Abstract
The human recombinant histamine-releasing factor (HrHRF) was previously shown to induce histamine release from human basophils from a subset of donors. The ability of HrHRF to directly induce histamine release from only certain basophils was thought to involve interaction between HrHRF and a particular kind of IgE, termed IgE+, on the surface of these cells. Recent studies disproved the hypothesis that the IgE molecule or its high-affinity receptor, FcεRI, is involved in secretion of histamine and cytokines by basophils stimulated with HrHRF. Rather, data suggest that HrHRF is a cytokine that stimulates basophils by binding to a cell-surface structure other than the IgE molecule. This report describes the effects of HrHRF on another inflammatory cell type: eosinophils from mildly allergic donors. In purified eosinophils primed with granulocyte-macrophage colony-stimulating factor, both tumor necrosis factor α (TNF-α) and HrHRF induced increased secretion of interleukin (IL) 8. In addition, both HrHRF and IL-5 enhanced secretion of IL-8 stimulated by TNF-α. Secretion of IL-8 reached a plateau level in less than 24 hours, was inhibited by cycloheximide, and required the presence of HrHRF throughout the culture period. In some eosinophil preparations, HrHRF induced calcium mobilization that was inhibited by pertussis toxin. Additionally, HrHRF caused secretion of IL-8 from the human eosinophilic cell line, AML14-3D10, which does not possess the α chain of FcεRI. These data provide evidence that HrHRF contributes to activation of eosinophils and thus suggest an additional role for HrHRF in the pathophysiologic mechanisms of allergic disease.
Introduction
Histamine-releasing factors (HRFs) comprise a group of molecules that induce basophil degranulation. Molecules with this activity include interleukins,1,2chemokines,3-5 and the previously subcloned IgE-dependent HRF.6 This human recombinant HRF (HrHRF) (also known as p237 or translationally controlled tumor protein8,9) was initially described as a complete secretagogue for secretion of histamine6 and interleukin (IL) 410 from basophils from a subset of allergic donors. Mediator release was thought to be the consequence of physical interaction between HRF and a certain type of IgE, termed IgE+, on the surface of the responding basophils.11 However, several lines of evidence suggest that the interaction with IgE may not be required for cell activation mediated by HrHRF. First, HrHRF enhanced anti-IgE–mediated histamine release, as well as IL-4 and IL-13 protein production, in donors with IgE− on the surface of their basophils, which by definition do not respond directly to HrHRF.12 Second, in IgE+ donors, HrHRF-induced histamine release can be modulated by agents that do not affect IgE-dependent histamine release.13 Third, rat basophilic leukemia cells transfected with the human α, β, and γ chains of FcεRI released histamine to polyclonal anti-IgE after passive sensitization with IgE+, but no histamine release occurred when HrHRF was the stimulus.14 These data indicate that HrHRF exerts its activity independently of IgE and strongly suggest the existence of an HrHRF-specific activation pathway other than through FcεRI. Therefore, we studied the role of HrHRF in activation of human eosinophils, inflammatory cells that do not normally express cell-surface FcεRI.
Although one study found that eosinophils from a subset of hypereosinophilic patients have FcεRI15 and another showed that the FcεRI α chain is up-regulated on eosinophils after antigen challenge in asthmatic subjects,16 this area of investigation remains controversial. A more recent study demonstrated that eosinophils from a variety of donors, including mildly allergic subjects, possess intracellular FcεRI α chain, but it was undetectable on the cell surface.17 Additionally, although Kita et al18 found extremely low levels of surface FcεRI, there was no effector function that could be attributed to this receptor. For these experiments, we used eosinophils from mildly allergic patients who had no detectable surface expression of FcεRI, the low-affinity IgE receptor FCεRII, or IgE, as determined by flow cytometry. Furthermore, we used the human eosinophilic cell line AML14-3D10, which has no observable FcεRI α chain on Western blot analysis of cell lysates.
Eosinophils are known to contribute to the pathophysiologic mechanisms of allergic diseases by secreting proinflammatory granule proteins, such as major basic protein and eosinophil cationic protein, that induce damage of bronchial epithelial cells.19,20Increased numbers of these cells are found in bronchoalveolar lavage (BAL) fluids and bronchial biopsy specimens from allergic asthmatic patients during the late phase of an allergic reaction (LPR).21 There is evidence that these cells are capable of synthesizing, storing, and in some cases, releasing cytokines that contribute to cell recruitment and activation. Transcription or translation of the following cytokines has been reported to occur in eosinophils: IL-1α, IL-3, IL-5, IL-6, IL-8, transforming growth factor (TGF) α and TGF-β, macrophage inflammatory protein 1α (MIP-1α), granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor α (TNF-α),22IL-4,23 IL-10,23 IL-16,24 the regulated upon activation, normal T-cell expressed and secreted (RANTES) cytokine,24 and IL-12.25 Although there is controversy about which of these cytokines are actually secreted,26 the eosinophils, but not the neutrophils, of patients with bronchial asthma or atopic dermatitis show up-regulated IL-8 protein.27 Furthermore, concentrations of IL-8 in BAL fluids from asthmatic patients are several times higher than in those from healthy subjects.28 Although IL-8 was originally defined as a neutrophil chemoattractant in vivo,29,30 it was found that eosinophils show chemotactic responses toward IL-8 in vitro31 and that this function is enhanced in eosinophils from antigen-challenged allergic subjects.32 Taken together, these observations suggest that the increased levels of IL-8 in BAL fluids from asthmatic patients may be at least partly due to eosinophil activation that could lead to further eosinophil recruitment to the inflammatory site.
In this study, we demonstrated that HrHRF directly caused the release of IL-8 or enhanced TNF-α–induced IL-8 secretion from human eosinophils and the eosinophilic AML14-3D10 cell line, primed with GM-CSF. Enhancement of cytokine secretion depended on both protein synthesis and the presence of HrHRF throughout the culture period. Additionally, HrHRF was chemotactic for eosinophils. In eosinophils from some donors, HrHRF also induced calcium (Ca++) mobilization, which was independent of the addition of exogenous GM-CSF or TNF-α.
Materials and methods
We used 10 × piperazine diethanesulfonic acid (PIPES) buffer that contained 250 mmol/L PIPES (Sigma, St Louis, MO), 110 mmol/L sodium chloride (NaCl), and 50 mmol/L potassium chloride (KCl), adjusted to pH 7.4. PIPES-albumin-glucose (PAG) buffer was made with 10% 10 × PIPES and contained 0.003% human serum albumin (Calbiochem-Novexbiochem Corp, La Jolla, CA) and 0.1%d-glucose. PAG-calcium-magnesium (PAGCM) buffer was made by adding 1 mmol/L calcium chloride (CaCl2) and magnesium chloride (MgCl2) to PAG buffer. Percoll (Pharmacia, Piscataway, NJ) was made isotonic by mixing 1 part 10 × PIPES with 9 parts Percoll. This solution was then adjusted to a density of 1.09 g/mL by adding approximately 38 mL of 1 × PIPES to 100 mL of isotonic Percoll and verifying the results with a densitometer.33
For preparation of cell lysates, 1 mL of lysis buffer was used; this consisted of 25 mmol/L HEPES, 5 mmol/L KCl, 119.4 mmol/L NaCl2, 1 mmol/L MgCl2, 0.5 mmol/L CaCl2 (pH 8), 1 mmol/L sodium orthovanadate, 1% Nonidet P-40, and 20 μL protease inhibitor mixture (PharMingen, San Diego, CA). GM-CSF, TNF-α, IL-5, and RANTES were purchased from R&D Systems (Minneapolis, MN).
HrHRF production
HrHRF was subcloned from the PGEX-2T vector by using the restriction enzymes EcoR1 and BamH1 used inEscherichia coli production.6 The 524 base-pair (bp) fragment was ligated into the baculovirus vector pBlueBac III, which coexpresses β-galactosidase, for color selection of successful recombination. In accordance with the manufacturer's specifications for the baculovirus system (MAXBAC; Invitrogen, San Diego, CA), plasmid DNA was transfected into Sf 9 cells for viral isolation and amplification and High 5 insect cells were subsequently used for protein production. The insect cells were grown commercially on a large scale in serum-free medium (Paragon Biotech, Baltimore, MD). The cell pellet from 14 L of insect cells was dissolved in 400 mL of NBB buffer (20 mmol/L sodium phosphate and 500 mmol/L NaCl [pH 7.8]) to which 400 μL of inhibitor mixture (PharMingen) was added. The suspension was freeze-thawed twice and centrifuged at 9000 rpm for 20 minutes. Purification of the supernatant, which contained the HrHRF protein, was accomplished by 2-step column chromatography. First, the supernatant was mixed with De-52 (Whatman, Maidstone, UK) in 0.02 mol/L Tris buffer (pH 8). The flow-through was screened for the presence of HrHRF by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting using a polyclonal anti-HRF antibody generated against the recombinant material produced in E coli.6 Positive fractions were concentrated and placed on a column (Sephadex G75; Pharmacia) in physiologic PIPES buffer. Again, SDS-PAGE and Western blotting were used to screen the column fractions. Fractions were pooled and concentrated and the bioactivity was confirmed by using the basophil histamine-release assay. The protein concentration of HrHRF was determined with a protein assay (Bio-Rad, Hercules, CA) and was found to be 160 μg/mL. Because the molecular weight of HrHRF is 23 kd, this stock solution is equivalent to 7 μmol/L. HrHRF was dialyzed against physiologic PIPES buffer for use in all assays. HrHRF had a single band on SDS-PAGE and was judged to be more than 95% pure.
Endotoxin levels in purified HrHRF were determined by a limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD), according to the manufacturer's specifications. Additionally, endotoxin was removed from certain aliquots of HrHRF by using a column containing polymyxin B immobilized on agarose (Detoxi-Gel; Pierce, Rockford, IL).
Purification of eosinophils
Granulocytes were isolated from EDTA-anticoagulated venous blood from mildly allergic donors by gradient centrifugation in isotonic Percoll (1.09 g/mL). Red blood cells were removed by hypotonic lysis, and CD16-positive cells (neutrophils) were removed with an immunomagnetic bead technique.33 Eosinophils were differentiated by using high-power light microscopy after staining (Diff-Quick Stain Kit; Dade, Düdingen, Switzerland). Eosinophil purity was always greater than 98%; in some experiments, it was 100%.
Cell cultures
Human eosinophils.
Purified eosinophils were resuspended in medium M199 (Gibco, Grand Island, NY) containing 20% fetal-calf serum (FCS; Sigma) with or without GM-CSF (10 ng/mL) and incubated for 30 minutes at 37°C in 5% carbon dioxide (CO2). Aliquots of 5 × 105 cells were transferred to 48-well plates containing TNF-α (50 ng/mL or 10 ng/mL), IL-5 (50 ng/mL), HrHRF (0.7 μmol/L), or a combination of these reagents. The final eosinophil concentration in each assay was 2 × 106/mL. In some experiments, either pertussis toxin (PT) or its inactive β-oligomer (List Biological Laboratories, Campbell, CA) was also added (1 ng/mL each). After 24 hours in culture at 37°C in 5% CO2, plates were centrifuged for 5 minutes at 1000 rpm and supernatants were collected and stored at −20°C until analysis by enzyme-linked immunosorbent assay (ELISA). In some experiments, cycloheximide (1 μmol/L) (Sigma) was added at the beginning of the culture. Cycloheximide did not affect cell viability as determined by staining with erythrosin B (Sigma).
AML14-3D10 cells.
The AML14-3D10 subline, a gift of Dr Cassandra Paul (Wright State University, Dayton, OH), is a human eosinophilic leukemic cell line that consistently shows eosinophilic granules in 95% of its cells.34 Cells were cultured in RPMI 1640 (Gibco BRL, Gaithersburg, MD), 8% FCS, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 50 μg/mL gentamicin (all from Biowhittaker), and 5 × 10−5 mol/L mercaptoethanol (JT Baker, Phillipsburg, NJ). Cells were incubated with the same cytokines used with the human eosinophils and cultured in the same manner. The final concentration of cells in the IL-8 production assays was 2 × 106/mL.
IL-8 determination
Supernatants from stimulated eosinophils were assayed with an IL-8–specific ELISA (Biosource, Camarillo, CA), according to the manufacturer's specifications. The threshold for cytokine detection was 10 pg/mL. In some cases, the values obtained were compared with values determined by using another commercial kit (R&D Systems). No significant differences were observed between the 2 assays.
Chemotaxis experiments
Chemotaxis experiments were performed by using the modified Boyden chamber technique described previously.35 Briefly, 25 μL of PAGCM buffer or various concentrations of the stimuli in the same buffer were placed in the lower chamber in triplicate. A 5-μm pore-sized polycarbonate membrane (Nucleopore Corp, Pleasanton, CA) separated the upper and lower chamber. Eosinophils (105) resuspended in PAGCM buffer were placed in each well of the upper chamber on top of the membrane. The chamber was then incubated for 30 minutes at 37°C in 5% CO2 and air, after which the chamber was disassembled. The membrane was removed, washed in PAG buffer to remove the nonmigrating eosinophils from the upper surface, scraped, and stained with Wright stain. Eosinophils from 10 high-power fields of triplicate wells were identified and counted.
Ca++ mobilization assay
The Ca++ mobilization assay we used was a modification of a procedure described for human basophils by MacGlashan et al.36 Purified eosinophils were loaded with 1 μmol/L Fura 2-AM (Molecular Probes, Eugene, OR) for 30 minutes at 37°C in RPMI-1640 (Gibco BRL) containing 2% FCS and 0.32 mmol/L EDTA. In some experiments, eosinophils were incubated for 2 hours at 37°C in 5% CO2 with 1 ng/mL of PT or its β-oligomer and Fura 2-AM was added during the last 30 minutes of the incubation. The cells (5 × 105) were washed once with PAG buffer and resuspended in 200 μL of PAG for loading in the microscope observation chamber. A 15-μL cell suspension was placed on a siliconized (Sigma Cote; Sigma) coverslip that comprised the base of the observation chamber. After 5 to 10 minutes of settling time, the cell drop was overlaid with 1 mL of PAGCM buffer at 37°C. The temperature, which was measured by a probe placed next to the settled cells, was brought to a stable 36.5°C and the stimulus (dissolved in 1 mL of prewarmed buffer) was added. Intracellular changes in Ca++ were monitored with a Zeiss Axiovert microscope with epifluorescence capacity as described previously.37
Flow cytometry to determine surface IgE expression
Cells were incubated for 30 minutes at 4°C in phosphate-buffered saline containing 0.2% bovine serum albumin (PBS-BSA) and 3.6 mg/mL human IgG with saturating concentrations of receptor-specific antibody or an equivalent concentration of an irrelevant isotype-matched control antibody. Surface IgE was detected by using a fluorescein isothiocyanate–conjugated polyclonal goat antihuman IgE (Kirkegaard and Perry, Gaithersburg, MD). The FcεRI α chain was investigated by using an IgG1 mouse antihuman monoclonal antibody (mAb), 22E7 (provided by Dr J. Kochan, Hoffman-La Roche Inc, Nutley, NJ). FcεRII (CD23) was assessed by using mAb 9P.25 (Immunotech Inc, Westbrook, ME). Anti-FcεRI mAb 22E7 is known to detect the high-affinity IgE receptor on human basophils,38 and anti-FcεRII mAb 9P.25 detects the low-affinity IgE receptor on human B cells.39 An IgG1 isotype control mAb was purchased from Sigma. Cells were washed with PBS-BSA, incubated with 1:150 dilutions of R-phycoerythrin–conjugated F(ab′)2 goat antimouse IgG antibody (Biosource) for 30 minutes at 4°C in the dark, washed, resuspended in PBS with 0.2% BSA, and assayed immediately with a flow cytometer (EPICS Profile II; Coulter, Hialeah, FL). Results were expressed as mean fluorescence intensity.
Cell lysates and Western blots to determine the presence of the FcεRI α chain
Human basophils were isolated by negative selection using magnetic beads from a basophil isolation kit (Miltenyi, Auburn, CA).13 Basophils (5.5 × 106) ranging in purity from 67% to 97% or AML14-3D10 cells (5.5 × 106) were lysed in 220 μL of lysis buffer for 20 minutes on ice. Lysed cells were spun at 14 000 rpm for 15 minutes in an Eppendorf centrifuge (Brinkman, Westbury, NY). Subsequently, 20 μL of each cell type (approximately 500 000 cells/lane) were combined with 20 μL of Tris-glycine 2 × sample buffer (Novex, San Diego, CA), boiled for 5 minutes, and electrophoresed (125 V) for 2 hours on a 4% to 20% Tris-glycine gel (Novex). SeeBlue molecular-weight markers (Novex) were used as a standard. Proteins from the cell lysates were transblotted on nitrocellulose (Schleicher & Schuell, Keene, NH; 30 V for 2 hours). The nitrocellulose was blocked in 10% nonfat dry milk in PBS with 05% Tween (PBS-T) for 1 hour. The blot was washed 3 times in PBS-T and incubated in PBS-T and 2% BSA for 3 hours with mAb 22E7 ascites, which detects the FcεRI α chain, at a dilution of 1:300. After a 15-minute washing, the secondary antibody, sheep antimouse horseradish peroxidase (Amersham Life Sciences, Piscataway, NJ), was diluted 1:20 000 in PBS-T and incubated for 1 hour. After the final washing step (6 times for 6 minutes each in PBS-T), the blot was developed by using SuperSignal chemiluminescent substrate (Pierce) and exposed to Hyperfilm ECL (Amersham Life Sciences).
Statistical methods
Results are expressed as the mean ± SEM. Where indicated, cells incubated under different conditions were compared by using the Student t test. In the Ca++ mobilization experiments, the Student t test was used to compare time points before and after the addition of the stimulus. Specifically, thet test was used to compare the area under the curve for a relevant time period by using the initial phase of the calcium response, which is important in the PT response.
Results
IL-8 production by HrHRF in eosinophils requires priming with GM-CSF
To determine the optimal conditions for cytokine production by eosinophils stimulated with HrHRF, freshly isolated eosinophils were incubated with HrHRF, TNF-α, and IL-5 alone and with a combination of these 3 cytokines. None of these cytokines alone induced significant IL-8 secretion. Only the combination of TNF-α and IL-5 induced significant cytokine production, which was only 400 pg/mL greater than the value for the medium control (data not shown). Although significant IL-8 production by peripheral blood eosinophils stimulated with TNF-α alone has been reported,23 other authors showed that priming of eosinophils with GM-CSF is required for optimal IL-8 secretion.28 Therefore, we tested whether preincubation of eosinophils with GM-CSF would alter IL-8 secretion. Incubation of eosinophils from 4 separate donors with GM-CSF for 30 minutes before stimulation greatly enhanced the responsiveness of these cells to TNF-α but not to IL-5 (Figure 1). In the presence of GM-CSF, HrHRF induced some IL-8 production above the value for the medium control; however, HrHRF enhanced IL-8 secretion induced by TNF-α or IL-5. The magnitude of the enhancement with HrHRF and TNF-α was greater than that with HrHRF and IL-5. Additionally, this enhancement of IL-8 production was comparable to that induced by the combination of TNF-α and IL-5 (Figure1).
Augmentation of interleukin (IL) 8 production in eosinophils primed with granulocyte-macrophage colony-stimulating factor (GM-CSF).
Cells were preincubated in 10 ng/mL GM-CSF. After 30 minutes, aliquots were distributed in wells containing medium, 50 ng/mL tumor necrosis factor α (TNF-α), 50 ng/mL IL-5, 0.7 μmol/L human recombinant histamine-releasing factor (HrHRF), or a combination of these cytokines, as indicated on the x-axis. After 24 hours of culture, supernatants were collected and IL-8 content was determined by enzyme-linked immunosorbent assay (ELISA). *P < .05 compared with cells incubated with medium alone (4 experiments).
Augmentation of interleukin (IL) 8 production in eosinophils primed with granulocyte-macrophage colony-stimulating factor (GM-CSF).
Cells were preincubated in 10 ng/mL GM-CSF. After 30 minutes, aliquots were distributed in wells containing medium, 50 ng/mL tumor necrosis factor α (TNF-α), 50 ng/mL IL-5, 0.7 μmol/L human recombinant histamine-releasing factor (HrHRF), or a combination of these cytokines, as indicated on the x-axis. After 24 hours of culture, supernatants were collected and IL-8 content was determined by enzyme-linked immunosorbent assay (ELISA). *P < .05 compared with cells incubated with medium alone (4 experiments).
Although the HrHRF preparations produced in baculovirus used for these experiments contained low but variable amounts of endotoxin, we do not believe that contaminating endotoxin accounted for our results. Although there is one report that 10 ng/mL of lipopolysaccharide (LPS) induced IL-8 secretion from human eosinophils,40 we used a polymyxin B column to remove endotoxin and our HrHRF preparation contained 10 000-fold less endotoxin than the amount reported as necessary to induce IL-8. Thus, HrHRF that contained 8.5 ng/mL of endotoxin on limulus amebocyte lysate assay was placed on a Detoxi-Gel column containing polymyxin B immobilized on agarose and an 8500-fold reduction in endotoxin was achieved. This resulted in a barely detectable level of 1 pg/mL of endotoxin in the assay with eosinophils. Additionally, preparations that contained almost undetectable amounts of endotoxin (1 pg/mL) behaved similarly to those with higher levels of endotoxin (350 ng/mL). Moreover, when preparations of HrHRF containing 350 ng/mL of endotoxin were used, LPS at a concentration of 350 ng/mL did not reproduce the results (n = 4, data not shown).
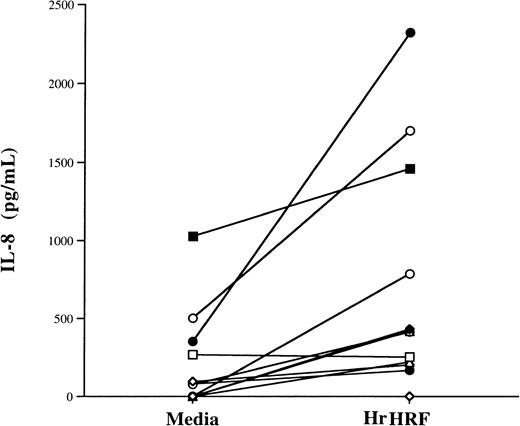
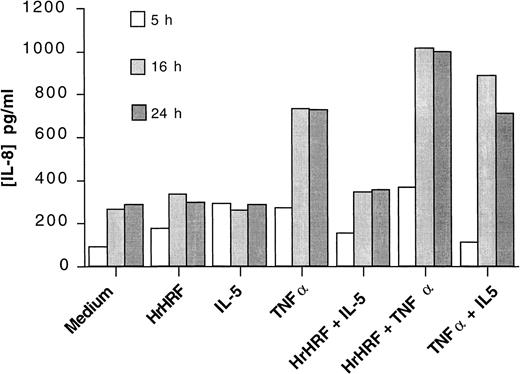
Although HrHRF alone did not significantly increase IL-8 production in the 4 donor samples used to produce the results shown in Figure 1, there was a trend toward an increase. Therefore, we examined samples from additional donors. In 12 experiments using eosinophil samples from 7 different donors, HrHRF induced significant production of IL-8 by eosinophils primed with GM-CSF, even in the absence of TNF-α (Figure2). All 12 experiments are plotted in Figure 2, and the same symbol is used to depict samples from the same donor. Although there was some variability, mean IL-8 levels were 250 ± 136 pg/mL without stimulation and 682 ± 202 pg/mL after exposure to HrHRF; thus, HrHRF significantly increased production of IL-8 (P < .03). Because of these results, priming or coculture with GM-CSF was used in subsequent experiments that demonstrated IL-8 production from eosinophils, the Ca++experiments, and the studies with AML14-3D10 cells. Additionally, the kinetics of cytokine production was studied by collecting supernatants of stimulated cells at 5, 16, and 24 hours. As shown in Figure3, the kinetics of IL-8 secretion was similar regardless of the stimulus used and reached a plateau level in less than 24 hours.
Induction of IL-8 secretion by HrHRF alone in eosinophils primed with GM-CSF.
Eosinophils were treated with GM-CSF and placed in medium alone or with 0.7 μmol/L HrHRF, and the supernatants were tested for IL-8 content. Twelve experiments using samples from 7 donors are represented. Each experiment is plotted; the same symbol is used to depict samples from the same donor. There was a significant difference in IL-8 production between HrHRF-stimulated cells and controls (P < .03).
Induction of IL-8 secretion by HrHRF alone in eosinophils primed with GM-CSF.
Eosinophils were treated with GM-CSF and placed in medium alone or with 0.7 μmol/L HrHRF, and the supernatants were tested for IL-8 content. Twelve experiments using samples from 7 donors are represented. Each experiment is plotted; the same symbol is used to depict samples from the same donor. There was a significant difference in IL-8 production between HrHRF-stimulated cells and controls (P < .03).
Kinetics of IL-8 production.
Eosinophils were incubated with GM-CSF for 30 minutes and then stimulated for 5 hours (open bars), 16 hours (gray bars), or 24 hours (closed bars) with the cytokines indicated on the x-axis. An experiment representative of 3 is shown.
Kinetics of IL-8 production.
Eosinophils were incubated with GM-CSF for 30 minutes and then stimulated for 5 hours (open bars), 16 hours (gray bars), or 24 hours (closed bars) with the cytokines indicated on the x-axis. An experiment representative of 3 is shown.
IL-8 production by eosinophils requires protein synthesis
It is known that some cytokines are both stored in a preformed state in granules and newly synthesized. For example, TNF-α is released from mast cells after cross-linking FcεRI as a result of both release from preformed cytoplasmic granule stores and de novo synthesis.41 We questioned whether de novo protein synthesis is required for cytokine-induced IL-8 production from eosinophils. Stimulated eosinophils were incubated in the presence or absence of 1 μmol/L cycloheximide. As shown in Figure4, cycloheximide inhibited IL-8 production by all the stimuli. However, in 2 of 3 experiments, the combination of HrHRF and TNF-α induced small but measurable amounts of IL-8 from cycloheximide-treated cells. Because cycloheximide did not decrease cell viability, the decrease in HrHRF–stimulated IL-8 production was probably due to decreased protein synthesis.
IL-8 production is dependent on protein synthesis.
Eosinophils were incubated in the presence of GM-CSF and cycloheximide (1 μmol/L; open bars) or GM-CSF alone (closed bars). After 30 minutes, cells were transferred to wells containing the stimuli indicated on the x-axis. Twenty-four hours later, the IL-8 content of the supernatants was determined by ELISA (3 experiments).
IL-8 production is dependent on protein synthesis.
Eosinophils were incubated in the presence of GM-CSF and cycloheximide (1 μmol/L; open bars) or GM-CSF alone (closed bars). After 30 minutes, cells were transferred to wells containing the stimuli indicated on the x-axis. Twenty-four hours later, the IL-8 content of the supernatants was determined by ELISA (3 experiments).
Continued presence of HrHRF in cultures is necessary for enhanced IL-8 production
We questioned whether incubation of eosinophils with HrHRF for short periods would provide the necessary signal to enhance IL-8 secretion induced by TNF-α. Therefore, eosinophils treated with GM-CSF were incubated for 1 hour with medium or HrHRF, washed, and stimulated with additional medium or TNF-α without HrHRF. The culture continued for 24 hours. When HrHRF was present during only the first hour of culture, no enhancing effect on TNF-α–induced IL-8 production was observed (1963 pg/mL versus 1778 pg/mL). In contrast, when HrHRF was present throughout the culture period, 3913 pg/mL of IL-8 was generated. Decreased IL-8 production in cells primed with HrHRF was not a consequence of cell washing because comparable levels of IL-8 were produced in cultures that had been washed and then replenished with the appropriate cytokines (3848 pg/mL versus 3913 pg/mL).
IL-8 production by stimulated eosinophils is inhibited by a modulator of Gαiprotein activity
We previously found evidence that HrHRF-induced histamine release from human basophils is insensitive to PT, a known inhibitor of G proteins bearing an αi subunit.13 In contrast to these results with basophils, PT (1 ng/mL) moderately reduced IL-8 secretion from GM-CSF–primed eosinophils that was induced by HrHRF, TNF-α, or the combination of HrHRF and TNF-α (Figure5). This reduction was a consistent finding. To provide a control, cells were also stimulated in the presence of the PT β-oligomer that does not affect Gαifunction. In the experiments shown in Figure 5, the PT β-oligomer did not affect IL-8 secretion induced by the various stimuli. These experiments used a suboptimal concentration of TNF-α (10 ng/mL), which was also shown to have synergy with HrHRF for IL-8 production.
Pertussis toxin inhibits IL-8 secretion by eosinophils.
Cells were stimulated with TNF-α (10 ng/mL), HrHRF (0.7 μmol/L), or both in the absence (open bars) or presence of 1 ng/mL of either PT (closed bars) or its inactive β-oligomer (stippled bars). Data are the mean values from 2 experiments.
Pertussis toxin inhibits IL-8 secretion by eosinophils.
Cells were stimulated with TNF-α (10 ng/mL), HrHRF (0.7 μmol/L), or both in the absence (open bars) or presence of 1 ng/mL of either PT (closed bars) or its inactive β-oligomer (stippled bars). Data are the mean values from 2 experiments.
HrHRF induces Ca++mobilization in eosinophils from some donors that is inhibited by PT
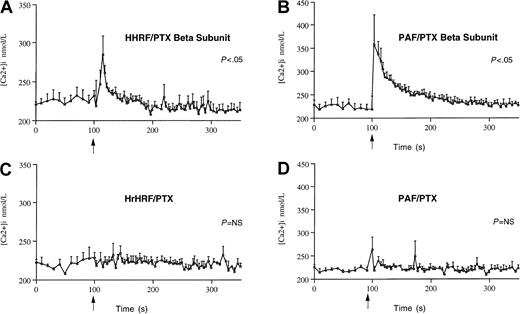
Because human eosinophils are known to possess the receptor for GM-CSF,42 we investigated the effect of HrHRF on the Ca++ response in the absence of GM-CSF. Unlike the previous experiments, the initial Ca++ experiments did not include use of GM-CSF to prime the eosinophils. Figure6 shows the average Ca++response of eosinophils (4 different donors) preincubated with HrHRF in the presence of PT or the PT β-oligomer. HrHRF induced an elevation in Ca++ in eosinophils preincubated with the PT β-oligomer (P < .05), whereas in cells pretreated with PT, the Ca++ response induced by either HrHRF or platelet-activating factor (PAF), the positive control, was completely ablated. Under these experimental conditions, Ca++mobilization occurred in 4 of 6 samples from different donors tested. However, in samples from a total of 9 donors examined over months, Ca++ responses to HrHRF occurred in only 25% (9 of 36 experiments). The lack of responsiveness of some cell preparations was not due to a general inability of the cells to mobilize Ca++ because eosinophils always responded to stimulation with PAF, regardless of the effect of HrHRF. In general, the HrHRF-induced Ca++ response was less vigorous than that induced by PAF.
Changes in cytosolic free calcium in response to HrHRF or a known positive stimulus, PAF.
Eosinophils were preincubated with either the inactive β-oligomer of PT (panels A,B) or PT (panels C,D) and loaded with Fura 2-AM. Cells were then stimulated (arrows) with 0.7 μmol/L HrHRF (panels A,C) or 1 μmol/L PAF (panels B,D). The average calcium response ± SEM (vertical bars) of eosinophils from 4 donors is expressed as the relative change in Fura 2-AM saturation. P values indicate differences between 8 time points before and after the addition of stimulus.
Changes in cytosolic free calcium in response to HrHRF or a known positive stimulus, PAF.
Eosinophils were preincubated with either the inactive β-oligomer of PT (panels A,B) or PT (panels C,D) and loaded with Fura 2-AM. Cells were then stimulated (arrows) with 0.7 μmol/L HrHRF (panels A,C) or 1 μmol/L PAF (panels B,D). The average calcium response ± SEM (vertical bars) of eosinophils from 4 donors is expressed as the relative change in Fura 2-AM saturation. P values indicate differences between 8 time points before and after the addition of stimulus.
Because HrHRF induced a Ca++ response only 25% of the time, we subsequently preincubated the cells with GM-CSF. Preincubation of cells with GM-CSF, TNF-α, or both did not change the response to HrHRF, nor did it induce Ca++ mobilization in cells otherwise unresponsive to HrHRF (data not shown). Again, LPS used at concentrations of up to 500 ng/mL as a control for endotoxin in the preparation failed to induce Ca++ mobilization in eosinophils (data not shown).
HrHRF is chemotactic for human eosinophils in vitro
Teshima et al43 reported that recombinant p26 HRF injected into the peritoneum of ovalbumin-sensitized mice caused eosinophil recruitment within 4 hours. Because of this report, we performed eosinophil chemotaxis studies. Purified eosinophils were stimulated with HrHRF or PAF, and chemotaxis was assessed by using Boyden microchambers. In 3 experiments, the number of eosinophils migrating in the medium-control condition was 24 ± 4. The number of eosinophils migrating in response to HrHRF (0.4 μmol/L) was 54 ± 14, or 225% of the medium-control value; to HrHRF (0.24 μmol/L), 58 ± 6, or 243%; and to HrHRF (0.12 μmol/L), 18 ± 7, which was not above the medium-control value. In comparison, the results with the 2 positive controls were that 473 ± 65 eosinophils (1971%) migrated in response to PAF (10−7mol/L) and 157 ± 105 eosinophils (653%) migrated in response to RANTES (100 ng/mL).
rHRF stimulates IL-8 production in the human eosinophilic cell line AML14-3D10
To demonstrate the effect of HrHRF on an eosinophilic cell line, AML14-3D10 cells were stimulated in the presence or absence of GM-CSF. Results were similar to those achieved with human eosinophils in the presence of GM-CSF: HrHRF stimulated IL-8 production to a level 2224 pg/mL above that with medium alone. Unlike eosinophils, AML14-3D10 cells did not need priming with GM-CSF for this to occur. This is not surprising, since these cells produce and use GM-CSF in an autocrine fashion.44 Even in the absence of GM-CSF, HrHRF produced a level of IL-8 in the AML14-3D10 cell line that was 1217 pg/mL above that with the medium control (n = 3).
The effect of HrHRF on eosinophils and AML14-3D10 cells is not dependent on IgE
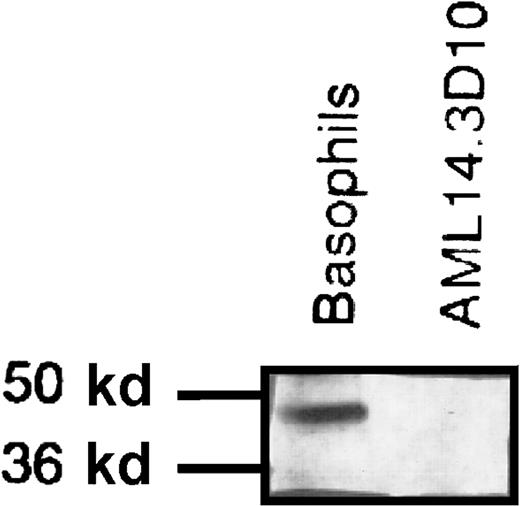
It was previously reported that human eosinophils do not express FcεRIα but do have an intracellular pool of it.17 We reproduced these data: our flow cytometry studies detected no extracellular expression of IgE, FcεRIα, or FcεRII on either human eosinophils or AML14-3D10 cells (data not shown). We next investigated whether the AML14-3D10 cells, like human eosinophils, had an intracellular pool of FcεRIα. Western blot analysis of AML14-3D10 cell lysates incubated with a mAb specific for FcεRIα showed no band, whereas the matched, positive-control basophil lysates had a distinct band of approximately 50 kd (Figure7). Therefore, unlike human eosinophils, AML14-3D10 cells have no detectable FcεRIα. Thus, HrHRF activates eosinophils and AML14-3D10 cells through a mechanism that does not depend on IgE or the FcεRI receptor.
Western blot of cell lysates incubated with monoclonal antibody to the FcεRI α chain.
For this experiment, 5.5 × 106 basophils or AML14-3D10 cells were lysed, and 500 000 cells per lane were loaded on a gel, electrophoresed, and assessed by Western blot analysis. Basophils have a band of approximately 50 kd that corresponds to FcεRIα; this is absent in the AML14-3D10 cells. Results shown are representative of 3 experiments.
Western blot of cell lysates incubated with monoclonal antibody to the FcεRI α chain.
For this experiment, 5.5 × 106 basophils or AML14-3D10 cells were lysed, and 500 000 cells per lane were loaded on a gel, electrophoresed, and assessed by Western blot analysis. Basophils have a band of approximately 50 kd that corresponds to FcεRIα; this is absent in the AML14-3D10 cells. Results shown are representative of 3 experiments.
Discussion
The principal findings of this study are that HrHRF can activate a cell type other than the basophil and that it acts like a cytokine. Our experiments demonstrated that HrHRF causes IL-8 production from eosinophils and AML14-3D10 cells primed with GM-CSF and from AML14-3D10 cells in the absence of GM-CSF. Additionally, HrHRF is chemotactic for eosinophils in vitro in the absence of GM-CSF, although it is not known whether the concentrations required for chemotaxis have in vivo relevance. HrHRF also induced Ca++ mobilization in a subset of allergic donors in the absence of GM-CSF coculture. Because there was no difference in viability or cell number in cultures incubated with GM-CSF, we believe that GM-CSF may provide an activation signal allowing cells to respond to HrHRF, TNF-α, or both. Only the combination of TNF-α and IL-5 induced significant IL-8 production in eosinophils not primed with GM-CSF. However, even in this case, priming with GM-CSF greatly enhanced the magnitude of the response.
The necessity of GM-CSF for IL-8 secretion from eosinophils stimulated by other stimuli, such as RANTES or PAF, was reported previously.28 In contrast, Nakajima et al23showed that preincubation with GM-CSF was not required for IL-8 secretion from eosinophils when 25 ng/mL TNF-α was the stimulus. Although slightly different experimental conditions were used, one major difference between their study and ours was the inability of TNF-α alone to induce IL-8 secretion from GM-CSF–primed eosinophils. The reason for this difference is unclear, although it might be explained by the source of the eosinophils. We routinely used eosinophils from mildly allergic donors, whereas the cells used by Nakajima et al were primarily from healthy donors. Of note, Nakajima et al23 also reported a significant enhancement by IL-5 of IL-8 induced by immobilized immunoglobulin, as well as a nonsignificant tendency of IL-5 to enhance TNF-α–induced IL-8 release. This parallels the results with the combination of HrHRF and TNF-α or TNF-α and IL-5 in our experiments.
In addressing the specificity of HrHRF activation of eosinophils, we also considered the following. Human eosinophils express Mac-1 (CD11b),45 a molecule capable of binding various ligands, including LPS.46 Our finding of no difference in IL-8 production between experiments using preparations containing barely detectable levels of endotoxin and experiments using preparations with high endotoxin levels rules out endotoxin as the stimulus for the results observed. Additionally, although both neutrophils47 and monocytes48 were previously shown to secrete IL-8 on stimulation with TNF-α, it is unlikely that these cells were responsible for the IL-8 production in our eosinophil preparations. Although eosinophils with greater than 98% purity were used in all our experiments, purity was essentially 100% in some experiments, and no differences in IL-8 production were observed.
It might be argued that IL-8 production by HRF that requires GM-CSF and TNF-α is only an in vitro phenomenon. However, HRF has been found in nasal lavages obtained during antigen-induced LPR49 and in fluids from skin blisters.50 Additionally, GM-CSF protein was a predominant cytokine in skin-blister fluids associated with LPR after an antigen challenge,51 and levels of messenger RNA (mRNA) for GM-CSF were significantly elevated in the bronchial mucosa of asthmatic subjects compared with healthy subjects.52 Furthermore, activated T cells53 and eosinophils in vivo54 have been reported as sources of GM-CSF. Although it remains unclear which signals induce GM-CSF production in eosinophils, it was shown that eosinophils from BAL fluids, but not from peripheral blood, have mRNA for GM-CSF.54 In antigen-challenged lungs, TNF-α can be released from mononuclear cells stimulated with IgE and antigen complexes55 and possibly from activated mast cells.56 Indeed, TNF-α was found at significantly higher concentrations in BAL fluids and sputum of symptomatic allergic subjects.57 58 Therefore, we speculate that after exposure to an antigen, the appropriate cytokine environment would be available for enhancement of IL-8 production by HRF.
HrHRF induced increases in intracytoplasmic Ca++ that were completely ablated by preincubation of eosinophils with PT. However, the Ca++ responses induced by HrHRF were observed in only 25% of donors. The reason for this low response rate is unclear. It may have been a consequence of variability among different eosinophil preparations. It is unlikely that it was due to differences in expression of a putative HRF receptor because HrHRF always had an effect on cytokine production that was independent of Ca++mobilization.
It is intriguing that both secretion and Ca++ mobilization in eosinophils induced by HrHRF were inhibited by PT, whereas the HrHRF-induced histamine release from basophils from IgE+donors was not inhibited by PT (data not shown). One possible explanation for these findings is that there may be differential HrHRF signaling in these 2 cell types. There is precedent for this. The CC chemokine, RANTES, showed 2 types of Ca++ signaling in T-cell lines.59 The initial transient peak was sensitive to PT, whereas the second, delayed Ca++ peak was inhibited by protein tyrosine kinase inhibitors. These different Ca++responses are associated with different functional responses. Although one could propose that this difference is due to the presence of 2 different HrHRF receptors, as is the case with chemokine receptors,59 another likely explanation is the use of different guanine nucleotide binding proteins (G proteins) in HrHRF signaling in these 2 cell types. Studies of cardiac phenomena and of bradykinins have found that different types of cells express different amounts of G proteins.60-62 Additionally, the CC chemokine MIP-1α is known to be coupled to multiple heterotrimeric G proteins—G(s), G(o), and G(z)—but not G(i).63 These differences in expression of G proteins account for different effector functions and are being investigated.
Another difference between HrHRF activation of basophils and HrHRF activation of eosinophils was observed in the HrHRF priming experiments. Priming of basophils with HrHRF for 15 minutes and subsequent washing of the cells did not diminish histamine release (data not shown). This suggests a high-affinity interaction between HrHRF and the basophil. On the other hand, preincubation of eosinophils with HrHRF for 1 hour and washing abolished the enhancement of TNF-α–induced production of IL-8. These findings might appear contradictory, but they could be due to the time needed to generate the response in the 2 cell types. Histamine release is essentially complete within 15 minutes, whereas augmentation of IL-8 production takes more than 5 hours.
Eosinophils from selected patients with hypereosinophilia15 and eosinophils obtained after antigen challenge16 were reported to express FcεRI. However, these results are contradicted by the data of Seminario et al17 and Kita et al.18 Additionally, our data demonstrating HrHRF activation of AML14-3D10 cells, which do not possess FcεRIα, suggest that neither IgE nor an IgE-binding structure played a role in Ca++ mobilization or regulation of cytokine production by HrHRF in our donors. Hence, we conclude that HrHRF contributes to eosinophil activation in a manner independent of FcεRI. Research is currently focused on molecular identification of the HRF-binding structure on the surface of inflammatory cells.
Acknowledgment
We thank Ms Nancy Dietz for secretarial support.
Supported by National Institutes of Health (NIH) grant RO1AI 20253 (D.W.M.) and NIH grant RO1 AI 32651 (S.M.M.) and partly supported by Bristol-Myers Squibb Pharmaceutical Research Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susan M. MacDonald, The Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Circle, Baltimore, MD; e-mail: smacdona@mail.jhmi.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal