Abstract
The study of the molecular bases of thrombophilia in a large family with 4 symptomatic members is reported. Three thrombophilic genetic components (FV R506Q, FV H1299R, and PT 20210G/A), all affecting the activity of the prothrombinase complex, were detected alone and in combination in various family members. In addition, a newly identified missense mutation (factor V [FV] Y1702C), causing FV deficiency, was also present in the family and appeared to enhance activated protein C (APC) resistance in carriers of FV R506Q or FV H1299R by abolishing the expression of the counterpart FV allele. The relationships between complex genotypes, coagulation laboratory findings, and clinical phenotypes were analyzed in the family. All symptomatic family members were carriers of combined defects and showed APC resistance and elevated F1 + 2 values. Evidence for the causative role of the FV Y1702C mutation, which affects a residue absolutely conserved in all 3 A domains of FV, factor VIII, and ceruloplasmin, relies on (1) the absolute cosegregation between the mutation and FV deficiency, both in the family and in the general population; (2) FV antigen and immunoblot studies indicating the absence of Y1702C FV molecules in plasma of carriers of the mutation, despite normal levels of the FV Y1702C messenger RNA; and (3) molecular modeling data that support a crucial role of the mutated residue in the A domain structure. These findings help to interpret the variable penetrance of thrombosis in thrombophilic families and to define the molecular bases of FV deficiency.
Introduction
The heterogeneity of clinical phenotypes and the variable manifestations of thrombosis observed in thrombophilic families have led to the hypothesis that predisposition to venous thrombosis results from the combination of several genetic defects.1-4 Functional polymorphisms that are relatively frequent in the population, such as factor V (FV) R506Q (Leiden mutation)5 and the prothrombin (PT) 20210G/A mutation,6 have been found to play a major role as risk factors.7,8 Both act by enhancing thrombin generation by the prothrombinase complex,9 via different mechanisms. FV R506Q impairs activated protein C (APC)-mediated FVa inactivation,10-12 a condition termed APC resistance,13 and the PT 20210G/A mutation causes an increase in the concentration of circulating PT.6Combinations of FV R506Q with the PT mutation and other thrombophilic defects have been repeatedly documented in thrombophilic patients.14-17
The crucial role of FV, strategically placed at the crossroads of the procoagulant and anticoagulant pathways,18,19 has prompted the quest for new candidate determinants of venous thromboembolism in the FV gene. A peculiar FV allele (FV H1299R, also known as R2), marked by an A→G transition at position 4070,20 has been described and found to be linked to a number of other FV gene polymorphisms (the HR2 haplotype), which encode several amino acid changes.21 Carriership of the FV H1299R allele is associated with mild APC resistance21 and with a relative excess22 of the more thrombogenic23 FV isoform24 (FV1) in plasma. Accordingly, it has been reported that the FV H1299R mutation increases the risk of venous thrombosis in carriers of the FV R506Q mutation25 and represents per se a thrombotic risk factor.26
Moreover, quantitative FV deficiency,27 a potentially anticoagulant defect,28 has been shown to enhance APC resistance in heterozygous carriers of the FV R506Q.29-32
We report here the molecular characterization of a thrombophilic family encompassing 3 thrombophilic mutations (FV R506Q,5 FV H1299R,20 and PT 20210G/A6) and quantitative FV deficiency. The relationships between combinations of mutations affecting components of the prothrombinase complex and coagulation and clinical phenotypes were evaluated in the family.
Patients, materials, and methods
Patients
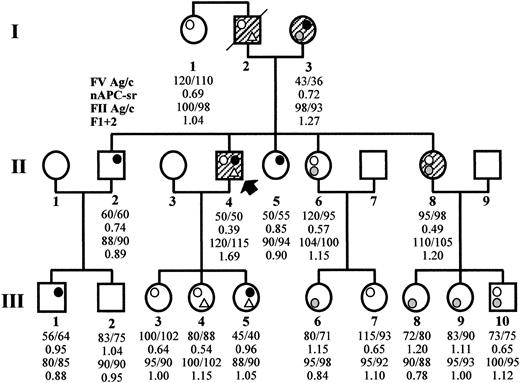
The thrombophilic family (Figure1) came to clinical observation via subject II4, a 51-year-old male who has experienced recurrent spontaneous superficial vein thrombosis (SVT) and a deep vein thrombosis (DVT) in the right leg. Three additional family members across 2 generations have experienced both SVT and DVT (I2, I3, II8) and, 2 of them (I2 and I3), also pulmonary embolism.
Family pedigree and coagulation laboratory data.
The propositus is subject II4 (arrow). Open circle, FV Leiden (R506Q); gray circle, FV R2 (H1299R); closed circle, FV defect (Y1702C); open triangle, PT 20210G/A. Symptomatic family members are hatched. The numbers reported below each family member express the FV antigen and activity levels (normal range, 70%-130%), the normalized APC ratio (APC resistance is defined by an APC ratio less than 0.84), the PT antigen and activity levels (normal range, 75%-125% and 80%-120%, respectively), and the F1 + 2 values in nmol/L (normal range, 0.4-1.1 nmol/L), respectively.
Family pedigree and coagulation laboratory data.
The propositus is subject II4 (arrow). Open circle, FV Leiden (R506Q); gray circle, FV R2 (H1299R); closed circle, FV defect (Y1702C); open triangle, PT 20210G/A. Symptomatic family members are hatched. The numbers reported below each family member express the FV antigen and activity levels (normal range, 70%-130%), the normalized APC ratio (APC resistance is defined by an APC ratio less than 0.84), the PT antigen and activity levels (normal range, 75%-125% and 80%-120%, respectively), and the F1 + 2 values in nmol/L (normal range, 0.4-1.1 nmol/L), respectively.
Normal controls from the general population
A sample of 252 subjects from northern Italy was recruited to perform a population-based screening for the FV Y1702C mutation. Informed consent to participate in the study was obtained from all subjects.
Coagulation laboratory investigations
The presence of PC, PS, and antithrombin III deficiencies in the family was excluded by conventional coagulation tests. PT antigen levels were determined by a specific enzyme linked immunosorbent assay (ELISA) as previously described.33 PT activity was detected in a thromboplastin-based assay using factor II–deficient plasma on a ACL 3000Plus analyser (Instrumentation Laboratories, Milan, Italy). Normal ranges, obtained from 100 healthy subjects of both sexes 15 to 75 years old, were 75% to 125% and 80% to 120% for PT antigen and activity, respectively. FV antigen (FV:Ag) and activity (FV:c) levels, as well as APC resistance, were measured as previously described.30 The normal ranges were 70% to 130% for FV:Ag and FV:c and more than 0.84 for the normalized APC ratio. F1 + 2 levels were determined in the family members using Enzygnost F1 + 2 micro (Dade-Behring, Marburg, Germany) as previously described.34 The normal range was 0.4 to 1.1 nmol/L.
DNA studies
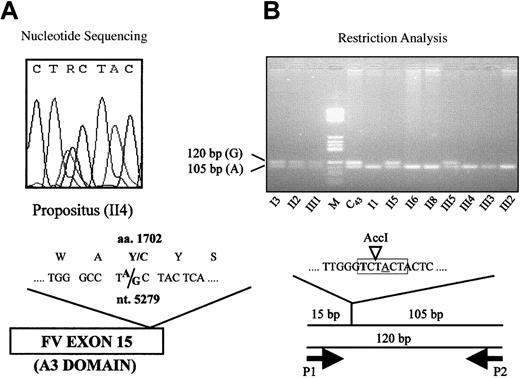
Exon scanning of the FV gene was performed by direct sequencing as described.31 Primers located in introns 14 (5′-AACCAGCCATTTTGACTTA-3′) and 15 (5′-GAAATAACCCCGACTCTTC-3′), respectively, were used to amplify and sequence (Figure2A) a 410–base pair (bp) DNA fragment spanning the whole exon 15. A restriction protocol for the detection of the 5279A/G mutation (Figure 2B) was obtained by means of a mutagenized primer (5′-CTGTCGGGCTTGGGTCT-3′, P1, nucleotides [nt.] 5262-5278) introducing an AccI restriction site in the normal allele. Forward primer P1 was used in combination with reverse primer P2 (5′-GAAATAACCCCGACTCTTC-3′, intron 15) to amplify a 120-bp DNA fragment, which was subsequently digested with AccI (New England Biolabs, Beverly, MA) under the conditions recommended by the manufacturer. The FV H1299R and PT 20210G/A polymorphisms were detected as reported.20 35
Detection of the 5279A/G mutation (FV Y1702C).
(A) Detection by direct sequencing: The heterozygous sequencing pattern of the propositus is shown. The “R” in the nucleotide sequence indicates that either an A or a G may be present at this position. (B) Detection by restriction analysis: Mutagenized (bold) primer P1, which creates an AccI restriction site (highlighted in gray) in the normal (A, underlined) allele, is used in combination with reverse primer P2 to amplify a 120-bp DNA fragment. The fragments obtained byAccI digestion of the polymerase chain reaction products are visualized by agarose gel electrophoresis. C43, individual from the general population; M, molecular weight marker.
Detection of the 5279A/G mutation (FV Y1702C).
(A) Detection by direct sequencing: The heterozygous sequencing pattern of the propositus is shown. The “R” in the nucleotide sequence indicates that either an A or a G may be present at this position. (B) Detection by restriction analysis: Mutagenized (bold) primer P1, which creates an AccI restriction site (highlighted in gray) in the normal (A, underlined) allele, is used in combination with reverse primer P2 to amplify a 120-bp DNA fragment. The fragments obtained byAccI digestion of the polymerase chain reaction products are visualized by agarose gel electrophoresis. C43, individual from the general population; M, molecular weight marker.
FV mRNA studies
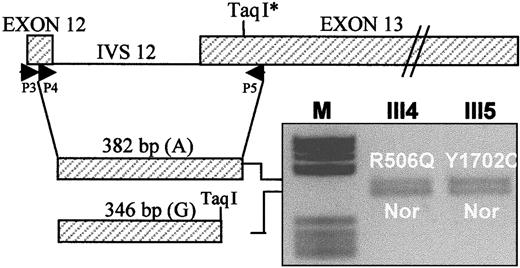
Total RNA was extracted from platelets, which are known to contain trace amounts of FV messenger RNA (mRNA), by conventional methods (RNAfast, Molecular Systems, San Diego, CA). FV mRNA was reverse transcribed using primer P5 (5′-AAGAATAATTTGAACCAACAAT-3′, nt. 2425-2404), located in exon 13 (Figure3), and Super Script II RT reverse transcriptase (GIBCO-BRL, Life Technologies). A 382-bp FV complementary DNA (cDNA) fragment spanning exons 12 to 13 was synthesized in 2 rounds of polymerase chain reaction using forward primers P3 (5′-TGACCCTCTTCCCCATG-3′, nt. 2012-2028) and P4 (5′-ACGGTCACAATGGATAATGT-3′, nt. 2044-2063), both in exon 12, and reverse primer P5 as described31,32 (Figure 3). This region contains a neutral TaqI restriction polymorphism (2391 G/A),36 the A allele of which marks both the FV R506Q allele and the FV-deficient allele (Y1702C) in this family. Heterozygosity for this marker in the propositus' daughters who carry the FV R506Q (III4) and Y1702C (III5) mutations, respectively, offers the opportunity to evaluate the relative expression at the mRNA level of both these alleles with respect to a normal FV allele. The cDNA fragments obtained from family members III4 and III5 were digested with TaqI and the products run on a 2.5% agarose gel and stained with ethidium bromide (Figure 3). The expression at the mRNA level of different FV alleles was evaluated by densitometric analysis of the obtained bands.
Expression at the mRNA level of the FV genes bearing the Leiden mutation (R506Q) and the FV defect (Y1702C), respectively, relative to a normal (Nor) FV allele.
Primers P3, P4, and P5 are used to amplify a nested FV cDNA fragment spanning exons 12 to 13 and containing a polymorphic TaqI restriction site. The 382-bp uncleaved cDNA, corresponding to the A allele of the TaqI polymorphism, marks the FV R506Q allele in subject III4 and the FV Y1702C allele in subject III5; the 346-bpTaqI restriction product, corresponding to the G allele of the TaqI polymorphism, marks the normal (Nor) FV allele in both subjects. The gene and cDNA schemes are drawn to different scales. IVS 12, intron 12.
Expression at the mRNA level of the FV genes bearing the Leiden mutation (R506Q) and the FV defect (Y1702C), respectively, relative to a normal (Nor) FV allele.
Primers P3, P4, and P5 are used to amplify a nested FV cDNA fragment spanning exons 12 to 13 and containing a polymorphic TaqI restriction site. The 382-bp uncleaved cDNA, corresponding to the A allele of the TaqI polymorphism, marks the FV R506Q allele in subject III4 and the FV Y1702C allele in subject III5; the 346-bpTaqI restriction product, corresponding to the G allele of the TaqI polymorphism, marks the normal (Nor) FV allele in both subjects. The gene and cDNA schemes are drawn to different scales. IVS 12, intron 12.
Protein studies
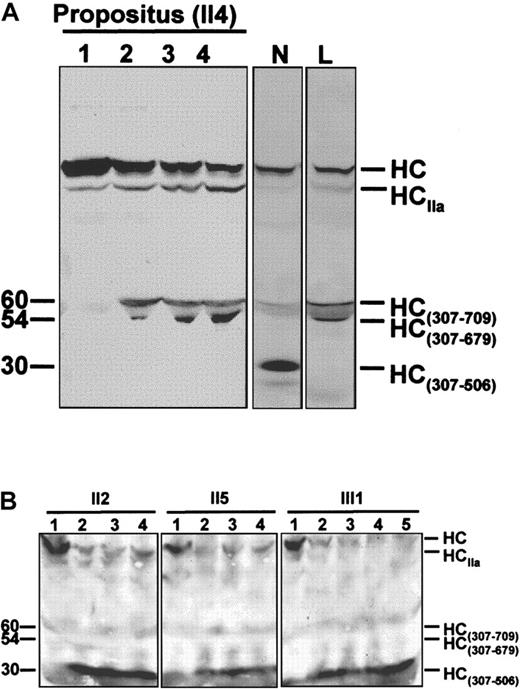
FV molecules present in plasma of the propositus and of family members II2, II5, and III1 were characterized by Western blotting (Figure 4) as described.32Briefly, 100 μL of plasma was diluted 10-fold with HBS Ca++ (20-mmol/L HEPES, 0.15-mol/L NaCl, 5-mmol/L CaCl2, pH 7.4) and incubated at 37°C in the presence of synthetic phospholipid vesicles (PCPS, 20 μmol/L). After clotting, the solution was centrifuged at 10 000 rpm for 30 seconds, and purified human APC (5-20 nmol/L) was added to the supernatant. Aliquots were drawn from the mixture at regular intervals and run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis to monitor FV inactivation. The gel was transferred to nitrocellulose and stained with monoclonal antibody αHFVaHC#17, which recognizes an epitope located between residues 307 and 506 of the FVa heavy chain. The FVa inactivation pattern obtained for the propositus was compared with those of normal and FV R506Q homozygous controls.
Time courses of APC-mediated FVa inactivation.
(A) Time course of FVa inactivation in the FV R506Q/Y1702C doubly heterozygous propositus. The inactivation end-point patterns of normal (N) and R506Q (L) FVa are reported for comparison. In the normal control, the FV heavy chain (HC) is cleaved by APC at Arg506 and Arg306, resulting in a 30-kd immunoreactive fragment (HC307-506). In the FV R506Q homozygote, cleavage of the FV heavy chain does not generate the HC307-506fragment, and a 60/54-kd doublet (HC307-679/709) appears instead. The propositus' FV shows an inactivation pattern indistinguishable from that of the R506Q FV homozygote, which indicates that the Y1702C FV is not expressed in plasma. HCIIa is a shorted heavy chain of FVa, due to the prolonged exposure of FVa to the thrombin generated following clot formation and characterized by considerably lower cofactor activity. It suggests a potentially alternative method for FVa inactivation in FVR506Q individuals.50 51 Lane 1, FVa in the presence of phospsholipid vesicles before adding APC; lanes 2 to 4, FVa 5, 10, and 20 minutes after the addition of APC. Molecular weights (kd) are reported on the left. (B) Time course of FVa inactivation in family members carrying the FV Y1702C mutation as a single heterozygous defect. The normal FVa inactivation pattern (presence of the 30-kd band and absence of the 60/54-kd doublet) indicates that the Y1702C FV molecules do not contribute to the altered FVa inactivation pattern (propositus, Figure 4A). Lane 1, FVa in the presence of phospholipid vesicles before adding APC; lanes 2 to 5, FVa 3, 5, 10, and 20 minutes after the addition of APC.
Time courses of APC-mediated FVa inactivation.
(A) Time course of FVa inactivation in the FV R506Q/Y1702C doubly heterozygous propositus. The inactivation end-point patterns of normal (N) and R506Q (L) FVa are reported for comparison. In the normal control, the FV heavy chain (HC) is cleaved by APC at Arg506 and Arg306, resulting in a 30-kd immunoreactive fragment (HC307-506). In the FV R506Q homozygote, cleavage of the FV heavy chain does not generate the HC307-506fragment, and a 60/54-kd doublet (HC307-679/709) appears instead. The propositus' FV shows an inactivation pattern indistinguishable from that of the R506Q FV homozygote, which indicates that the Y1702C FV is not expressed in plasma. HCIIa is a shorted heavy chain of FVa, due to the prolonged exposure of FVa to the thrombin generated following clot formation and characterized by considerably lower cofactor activity. It suggests a potentially alternative method for FVa inactivation in FVR506Q individuals.50 51 Lane 1, FVa in the presence of phospsholipid vesicles before adding APC; lanes 2 to 4, FVa 5, 10, and 20 minutes after the addition of APC. Molecular weights (kd) are reported on the left. (B) Time course of FVa inactivation in family members carrying the FV Y1702C mutation as a single heterozygous defect. The normal FVa inactivation pattern (presence of the 30-kd band and absence of the 60/54-kd doublet) indicates that the Y1702C FV molecules do not contribute to the altered FVa inactivation pattern (propositus, Figure 4A). Lane 1, FVa in the presence of phospholipid vesicles before adding APC; lanes 2 to 5, FVa 3, 5, 10, and 20 minutes after the addition of APC.
Molecular modeling
The FV Y1702C mutation in the A3 domain of FV was investigated using the coordinates of the highly homologous A3 domain of ceruloplasmin (CP)37 (PDB 1KCW), by means of the shareware software Swiss PdbViewer v3.5b3, developed by Nicholas Guex, Torsten Schwede, and Alexander Diemand for the Glaxo Wellcome Experimental Research.
Results
Identification and characterization of a new missense mutation causing FV deficiency
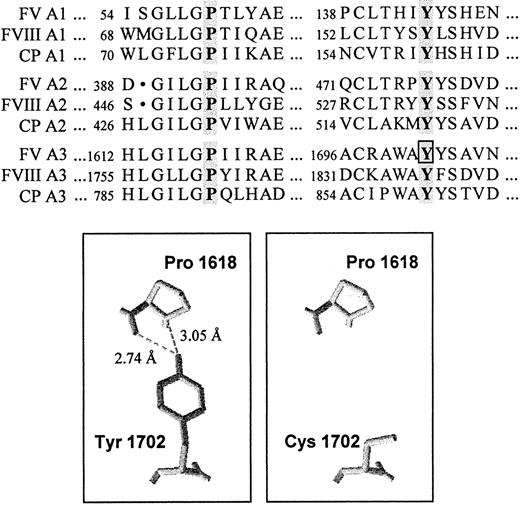
To spot the mutation responsible for FV deficiency in the propositus, coding regions and splicing junctions of the FV gene were scanned by direct sequencing. A novel A→G transition, which predicts the substitution of Y1702 by a cysteine in the A3 domain of FV, was identified at nucleotide 5279 in exon 15 in the heterozygous state (Figure 2A). Alignment of the FV A domains with the homologous counterparts38,39 of factor VIII (FVIII) and CP showed the absolute conservation of the tyrosine residue affected by the mutation and a high degree of homology among the surrounding amino acid sequences (Figure 5).
Conservation and structural role of the mutated tyrosine (FV Y1702) in the A domains.
(Top) Alignment of a portion of the A domains of human FV, FVIII, and CP. The conserved tyrosine and proline residues are highlighted. The tyrosine residue affected by the FV 5279A/G mutation is boxed. (Bottom) Three-dimensional model of a portion of the A3 domain of FV based on the coordinates of CP. The highly conserved Y1702 residue forms 2 hydrogen bonds with Pro1618. The substitution of the tyrosine residue by a cysteine, predicted by the FV 5279A/G mutation, causes the loss of these interactions.
Conservation and structural role of the mutated tyrosine (FV Y1702) in the A domains.
(Top) Alignment of a portion of the A domains of human FV, FVIII, and CP. The conserved tyrosine and proline residues are highlighted. The tyrosine residue affected by the FV 5279A/G mutation is boxed. (Bottom) Three-dimensional model of a portion of the A3 domain of FV based on the coordinates of CP. The highly conserved Y1702 residue forms 2 hydrogen bonds with Pro1618. The substitution of the tyrosine residue by a cysteine, predicted by the FV 5279A/G mutation, causes the loss of these interactions.
Because the FV Y1702C mutation is not detectable by restriction enzyme digestion, a mutagenized primer introducing an AccI restriction site in the normal (A) allele (Figure 2B) was designed to allow rapid screening for the mutation (see “Materials and methods”). The FV Y1702C substitution was found (Figure 2B) in all family members whose FV levels (Figure 1) were compatible with heterozygous FV deficiency, but it was absent in all others.
To substantiate the causative role of the FV Y1702C mutation, 252 healthy individuals from the general population were tested for the presence of the Y1702C substitution and, in parallel, characterized for FV:c levels (mean 99%, SD 24%, range 30%-187%). One of these controls (C43 in Figure 2B) was found to be a carrier, which was confirmed by direct sequencing, and showed reduced FV levels (FV:c 58%).
The expression of the FV allele bearing the Y1702C mutation was investigated both at the mRNA and protein levels. Total RNA extracted from platelets was reverse transcribed and a FV cDNA fragment, spanning exons 12 to 13 and containing a TaqI restriction polymorphism in linkage with both the FV R506Q and the FV-deficient (Y1702C) alleles, was amplified (Figure 3). Heterozygosity for this marker in the propositus' daughters who carry the FV R506Q (III4) and the Y1702C (III5) mutations, respectively, made it possible to compare the expression at the mRNA level of both mutant genes with that of a normal FV allele. Densitometric analysis of theTaqI-restricted FV cDNA fragments obtained from subjects III4 and III5 indicated that the FV genes carrying either mutation (Y1702C or R506Q) were normally expressed at the mRNA level (Figure 3).
The expression of the FV Y1702C mutation at the protein level was studied in the propositus, who carries the R506Q mutation on the counterpart FV allele. This doubly heterozygous condition offers the opportunity to evaluate the protein expression of the FV Y1702C allele, because plasmatic FV bearing the R506Q substitution is clearly recognizable on an immunoblot by its characteristic APC-mediated inactivation pattern.40 The time course of APC-mediated FVa inactivation in vitro showed a pattern indistinguishable from that of a FV R506Q homozygote (Figure 4A), providing direct evidence for the impaired secretion of FV bearing the Y1702C substitution. However, to further investigate the possibility that FV molecules encoded by the FV Y1702C allele contribute to the abnormal FVa inactivation pattern observed in the propositus, family members who carry the FV Y1702C mutation as a single defect (II2, II5, and III1) were also studied as controls (Figure 4B). These experiments indicate completely normal APC-mediated FVa inactivation patterns for all 3 subjects (Figure 4B), which confirms that the FV allele bearing the Y1702C mutation is not expressed at the protein level in plasma and thus does not participate in the abnormal FVa inactivation pattern.
Genotype, phenotype relationships in family members
The presence of FV gene mutations (R506Q, H1299R, and Y1702C) and of the PT gene variant (PT 20210G/A) was investigated and coagulation data collected in the propositus' family (Figure 1).
FV R506Q was detected in the heterozygous condition in 7 family members, once in combination with the PT 20210G/A mutation and 3 times with FV H1299R (Figure 1). The presence of FV R506Q was also inferred in the deceased propositus' father, again in combination with PT 20210G/A (paternity was confirmed by FV microsatellite analysis in the family). As expected, all carriers of the FV R506Q mutation showed APC resistance (Figure 1) that was particularly marked in double heterozygotes for FV Y1702C (the propositus, APC ratio 0.39) or for FV H1299R (mean APC ratio 0.57), which is known to enhance APC resistance in FV R506Q carriers.21
The FV H1299R substitution was present in 7 family members, both alone and in combination. In accordance with previous findings,20 carriers of this mutation had FV levels (Figure 1) in the lower end of the normal range (mean FV:Ag 87, mean FV:c 85).
The FV Y1702C substitution was found as a single defect in 3 family members, in combination with FV H1299R in the propositus' mother, and in combination with PT 20210G/A in one of his daughters (Figure 1). In accordance with the results of the Western blot, it caused (Figure 1) a 50% reduction of plasma FV antigen and activity levels in all carriers (mean FV:Ag 51, mean FV:c 51). The lowest FV levels (FV:c 36%) were observed in the propositus' mother who, in addition to the FV Y1702C mutation, carried the FV H1299R variant.
The presence of the PT 20210G/A mutation was detected in the propositus and in 2 of his daughters, and PT antigen and activity levels were measured in all family members (Figure 1). All subjects showed PT levels within the normal range, and only a weak correlation was observed between carriership of the PT 20210G/A mutation and high PT levels. F1 + 2 levels (Figure 1) were also determined as an integrated measure of prothrombinase activity,41 and the highest values were observed in carriers of double defects and particularly in those who had experienced venous thromboembolism.
Discussion
Thrombin generation by the prothrombinase complex represents a key step of the coagulation cascade. The rate of PT conversion depends on the concentration and activity of the various components of this macromolecular complex.9,42 A prominent regulatory role is played by FV and FVa,19 which are subject to43,44 and themselves participate in45,46 the negative control by the APC system.
Three thrombophilic mutations (FV R506Q, FV H1299R, and PT 20210G/A) were found in different combinations in various members of a large thrombophilic family. All of them affect the activity of the prothrombinase complex (Figure 6), by increasing either the substrate concentration (PT 21210G/A)6 or the survival of FVa in plasma (FV R506Q and FV H1299R). Elevated PT levels, predicted by the PT 21210G/A mutation, have been shown to enhance thrombin generation significantly in a reconstructed plasma system.42 Differently, both FV mutations predict a delay in the APC-mediated inactivation of FVa, either (FV R506Q) by suppressing one of the APC cleavage sites on FV5,10-12 or (FV H1299R) by determining a relative increase22 of the poorly phospholipid-binding FVa1 form in plasma.23,24 In addition, they might also act via reduced cofactor activity in the APC-mediated inactivation of FVIIIa.46,47
Mutations affecting the prothrombinase complex in the family under study.
(Top) Schematic representation of FV molecules characterized by the Leiden mutation (FV R506Q), the R2 haplotype (FV H1299R), and the FV defect (FV Y1702C), respectively. The amino acid substitutions linked to the FV H1299R mutation are indicated by the smaller triangles. (Bottom) Expected combined effects of the FV and PT mutations on the activity of the prothrombinase complex. The arrows indicate the increased cofactor activity for the R506Q and H1299R FV molecules and the increased PT levels for the 20210G/A mutation. The crossed arrow indicates the lack in plasma of the FV molecules bearing the Y1702C substitution.
Mutations affecting the prothrombinase complex in the family under study.
(Top) Schematic representation of FV molecules characterized by the Leiden mutation (FV R506Q), the R2 haplotype (FV H1299R), and the FV defect (FV Y1702C), respectively. The amino acid substitutions linked to the FV H1299R mutation are indicated by the smaller triangles. (Bottom) Expected combined effects of the FV and PT mutations on the activity of the prothrombinase complex. The arrows indicate the increased cofactor activity for the R506Q and H1299R FV molecules and the increased PT levels for the 20210G/A mutation. The crossed arrow indicates the lack in plasma of the FV molecules bearing the Y1702C substitution.
Quantitative FV deficiency, a potentially anticoagulant defect that might in principle decrease the activity of the prothrombinase complex and that actually prolongs the initiation phase of coagulation,42 was also present in the propositus and other family members. The molecular investigations led to the identification of a candidate defect (5279A/G, Y1702C) in the FV gene. Genetic, functional, and structural evidence supports the association of the FV Y1702C mutation with FV deficiency. First of all, the family studies and general population screening indicate a complete cosegregation between the mutation and low FV levels; in fact, the only carrier of the mutation detected in the general population had FV levels reduced by half. However, to exclude that the FV Y1702C mutation is a mere neutral variant in absolute linkage disequilibrium with the causative mutation, the role of the mutated tyrosine residue, absolutely conserved in all 3 A domains of FV, FVIII, and CP (Figure5), was investigated by inspection of the 3-dimensional structure37 of CP. This analysis revealed that the homologous tyrosine residue (Y860) in this protein is buried in the domain core, belongs to a conserved β-sheet, and interacts via 2 hydrogen bonds with a proline residue (P791), which is also absolutely conserved (Figure 5) in all A domains. Molecular modeling of the FV A domains,48 based on the approximate 40% homology with the A domains of CP, indicates that FV Y1702 has the same features. Substitution of the tyrosine residue by a cysteine would lead to the loss of these interactions (Figure 5), potentially disrupting the domain scaffold. Although a disulfide bridge formed between this cysteine and one of the other free cysteines in factor V49would stabilize the abnormal structure, the FV antigen levels and Western blot analyses indicate that the product of the FV Y1702C allele is not detectable in plasma, although its expression at the mRNA level is normal.
Although the Y1702C FV does not participate in the abnormal FVa inactivation, FV deficiency caused by the Y1702C mutation turned out to contribute to shape the thrombotic risk profile in the family. In carriers of the FV R506Q mutation (propositus) and of the FV H1299R mutation (propositus' mother), quantitative FV deficiency due to the FV Y1702C mutation, far from protecting from thrombosis, proved to increase APC resistance and thus thrombotic risk via a pseudohomozygous APC resistance mechanism.30-32 As suggested by the Western blot, increased APC resistance is attributable to the exclusive presence of R506Q (propositus) or H1299R (propositus' mother) FV molecules in plasma. In this respect, the propositus' mother is the first case of “FV H1299R pseudohomozygous APC resistance” ever described.
Plasma levels of F1 + 2 represent an integrated measure of the activity of the prothrombinase complex. Although the family-based evaluation of F1 + 2 levels is poorly reliable because of the assay variability, F1 + 2 values were found to be higher in carriers of a double defect and appeared to correlate with thrombotic risk in this family. It was also observed that all symptomatic family members showed more or less pronounced APC resistance (Figure 1).
The relationships between clinical phenotypes and combined genotypes indicated that all family members who had developed thrombosis were carriers of at least 2 defects (Figure 1), the combination of APC resistance and high PT levels being associated with recurrent thrombotic episodes. This valuable information is of potential help for the prevention of thrombosis in the young combined heterozygotes of the third generation of this family.
Supported by Telethon, Italy (grant E.675) and MURST. Also supported in part by a grant from Veneto Region, RSF No. 783/01/97 (P.S.) and by start-up funds from the Department of Chemistry at Cleveland State University (M.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesco Bernardi, Department of Biochemistry and Molecular Biology, Via L. Borsari 46, 44100 Ferrara, Italy; e-mail:ber@unife.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal