity on the cell surface | 183 |
| Neisseria meningitidis | Gram-negative | Determination of the number of bacterial binding sites for plasminogen, activation by tPA, and measurements of plasmin activity on the cell surface | 183 |
| Pseudomonas aeruginosa | Gram-negative | Degradation of plasminogen and plasmin by an extracellular protease | 184, 185 |
| Salmonella enteritidis | Gram-negative | Binding of plasminogen to thin aggregative fimbriae followed by activation by tPA | 131 |
| Salmonella typhimurium | Gram-negative | Binding of plasminogen to type 1 fimbria | 179 |
| Staphylococcus aureus | Gram-positive | Determination of the number of bacterial-binding sites for plasminogen, activation by tPA, and measurements of plasmin activity on the cell surface | 186 |
| Streptococcus species; group A, C, and G | Gram-positive | Binding of plasminogen to M and M-like proteins, activation of bound plasminogen by streptokinase | 134, 187, 188 |
| Yersinia pestis | Gram-negative | Studies on the role of an outer membrane plasminogen activator in systemic infection | 128,129 |
| Bacterial species . | Gram reaction . | Interactions investigated . | Reference . |
|---|---|---|---|
| Borrelia burgdorferi | Gram-negative | Activation of bound plasminogen in the presence of host-derived plasminogen activators | 130, 132, 135 |
| Escherichia coli | Gram-negative | Binding of plasminogen to G fimbria and curli organelles followed by activation by tPA | 131, 179 |
| Fusobacterium nucleatum | Gram-negative | Activation of bound plasminogen by streptokinase, uPA, or Porphyromonas gingivalis culture supernatant | 180 |
| Helicobacter pylori | Gram-negative | Determination of binding constants and mapping of bacterial-binding site in plasminogen | 122, 181 |
| Mycoplasma fermentans | Gram-negative | Activation of bound plasminogen by tPA | 182 |
| Neisseria gonorrhoeae | Gram-negative | Determination of the number of bacterial binding sites for plasminogen, activation by tPA, and measurements of plasmin activity on the cell surface | 183 |
| Neisseria meningitidis | Gram-negative | Determination of the number of bacterial binding sites for plasminogen, activation by tPA, and measurements of plasmin activity on the cell surface | 183 |
| Pseudomonas aeruginosa | Gram-negative | Degradation of plasminogen and plasmin by an extracellular protease | 184, 185 |
| Salmonella enteritidis | Gram-negative | Binding of plasminogen to thin aggregative fimbriae followed by activation by tPA | 131 |
| Salmonella typhimurium | Gram-negative | Binding of plasminogen to type 1 fimbria | 179 |
| Staphylococcus aureus | Gram-positive | Determination of the number of bacterial-binding sites for plasminogen, activation by tPA, and measurements of plasmin activity on the cell surface | 186 |
| Streptococcus species; group A, C, and G | Gram-positive | Binding of plasminogen to M and M-like proteins, activation of bound plasminogen by streptokinase | 134, 187, 188 |
| Yersinia pestis | Gram-negative | Studies on the role of an outer membrane plasminogen activator in systemic infection | 128,129 |
TPA indicates tissue plasminogen activator; uPA, urokinase-type plasminogen activator.
Treatments using coagulation inhibitors
Blood coagulation is normally limited to a site of vascular injury, indicating that clotting is regulated in a highly controlled manner. The mechanism requires, apart from procoagulative factors, a number of specific proteinase inhibitors or enzymes that down-regulate clotting activity.137 It is important to stress that the correct ratio of procoagulative enzymes and their inhibitors is necessary to ensure that clotting is restricted to a damaged site. Once this delicate balance is disturbed, an efficient wound-healing process is hindered either by the inability to form a fibrin network, by uncontrolled coagulation, or by impaired fibrinolysis. In patients with sepsis, severe sepsis, or septic shock, the levels of many natural inhibitors are markedly lowered, and this may result in an unfavorable outcome for the disease.138-140 In several animal models and clinical trials, therapeutic substitution of coagulation inhibitors has been demonstrated to improve survival in cases of severe infection. In what follows, some of these treatments are described.
Tissue factor pathway inhibitor
Expression of TFPI is, under normal conditions, restricted to megakaryocytes, to endothelium of the small capillaries, and to macrophages. TFPI levels in plasma are increased under inflammatory conditions.50 However, despite the elevated levels of plasma TFPI in sepsis patients, efficacy studies indicate that in addition, administration of a 10-fold higher concentration of recombinant TFPI might be needed to compensate for an uncontrolled activation of the extrinsic pathway of coagulation.141 Treatments with recombinantly expressed variants of TFPI showed improved survival rates in several rabbit and baboon sepsis models,142-145 whereas, in pigs, TFPI treatment attenuated the response of the inflammatory mediators TNF-α and IL-8, but did not provide significant survival benefit.146 The anti-inflammatory effect of TFPI can be explained by recent observations made by Park et al,147who showed that TFPI binds to endotoxin, suggesting that this interaction can depress cellular responses to the bacterial component. Infusion of TFPI in humans who received an intravenous injection of endotoxin resulted in an attenuation of thrombin generation. However, endotoxin-induced initiation of the fibrinolytic system and release of cytokines (TNF-α and IL-6) was not affected by TFPI treatment.148 Currently, a phase 2 clinical trial of recombinantly expressed TFPI in patients with sepsis is in progress.141
Antithrombin III
AT III is the main inhibitor of thrombin and F X, but other serine proteinases, including F IX, F XI, F XII, PK, uPA, tPA, and plasmin, are also inactivated by AT III.149 In septic shock, AT III levels drop drastically in plasma, and low AT III levels are predictive of a fatal outcome for the disease.139 Several mechanisms can cause low AT III levels, such as consumption of AT III, degradation by elastase released from neutrophils, and extravascular leakage due to increased vascular permeability.150Experiments with baboons infected with a lethal dose of E coli showed that treatment with AT III increased the formation of thrombin–AT III complexes and diminished fibrinogen consumption as compared with a nontreated control group. The analysis of the cytokine response to the AT III administration revealed that levels of IL-6, IL-8, and IL-10 in plasma were significantly reduced in the AT III–treated group, whereas TNF-α concentrations were enhanced.151 The finding that survival was improved in the group of infected baboons treated with AT III indicates that the anticoagulant and anti-inflammatory effects of an AT III treatment may improve the outcome in severe infectious diseases.151Application of AT III has also been tested in humans suffering from severe sepsis or septic shock in several studies.150Generally, high doses are required to maintain supranormal antithrombin levels and to overcome the problem of antithrombin consumption.149 Recently, Baudo et al152published a double-blind, randomized, multicenter study of 120 patients receiving AT III or placebo treatment. They concluded from their study that AT III reduces mortality only in a subgroup of septic shock patients. Other studies by Inthorn et al153 showed that long-term treatment with AT III attenuates the systemic inflammatory response and leads to a down-regulation of IL-6 in patients with severe sepsis. A double-blind placebo-controlled study involving 120 patients was carried out in Italy in 1999. Within the first 30 days, this trial showed that AT III therapy had a favorable effect on the survival of patients suffering from severe sepsis and/or post-surgery complications. However, this effect was not significant in terms of the overall survival.3
C1 inhibitor
C1 INH, the major plasma inhibitor of activated complement C1-esterase, PK, and F XII, is an acute-phase protein, and its levels can increase up to 2-fold during uncomplicated sepsis.154However, the ratio between functional and total levels of C1 INH is lower in patients with sepsis than in healthy volunteers.155 It has been assumed that degradation of the active inhibitor, probably by neutrophil elastase, is the cause of this phenomenon.154 Administration of C1 INH to baboons suffering from lethal E coli sepsis results in a reduction of circulating cytokine levels, such as TNF-α, IL-6, IL-8, and IL-10. Anatomical examination of C1 INH–treated animals revealed less severe damage to various organs than in control animals. However, the C1 INH therapy failed to result in a significant increase in survival.156 The effect of a combined C1 INH and AT III treatment after intravenous injection of endotoxin in rabbits was investigated by Giebler et al.157 This study found that the treatment significantly decreased clot formation in lungs and livers and resulted in an improved cardiovascular stability. There have also been reports that C1 INH treatment improves the clinical outcome in patients suffering from septic shock or streptococcal toxic shock syndrome. However, no controlled double-blind study of the effect of C1 INH in severe sepsis or septic shock has been evaluated so far.158 159
Thrombomodulin, protein C, protein S, and C4b-binding protein
TM is a cofactor expressed on endothelial cell surfaces, which modifies the activity of thrombin, leading to an activation of protein C and subsequent initiation of the anticoagulative pathway. The expression of TM is down-regulated by endotoxin and cytokines such as IL-1.160 Several animal studies provide evidence that application of a recombinantly produced soluble variant of TM prevents fatal acute thromboembolism and diminishes glomerular fibrin deposition in endotoxin-induced disseminated intravascular coagulation (DIC).161 A double-blind study to evaluate the effect of recombinant soluble TM in DIC is in progress.161
Protein C and its cofactor protein S function as important regulatory factors of the blood coagulation cascade.162Protein S is found in 2 forms, as free protein or in a noncovalent bimolecular complex with C4b-binding protein (C4BP).163The latter protein is a regulatory protein in the complement system that down-regulates the classical pathway of complement activation. C4BP binds approximately 60% of the circulating protein S and inactivates its anticoagulant properties.162 During sepsis, protein C levels are lowered, and especially in meningococcal septic shock, severely reduced protein C levels contribute to mortality and morbidity.94,164,165 Levels of C4BP and protein S, however, are within normal range, indicating that it is mainly the deficiency of protein C that determines the severity of meningococcal septic shock.165 In animal models, the administration of protein C had favorable effects on the hemodynamic parameters of endotoxin-treated animals and led to an improved survival.166,167 Smith and White168 recently published a clinical study involving 30 patients with meningococcaemia and severe protein C deficiency. Treatment of the patients with protein C resulted in a significant improvement of the host response to meniningococcal infection. Protein C and activated protein C are currently being investigated in clinical trials for septic shock.169 The receptor for protein C (EPCR) seems to play an important role in regulating the activity of protein C in infectious diseases. As shown in baboons that were challenged with a sublethal dose of E coli, the administration of a monoclonal antibody that blocks protein-C binding to EPCR converts the response to sublethal concentrations of bacteria into a lethal challenge.169 Interestingly, C4BP has been shown to bind to most strains of the species S pyogenes andBordetella pertussis.170 171 Functional studies indicate that the bacteria could interact with C4BP in order to protect themselves from a complement-mediated attack. However, any possible effect on the coagulation cascade was not studied.
Concluding remarks
Despite an improved intensive care system, morbidity and mortality associated with severe bacterial infections constitute an increasing problem. This dilemma is caused by, among other factors, the ability of bacteria to develop antibiotic resistance and by an increasing number of immunocompromised patients. In order to develop novel therapeutic strategies that can successfully fight severe diseases caused by bacteria, it is necessary to have a thorough understanding of the molecular mechanisms involved in their interactions with host effector systems. Much knowledge about sepsis and septic shock has been achieved by studying the effect of endotoxin in different in vitro and in vivo models. However, Gram-positive bacteria are increasing in prevalence in sepsis patients, and not all complications that occur in Gram-negative sepsis can be explained by the effect of endotoxin.172-175An increasing knowledge of the role of microbial products in hemostasis may lead to novel treatments in life-threatening infectious diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Heiko Herwald, Department of Cell and Molecular Biology, Section for Molecular Pathogenesis, Lund University, PO Box 94, S-221 00 Lund, Sweden; e-mail:heiko.herwald@medkem.lu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal