Abstract
Two prospectively studied patients with polycythemia vera (PV) whose platelet counts showed marked periodic fluctuation during treatment with hydroxyurea (HU) are reported. Cycle lengths in both were approximately 28 to 30 days. In one patient, the cyclic process was no longer evident when treatment with HU was withheld, and it reappeared on treatment rechallenge. Circulating thrombopoietin (TPO) levels fluctuated out of phase with the platelet count despite markedly reduced TPO-receptor (c-Mpl) expression in bone marrow megakaryocytes. These observations suggest that the cyclic phenomenon may be related to both a transient state of HU-induced depletion of megakaryocytes and a concentration-dependent mitigation by TPO of the HU effect on megakaryocytes and their precursors. It is conceivable that the affected patients harbor a megakaryocyte progenitor pool whose apoptotic activity is differently modulated by either HU or high concentrations of TPO.
Introduction
Hydroxyurea (HU) is a nonalkylating myelosuppressive agent that impairs DNA synthesis through the inhibition of ribonucleotide reductase.1 The drug is often used to supplement phlebotomy for patients with polycythemia vera (PV) who are at high risk.2 Similarly, treatment with HU has been shown to reduce significantly the incidence of recurrent thrombosis in patients with essential thrombocythemia who are at high risk.3 In addition, though the therapeutic use of HU may be declining in patients with chronic myeloid leukemia,4it is gaining momentum in those with sickle cell disease.5Side effects of HU are usually limited to the integument (leg ulcers, skin and nail hyperpigmentation, aphthous ulcers) and have been said to occur in approximately one third of treated patients.6 In addition, HU-induced cytopenia(s) may occur in some patients with PV if the drug is not used judiciously but is usually transient and dose dependent. In this report, we describe an unusual cycling of platelet counts with marked peak-to-trough amplitude, which is associated with HU therapy in patients with PV.
Study design
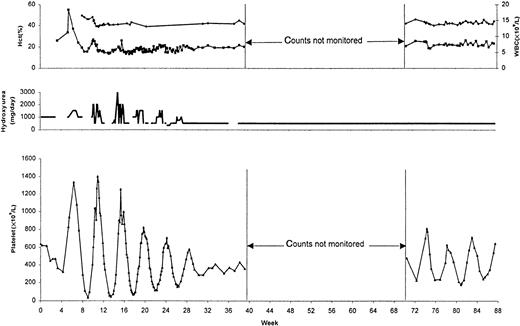
Patient 1
A 54-year-old woman was diagnosed with PV in 1996 when she sought treatment for migraine headaches, aquagenic pruritus, and increased hemoglobin concentration (17.5 g/dL; baseline, 13.5 g/dL). Hematocrit was 53%, white blood cell count was 7.4 × 109/L, and platelet count was 534 × 109/L. Additional studies revealed an increased red cell mass, a reduced serum erythropoietin (EPO) level, and an increased neutrophil alkaline phosphatase score. The spleen was not palpable. The patient was initially treated with phlebotomy alone, during which time her platelet count remained stable (Figure1). In July 1998, HU therapy (1 g/d) was initiated because she had a transient ischemic attack. Shortly afterward, cyclical fluctuations of platelet count developed that were sustained as long as the patient remained on HU treatment (Figure 1; highest and lowest platelet counts of 1412 and 50 × 109/L, respectively). Keeping the HU dose constant reduced the interval variations and produced a higher nadir in the platelet count (highest and lowest platelet counts of 693 and 91 × 109/L, respectively). Withholding treatment with HU resulted in prompt cessation of the cyclic changes, which reappeared with the resumption of HU therapy (Figure 1; highest and lowest platelet counts of 775 and 42 × 109/L, respectively). It is underscored that the daily dose of HU from week 8 through week 24 (1000 mg/d) and from week 39 through week 49 (1500 mg/d) was not altered at any point despite the marked fluctuations in the platelet count. Serum thrombopoietin (TPO), but not EPO, levels fluctuated out of phase with the platelet count during a therapeutic rechallenge (Figure 1). Serum TPO was measured by “in-house” immunochemiluminometric assay. A plastic bead-immobilized monoclonal anti-TPO antibody was used as the capture antibody, and an acridinium ester-labeled, immunopurified polyclonal anti-TPO antibody was used as the detection antibody (R&D Systems, Minneapolis, MN). Recombinant human TPO was used as a standard. The detection limit of the assay was 10 pg/mL, with an intra-assay and an interassay coefficient of variation of 5% and 10%, respectively. Immunohistochemical staining of the bone marrow revealed reduced megakaryocyte expression of TPO receptor (c-Mpl). The patient did not respond to subsequent treatment with either anagrelide (up to 4 mg/d for 10 weeks) or α-interferon (5 million U subcutaneously 3 times/wk for 6 weeks [Figure 1]).
Hydroxyurea-induced cyclic variations of platelet count and serum thrombopoietin concentration (TPO) in a patient with polycythemia vera.
The reference range for TPO is 11 to 70 pg/mL, and for serum EPO concentration it is 4 to 24 pg/mL. No remarkable variations were noted in hematocrit (Hct), white blood cell count (WBC), or EPO concentration. Alpha INF, α-interferon.
Hydroxyurea-induced cyclic variations of platelet count and serum thrombopoietin concentration (TPO) in a patient with polycythemia vera.
The reference range for TPO is 11 to 70 pg/mL, and for serum EPO concentration it is 4 to 24 pg/mL. No remarkable variations were noted in hematocrit (Hct), white blood cell count (WBC), or EPO concentration. Alpha INF, α-interferon.
Patient 2
A 59-year-old man was diagnosed with PV in 1995 when he sought treatment for a spontaneous left groin hematoma. Hemoglobin concentration was 21.0 g/dL, hematocrit was 64%, and platelet count was 636 × 109/L. Additional studies revealed an increased red cell mass, a reduced serum EPO level, an endogenous erythroid colony growth, and a normal spleen. Initial treatment consisted of phlebotomy supplemented by HU 1 g/d. In the following 3 weeks, the platelet count normalized and HU was withheld. After another 2 weeks, the patient was admitted to the hospital because of a syncopal episode; the platelet count at the time was 936 × 109/L. Treatment with HU was restarted. During the first 5 months the platelet count fluctuated from a 28- to 30-day cycle, with doses of HU ranging between 0.0 and 3.0 g/d (Figure 2; highest and lowest platelet counts of 1400 and 35 × 109/L, respectively). Subsequently, the HU dose was kept steady at 500 mg/d and the platelet cycle persisted, albeit with a higher nadir count and a lower peak value (Figure 2; highest and lowest platelet counts of 810 and 179 × 109/L, respectively).
Hydroxyurea-associated cyclic variations in the platelet count of a patient with polycythemia vera.
Use of constant drug dosage resulted in a higher nadir of the platelet count and a smaller interval variation. Hematocrit (Hct) and white blood cell count (WBC) did not show similar periodic fluctuations.
Hydroxyurea-associated cyclic variations in the platelet count of a patient with polycythemia vera.
Use of constant drug dosage resulted in a higher nadir of the platelet count and a smaller interval variation. Hematocrit (Hct) and white blood cell count (WBC) did not show similar periodic fluctuations.
Results and discussion
Unprovoked periodic oscillations of the platelet count have been reported in both the absence7 and the presence of chronic myeloid disorders, including PV.8,9 The periods of cycling in these patients varied from 27 to 66 days. To our knowledge, the current study is the first to report a well-documented case of drug-induced cyclic variations in the platelet count of a patient with PV (patient 1). A similar phenomenon was demonstrated in our second patient with PV, but the causal role of HU could not be confirmed because serial platelet counts in the absence of treatment with HU were not documented. Use of HU has previously been associated with cyclic variations of granulocyte count in patients with chronic myeloid leukemia (cycle length, 30 to 50 days).10 However, it is unclear whether the reported cases were observed with patients off therapy to confirm the pathogenic role of HU in inducing the cycling process. Nevertheless, periodic oscillations of the granulocyte count have also been associated with the use of myeloid growth factors11 and melphalan-based intermittent chemotherapy.12
The induction of oscillatory changes in hematopoiesis may require the presence of a destabilized stem cell pool and a cytokine-mediated feedback control that feeds into a compulsory delay in mitotic response.13 In the currently described patients, the former situation may have been created by HU, whereas the out-of-phase variation in serum TPO level suggests retention of an autoregulatory response despite the demonstrated, and expected,14reduction in megakaryocyte c-Mpl expression.15,16 It is conceivable that the affected patients harbor a megakaryocyte progenitor pool that either is unusually sensitive to HU or displays a different apoptotic response to high concentrations of TPO.17,18 Inhibition of either endogenous or drug-induced apoptosis by markedly elevated levels of TPO may allow megakaryocyte survival and platelet production despite continuous exposure to the drug. Accordingly, the cycle is completed when drug sensitivity is reestablished by the subsequent reduction in TPO level. The possibility of an unusual drug–megakaryocyte interaction was further suggested by the lack of response to subsequent treatment with either anagrelide or α-interferon.19 20
The currently described phenomenon may account for some, at least, of the difficulty in adjusting the doses of HU in certain patients with PV or related disorders. In such instances, our experience suggests that frequent adjustments in the HU dose may produce a “bouncing ball” effect with marked peak-to-trough amplitudes. Keeping the HU dose steady, on the other hand, may reduce the severity of both the platelet nadir and the interval variation. Conversely, an alternative platelet-lowering agent, though not successful in one of our patients, may be tried.19 20
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ayalew Tefferi, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal