Abstract
Ferrochelatase (FECH; EC 4.99.1.1) catalyzes the terminal step of the heme biosynthetic pathway. Defects in the human FECHgene may lead to erythropoietic protoporphyria (EPP), a rare inherited disorder characterized by diminished FECH activity with protoporphyrin overproduction and subsequent skin photosensitivity and in rare cases liver failure. Inheritance of EPP appeared to be autosomal dominant with possible modulation by low expression of the wild-type FECH allele. Animal FECHs have been demonstrated to be [2Fe-2S] cluster-containing proteins. Although enzymatic activity and stability of the protein appear to be dependent on the presence of the [2Fe-2S] cluster, the physiologic role of the iron-sulfur center remains to be unequivocally established. Three of the 4 [2Fe-2S] cluster-coordinating cysteines (ie, C403, C406, and C411 in the human enzyme) are located within the C-terminal domain. In this study 5 new mutations are identified in patients with EPP. Three of the point mutations, in 3 patients, resulted in FECH variants with 2 of the [2Fe-2S] cluster cysteines substituted with tyrosine, serine, and glycine (ie, C406Y, C406S, and C411G) and with undetectable enzymatic activity. Further, one of the patients exhibited a triple point mutation (T1224→A, C1225→T, and T1231→G) leading to the N408K/P409S/C411G variant. This finding is entirely novel and has not been reported in EPP. The mutations of the codons for 2 of the [2Fe-2S] cluster ligands in patients with EPP supports the importance of the iron-sulfur center for the proper functioning of mammalian FECH and, in at least humans, its absence has a direct clinical impact.
Introduction
Ferrochelatase (FECH; protoheme ferrolyase, EC4.99.1.1) catalyzes the insertion of ferrous iron into protoporphyrin IX macrocycle, the terminal step in the heme biosynthetic pathway.1,2 Decreased FECH activity is a hallmark of patients with erythropoietic protoporphyria (EPP; MIM 177,000). EPP is an inherited disorder of heme biosynthesis caused by a partial deficiency of FECH leading to an accumulation of free protoporphyrin, predominantly in erythrocytes.3,4 Clinically, the disorder is manifested starting in childhood, mainly as painful photosensitivity due to accumulation of free protoporphyrin in the skin.4,5However, in fewer than 5% of the patients accumulation of protoporphyrin in the liver may lead to liver injury and even terminal liver failure.4 5
Eukaryotic mature FECH is associated with the inner mitochondrial membrane, with the active site facing the mitochondrial matrix.1,6 FECHs isolated from human,7mouse,8 chicken,9 frog,9 andDrosophila melanogaster10 have been reported to be metalloenzymes, with a [2Fe-2S] cluster as the cofactor. This is in striking contrast to bacteria, yeast, and plant FECHs, which are devoid of iron-sulfur centers.1,2 Although a direct catalytic role for the [2Fe-2S] cluster apparently can be ruled out,1,7,8,11,12 the ultimate physiologic role of the metal center remains to be established.11,12 However, despite the lack of a direct catalytic involvement for the [2Fe-2S] cluster, mammalian FECH activity does appear to be dependent on the presence of the [2Fe-2S] cluster,11 suggesting that the metal center may play a structural role in keeping the enzyme in the correct and stable conformation.
The ligands of the [2Fe-2S] cluster are 4 cysteines,10,13 with 3 of the residues being present in the C-terminal domain.10,13 In the human enzyme, the cysteines are C146, C403, C406, and C411.10,13 In contrast, in bacteria, yeast, and plant FECHs, the 3 terminal cysteines coordinating the [2Fe-2S] cluster are either substituted with other residues or the 30 to 50 amino acid C-terminal domain is absent.12,13 The 3-dimensional structure of Bacillus subtilis FECH, determined to 1.9-Å resolution, revealed that the protein contains 2 similar domains, each with a 4-stranded parallel β sheet flanked by a helix.14 Proposed conserved active site amino acids are present in a cleft defined by the 2 domains.14 Although no 3-dimensional structure has been published for mammalian or any other FECH, a recent report on the crystallization of the human FECH indicates that the mammalian enzyme is a homodimer.15
The human FECH gene encompasses 11 exons and spans approximately 45 kb,15,16 and it has been assigned to chromosome 18q21.316-18 (GenBank No. D00726). Heterogeneous molecular defects in the FECH gene have been described to be associated with EPP.4,5,19 Albeit the mode of EPP inheritance has been considered to be mainly autosomal dominant with variable clinical expression,5 2 cases of autosomal recessive inheritance have been reported.20,21 In the dominant type of EPP, the inheritance does not strictly follow the mendelian rules.19 As recently demonstrated by Gouya and colleagues, the clinical expression of EPP results from coinheritance of a mutated FECH gene and a wild-type “low expressed” allele.19 This conclusion supported the hypothesis of a “triallelic system,” proposed by Went and Klasen,22that an additional factor or a “third allele” was required for the clinical manifestation of EPP. In this study, 5 new FECHgene mutations associated with EPP are identified. Significantly, 3 of the mutated codons correspond to 2 of the [2Fe-2S] cluster ligands.
Materials and methods
Study subjects
Three patients with EPP, representing 3 families, and their healthy family members were investigated. The clinical diagnosis of symptomatic EPP was based on skin photosensitivity and increased erythrocyte protoporphyrin. Peripheral blood samples were collected from the patients and their relatives, with informed consent.
In vitro amplification of genomic DNA
Genomic DNA was isolated and purified according to the directions supplied with Wizard Genomic DNA Purification kit (Promega-Biotech, Madison, WI). All 11 exons of the FECHgene of index patients in families I and III, as well as patient II, were amplified by polymerase chain reaction (PCR) and then analyzed in denaturing gradient gel electrophoresis (DGGE) according to a procedure described previously.5 The entire exon 11 as well as the intron 10-exon 11 boundary of the FECH gene was amplified in 2 overlapping fragments, 11a and 11b (according to FECHpublished sequence No. AJ 290235, 11a: nt 37315-37486, 11b: nt 37426-37554). DNA fragments with an abnormal mobility shift in the DGGE gel were subjected to direct sequencing using the fMol DNA sequencing kit (Promega-Biotech) with 35S-labeled deoxyadenosine triphosphate (dATP).
Genotyping using 2 single nucleotide polymorphisms (SNPs)
Construction and synthesis of normal and mutated FECHs inEscherichia coli
Construction and expression of normal and mutated FECH complementary DNAs (cDNAs) have been described previously.5 Briefly, the mutations corresponding to those observed in the patients with EPP were introduced in the expression plasmid containing the human wild-type FECH (pGEX-FECH5,24) using the method provided with the transformer site-directed mutagenesis kit (Clontech Laboratories, Palo Alto, CA). The sequences of the primers used in the mutagenesis of the 6 FECH mutants are listed in Table 1. The introduced mutations were verified by DNA sequencing. E coli DH5α cells harboring either the FECH wild-type expression plasmid or any of the FECH variants were grown in Luria-Bertani medium and induction of recombinant protein production was performed as described previously.5 FECH activity in bacterial lysates was measured fluorometrically by monitoring zinc-mesoporphyrin formation, according to the method described by Li and coworkers.25
Oligonucleotide-directed mutagenesis of the FECH-encoding plasmid
| Mutant . | Oligonucleotide sequence . |
|---|---|
| C406Y | 5′-GC TGT CCG CTC TAT GTC AAT CCT GTC TGC AGG GAG-3′ |
| C406S | 5′-GC TGT CCG CTC TCT GTC AAT CCT GTC TGC AGG GAG-3′ |
| N408K | 5′-CTG TCC GCT CTG TGT CAAACC TGT CTG CAG-3′ |
| P409S | 5′-GCT GTC CGC TCT GTG TCA ATT CTG TCT GCA GGG-3′ |
| C411G | 5′-CTG TCG GCA GGG AGA CTA AAT CCT TCT TCA-3′ |
| N408K-P409S- C411G | 5′-CTG TGT CAAATC TGT CGG CAG GGA GAC TAA ATC CT-3′ |
| Mutant . | Oligonucleotide sequence . |
|---|---|
| C406Y | 5′-GC TGT CCG CTC TAT GTC AAT CCT GTC TGC AGG GAG-3′ |
| C406S | 5′-GC TGT CCG CTC TCT GTC AAT CCT GTC TGC AGG GAG-3′ |
| N408K | 5′-CTG TCC GCT CTG TGT CAAACC TGT CTG CAG-3′ |
| P409S | 5′-GCT GTC CGC TCT GTG TCA ATT CTG TCT GCA GGG-3′ |
| C411G | 5′-CTG TCG GCA GGG AGA CTA AAT CCT TCT TCA-3′ |
| N408K-P409S- C411G | 5′-CTG TGT CAAATC TGT CGG CAG GGA GAC TAA ATC CT-3′ |
Variants of FECH-encoding plasmid, pGEX-FECH,23 were constructed as described under “Materials and methods” using the oligonucleotides shown above. The nucleotide substitutions introduced by mutagenesis are underlined and in bold.
Results
Five novel mutations in the FECH of EPP patients
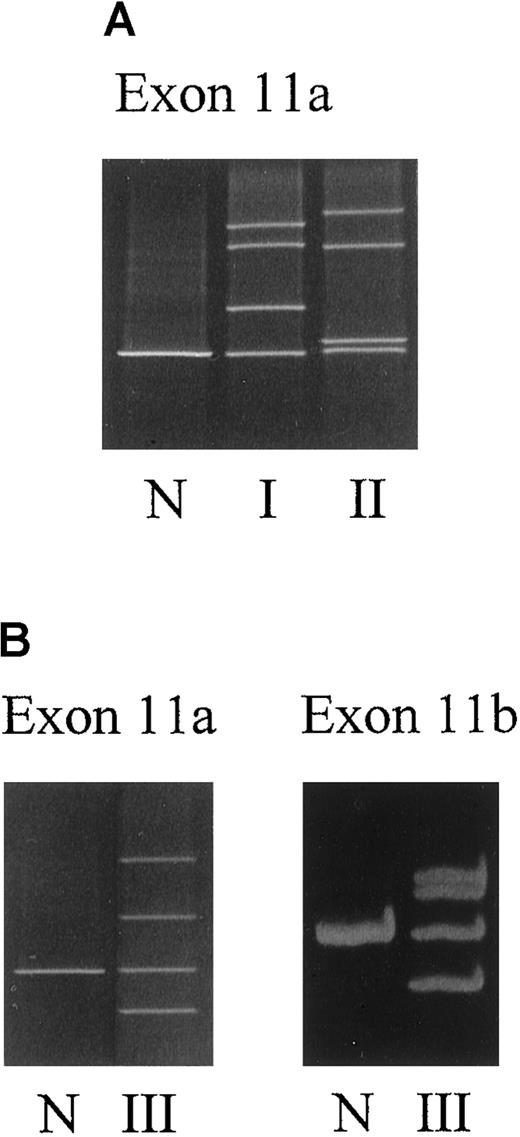
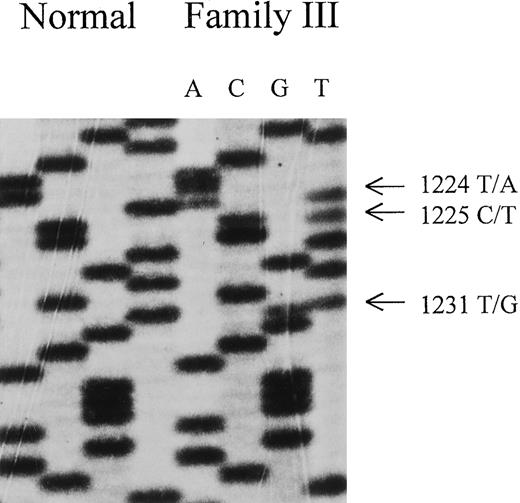
Three different abnormal DGGE patterns, one in each patient, were observed in the DGGE gel of fragment 11a (Figure1A,B). Direct sequencing of the genomic DNA unveiled a point mutation G1217→A that converted Cys-406 into Tyr (ie, C406Y) in patient I. In patient II, the same nucleotide G1217 was mutated into a C, resulting in a substitution of Cys-406 to Ser (ie, C406S). Besides the abnormality in 11a, the DNA sample from patient III exhibited another mobility shift in fragment 11b (Figure 1B and Table2). Sequencing of exon 11 disclosed 3 point mutations located in a close vicinity, T1224→A, C1225→T, and T1231→G, predicating amino acid substitutions of N408K, P409S, and C411G, respectively (Figure 2). Interestingly, the mutated cysteine codons correspond to the codons of 2 of the [2Fe-2S] cluster ligands.12 13 No additional sequence abnormalities were identified in the other 10 exons of the FECH gene among all 3 patients by DGGE analysis.
DGGE analysis of exon 11a and exon 11b of the FECHgene.
(A) Family I (I) and patient II (II). (B) Family III (III). N indicates normal sample. Sample preparation is as described in “Materials and methods.”
DGGE analysis of exon 11a and exon 11b of the FECHgene.
(A) Family I (I) and patient II (II). (B) Family III (III). N indicates normal sample. Sample preparation is as described in “Materials and methods.”
Biochemical and genetic abnormalities in patients with EPP
| Subject . | Age . | Photosensitivity . | Erythrocyte protoporphyrin*(μg/dL) . | FECH mutation . | FECH mutation . |
|---|---|---|---|---|---|
| Family I | |||||
| Index patient | 10 | + | 491 | G1217 → A | C406Y |
| Sister | 14 | + | 775 | ||
| Uncle | 43 | + | 46 | ||
| Patient II | 53 | + | 2744 | G1217 → C | C406S |
| Family III | Triple mutation | ||||
| Index patient | 35 | + | 711 | T1224 → A/ | N408K/ |
| Brother | 28 | + | 470 | C1225 → T/ | P409S/ |
| Daughter | 6 | − | ND | T1231 → G | C411G |
| Father | 59 | − | ND |
| Subject . | Age . | Photosensitivity . | Erythrocyte protoporphyrin*(μg/dL) . | FECH mutation . | FECH mutation . |
|---|---|---|---|---|---|
| Family I | |||||
| Index patient | 10 | + | 491 | G1217 → A | C406Y |
| Sister | 14 | + | 775 | ||
| Uncle | 43 | + | 46 | ||
| Patient II | 53 | + | 2744 | G1217 → C | C406S |
| Family III | Triple mutation | ||||
| Index patient | 35 | + | 711 | T1224 → A/ | N408K/ |
| Brother | 28 | + | 470 | C1225 → T/ | P409S/ |
| Daughter | 6 | − | ND | T1231 → G | C411G |
| Father | 59 | − | ND |
The normal range for erythrocyte protoporphyrin concentration is 1-10 μg/dL.
ND indicates not determined.
Nucleotide sequence of FECH cDNA derived from direct sequencing of PCR products of index patient of family III.
Note triple mutation: T →A at nucleotide 1224, C →T at nucleotide 1225, and T →G at nucleotide 1231. These 3 mutations lead to a FECH variant with N408K, P409S, and C411G.
Nucleotide sequence of FECH cDNA derived from direct sequencing of PCR products of index patient of family III.
Note triple mutation: T →A at nucleotide 1224, C →T at nucleotide 1225, and T →G at nucleotide 1231. These 3 mutations lead to a FECH variant with N408K, P409S, and C411G.
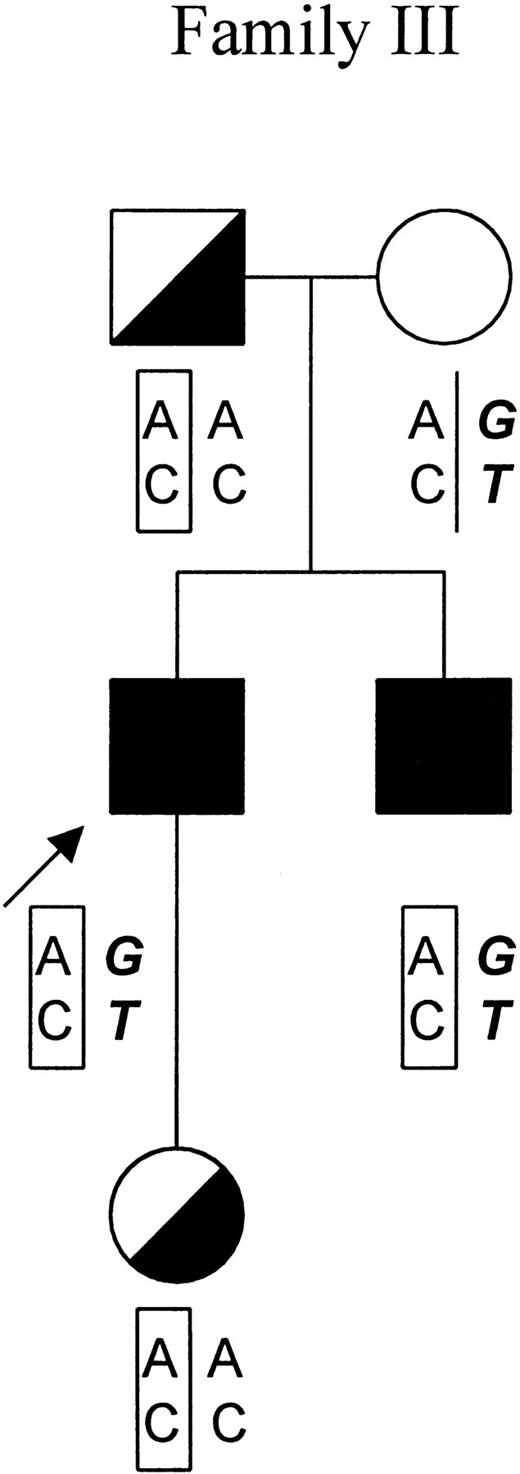
Subsequently, members of family III were screened for the triple mutations by sequencing of exon 11. As indicated in the pedigree of family III, 4 of the 5 tested individuals were positive for these mutations (Figure 3). The fact that theFECH gene of the patient's mother was totally absent for any of the mutations suggested that all 3 mutations aligned on a singleFECH gene allele from the father.
Genotypic analysis of family III.
In the pedigree of family III, the proband is indicated by an arrow. Symptomatic patients and asymptomatic carriers are represented by solid and in half-filled symbols, respectively. The results of genotypic analysis of −251A/G in the promoter region and IVS1-23C/T of theFECH gene are shown below the symbols of each individual. The mutated FECH allele featuring the haplotype −251A, IVS-23C is indicated by “AC” in a rectangular frame. The allele that is responsible for the clinical manifestation of EPP featuring the haplotype −251G, IVS-23T is indicated by “GT” in bold and italic.
Genotypic analysis of family III.
In the pedigree of family III, the proband is indicated by an arrow. Symptomatic patients and asymptomatic carriers are represented by solid and in half-filled symbols, respectively. The results of genotypic analysis of −251A/G in the promoter region and IVS1-23C/T of theFECH gene are shown below the symbols of each individual. The mutated FECH allele featuring the haplotype −251A, IVS-23C is indicated by “AC” in a rectangular frame. The allele that is responsible for the clinical manifestation of EPP featuring the haplotype −251G, IVS-23T is indicated by “GT” in bold and italic.
Clinical and biochemical characterization of the EPP patients with identified mutations
All of the EPP patients exhibited photosensitivy, although the intensity and onset of photosensitivity varied within the families. Erythrocyte protoporphyrin concentration was increased as is characteristic of patients with EPP (Table 2).
In vitro expression of mutated FECHgenes
The expression of the mutated FECH genes was assessed by engineering the mutations observed in the EPP patients in an E coli expression plasmid containing the FECHwild-type gene. Extracts of bacterial cells harboring the different FECH variants indicated no detectable enzymatic activity (Table3) confirming the importance of the cysteine residues to the proper functioning of FECH. Curiously, the 2 other mutations (ie, N408K and P409S) present in the family III carrier of the triple mutation had no effect on the enzymatic activity (Table 3).
In vitro expression of FECHmutations
| Patient . | FECH variant . | Residual activity of humanFECH (%) . |
|---|---|---|
| Wild type | 100 | |
| Index patient of family I | C406Y | 0.6 |
| Patient II | C406S | 0.9 |
| Index patient of family III | N408K | 100 |
| P409S | 100 | |
| C411G | 0.1 | |
| N408K-P409S-C411G | 0.1 |
| Patient . | FECH variant . | Residual activity of humanFECH (%) . |
|---|---|---|
| Wild type | 100 | |
| Index patient of family I | C406Y | 0.6 |
| Patient II | C406S | 0.9 |
| Index patient of family III | N408K | 100 |
| P409S | 100 | |
| C411G | 0.1 | |
| N408K-P409S-C411G | 0.1 |
Discussion
To date, more than 60 different mutations have been identified in the FECH gene of patients with EPP. The majority of them (around 80%) are the so-called “null allele” mutations, namely, mutations that lead to formation of a truncated, and therefore, inactive enzyme. The “null allele” mutations include nonsense mutations and all frameshift mutations resulting from short nucleotide deletions or insertions and exon skippings.
Thirteen different point mutations in the FECH gene have been identified previously in EPP; in this study, we describe 5 additional point mutations. It should be emphasized that not all point mutations inhibit enzymatic activity. For example, mutation M267I (G801→A) was one of the 2 point mutations identified in an EPP patient.20 On expression in E. coli and subsequent purification of the recombinant mutant protein, M267I mutant exhibited identical kinetic parameters (ie, Kms and Vmax) to those of the wild-type FECH. However, the M267I mutant had a slightly higher thermolability than that of normal human FECH.26Similarly, the residual enzyme activity of mutant Y191H (T571→C) was measured as 72% that of the wild-type enzyme.5 Indeed, in both cases, a second, and deleterious, mutation was identified in theFECH gene, which was most likely responsible for the EPP condition. In the case of family III in the present study, among the 3 mutations aligning on the same FECH allele, only C411G was apparently the cause of enzymatic deficiency because both N408K and P409S mutants were 100% active. The fact that both N408K and P409S have so far not been identified in over 40 additional patients with EPP and nearly 100 normal subjects (data not shown) suggests that these amino acid substitutions are rare silent mutations in the FECH gene.
These findings stress the need for an in vitro characterization of individual point mutations to differentiate causal mutations from rare polymorphisms. Typically, the assessment of the impact of the EPP mutations on FECH activity has been carried out with recombinant FECH variants, which mimic the mutations identified in patients with EPP and are overproduced in prokaryotic expression systems. Unlike the “null allele” mutations, point mutations provide information on the function of individual amino acids on the overall enzymatic activity. In this study, EPP phenotypes bearing mutations of cysteine codons corresponding to the codons of 2 of the [2Fe-2S] cluster ligands demonstrated the crucial role that these cysteine residues played in vivo. Previous site-directed mutagenesis studies of the iron-sulfur cluster ligands have shown that the metal center is essential for the stability, and consequently, for the function of mammalian FECH13 (and A. Pereira, P. Tavares, and G. C. Ferreira, unpublished results). The physiologic role of the [2Fe-2S] cluster in mammalian FECH remains to be established unequivocally, but it is clear from this study that its absence leads to FECH variants with altered biochemical properties and with serious clinical implications.
A rather peculiar feature of EPP is that only about 10% of the individuals with defective FECH develop clinical symptoms. In other words, EPP has a clinical penetrance of approximately 10%. The recent molecular genetic studies on EPP have not only led to the unveiling of numerous mutations in the FECH gene, but also have shed light on the mechanism of clinical manifestation in EPP.5,19,23 The genotype of a patient with overt EPP is a combination of a mutated FECH gene allele, which is totally devoid of enzyme activity, and a “low expressed” FECHgene allele, which produces about 50% of the normal amount of messenger RNA (mRNA). Had an individual the combination of a mutated allele and a normal FECH gene allele, he or she would be asymptomatic. These conclusions were drawn based on the results of mRNA quantification in 5 EPP families.19 However, direct measurement of the mRNA output from the FECH gene alleles to determine the clinical outcome of an individual, besides being a complex procedure, is not always possible because it requires the individual being a heterozygote for either 798G/C or 1520C/T dimorphisms.
Two other SNPs in the FECH gene, −251A/G in the promoter region and IVS1-23C/T, could well serve the purpose of predicting the clinical outcome of EPP as shown in the recent publication by Gouya and coworkers.19 Genotypic analysis of 39 unrelated EPP families indicated that haplotype (−251G, IVS1-23T) is strongly associated (P < 10−6) with the allele that is in trans to the mutated FECH allele—assuming it is “low-expressed” in all patients. Furthermore, there is an association (P < 10−6) between haplotype (−251A, IVS1-23C) and the normal FECH gene allele in asymptomatic carriers.19
In this study, haplotyping analysis was not possible in families I and II for which only DNA from the respective probands was available to us. However, we conducted genotypic analysis in family III at the request of the patient. As shown in Figure 3, both symptomatic patients (the index patient and his brother) exhibited the haplotype (−251G, IVS1-23T) in the allele in trans to the mutated allele (the mutated allele features a −251A, IVS1-23C haplotype in this family). In contrast, the 2 asymptomatic mutation carriers (the daughter and the father of the index patient) carried a normal FECH gene allele with haplotype (−251A, IVS1-23C). These results corroborate the previous and above-described findings. The daughter of the index patient, who is now 6 years old, has not developed any clinical symptoms. Most likely she will remain asymptomatic throughout her life because the chance of her becoming a patient is less than 2%.19
Moreover, the clinical expression or the severity of the disease varies among patients with EPP. The majority of the symptomatic patients manifest only a photosensitivity of the skin. Fewer than 5% of the patients develop in addition to cutaneous photosensitivity, liver complications in the form of a progressive liver cirrhosis and liver failure due to a massive accumulation of protoporphyrin in the liver—a life-threatening situation in EPP.4
More than 60 different mutations have so far been identified in approximately 100 EPP patients from over 80 unrelated EPP families, among which 19 patients suffered from EPP-related liver disease. Some interesting facts can be observed among these cases. Namely, all known patients with a liver complication carried a “null allele” mutation. In contrast, none of the 18 patients carrying a missense mutation have developed liver complications. This observation suggests a genotype-phenotype correlation between the FECH gene mutations and the EPP manifestation.27
In this study, all symptomatic patients carried a missense mutation. Except for patient II, from whom no data regarding the liver function was available to us, the rest of the patients had normal liver function. At this stage, it is premature to conclude that patients with a missense mutation will not ever develop liver complications because the total number of patients studied, especially those with liver complications, is limited.
Molecular studies continue to make important contributions to the understanding of the pathogenesis of EPP. However, both the “low expression” and the “null allele” mechanisms stemmed from limited experimental and clinical data. Molecular genetic analysis in a large number of EPP families should therefore remain an important objective for EPP research.
Supported by grants from the American Cancer Society (BE-248) and the National Institutes of Health (DK51186) (to G.C.F.), SNf 31-53799.98 and die Stiftung für Wissenschaftliche Forschung an der Universität Zürich (to X.S.-Y.) and INSERM U409 and Université Paris 7 (to J.-C.D.).
X.S.-Y. and L.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean-Charles Deybach, Centre Français des Porphyries, INSERM U409, Faculté X. Bichat, Hôpital Louis Mourier, Colombes, France; e-mail:jc.deybach@wanadoo.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal