Abstract
Activation of the serine/threonine kinase Akt and the regulation of its activation are recognized as critical in controlling proliferative/survival signals via many hematopoietic receptors. In B lymphocytes, the B-cell receptor (BCR)-mediated activation of Akt is attenuated by co–cross-linking of BCR with the inhibitory receptor FcγRIIB1, and the binding of the SH2 domain-containing inositol phosphatase, SHIP, to FcγRIIB1. Because SHIP dephosphorylates phosphatidylinositol 3,4,5-trisphosphate (PIP3) and activation of Akt requires PIP3, the destruction of this phospholipid has been proposed as the mechanism for Akt inhibition. However, upstream kinases that activate Akt, such as PDK1, also require PIP3 for activation. In this report, we addressed whether SHIP inhibits Akt directly at the level of Akt recruitment to the membrane, indirectly through PDK recruitment/phosphorylation of Akt, or both. We generated stable B-cell lines expressing a regulatable, but constitutively membrane-bound Akt that still required PDK-dependent phosphorylation for activation. Several lines of evidence suggested that activation of this membrane-targeted Akt is not inhibited by FcγRIIB1/SHIP and that PDK is not a target for SHIP-mediated inhibition. These data demonstrate that SHIP inhibits Akt primarily through regulation of Akt membrane localization. We also observed during these studies that FcγRIIB1/SHIP does not inhibit p70S6k activation, even though several other PIP3-dependent events were down-regulated. Because the enhanced activation of Akt in the absence of SHIP correlates with hyperproliferation in the myeloid lineage, our data have implications for SHIP and Akt-dependent regulation of proliferation in the hematopoietic lineage.
Introduction
In the early phase of the immune response, the binding of the B-cell receptor (BCR) to antigen leads to receptor cross-linking and activation. This results in intracellular calcium flux, gene expression, protein synthesis, and cell survival.1 Activation of phosphatidylinositol 3-kinase (PI-3K) and the generation of the phospholipid phosphatidylinositol 3,4,5-trisphosphate (PIP3), are critical for optimal BCR-mediated signaling.2 The rise in intracellular calcium, as well as the activation of the serine/threonine kinase, protein kinase B (PKB), or Akt (implicated in antiapoptotic cell survival signals), have been shown to be downstream of PI-3 kinase.2-6 Thus, negative regulation of PI-3 kinase–mediated signals is used as a mechanism for down-modulation of B-cell responses (see below).
Akt/ PKB was identified originally as part of an oncogene responsible for spontaneously arising leukemias and lymphomas in mice.7-11 Since then, many cytokines, growth factor receptors, and antigen receptors have been shown to induce Akt kinase activity.4,12,13 Akt activation has been linked to PI-3 kinase-dependent antiapoptotic signals delivered through these receptors,14-18 as well as glucose metabolism,19-21 protein synthesis,21,22 and gene expression.19,23-25 The human Akt locus has been mapped to chromosome 14q32, proximal to the immunoglobulin heavy chain locus.26 Because frequent translocations and inversions in this region have been linked to T-cell leukemia/lymphoma and childhood leukemia,8 27 elucidating the mechanism of Akt activation and its regulation could have potential implications for tumorigenesis.
Akt contains an N-terminal pleckstrin homology (PH) domain that binds the phospholipid PIP3, a glycine-rich region, followed by a kinase domain and a C-terminal regulatory region.8,28 Generation of PIP3 by PI-3 kinase in response to receptor activation results in the Akt-PH domain binding to PIP3, and Akt recruitment to the membrane.8,28,29 This membrane translocation is thought to cause a conformational change in Akt, allowing its phosphorylation and subsequent activation.13,29-32 The phosphorylation of Akt occurs on 2 specific sites, threonine 308 and serine 473, both of which are required for maximal Akt activation.33 Phosphorylation of Akt on Thr308 is mediated by phosphoinositide-dependent kinase1 (PDK1), whereas phosphorylation of Ser473 is mediated by PDK2.34 Recent studies suggest that PDK2 may in fact be composed of PDK1 in complex with another protein.35 36
PDK1 also contains a PH domain,37-40 and PDK1 recruitment to the membrane also requires the binding of its PH domain to PIP3. It appears that PDK1 can be constitutively localized on the membrane through the resting levels of PIP338; however, additional PDK1 may be recruited to PIP3 generated on receptor activation.41 In contrast, Akt is not constitutively on the membrane and its membrane recruitment requires receptor activation.8,34,38 Artificial membrane targeting of Akt through the addition of a myristoylation sequence at its N-terminus, leads to a constitutively active Akt and is sufficient for protection of cells against apoptosis.15,16,18 In fact, myristoylation of the gag sequence in v-Akt and its localization to the membrane have been attributed to its oncogenic potential.42 Thus, regulation of Akt membrane localization and its activation status may be critical in regulating cellular proliferation and oncogenesis.
In latter stages of the antibody responses, B lymphocytes are negatively regulated by excess circulating antibody through simultaneous engagement of the BCR and the FcγRIIB1 receptor.43,44 This leads to cessation of specific gene expression, and inhibition of immunoglobulin (Ig) synthesis, as well as apoptosis of the B cells. This FcγRIIB1-mediated inhibition requires tyrosine phosphorylation of the cytoplasmic tail of this receptor and the subsequent binding of the SH2 domain–containing inositol phosphatase, SHIP.45,46 SHIP has 5′-inositol phosphatase activity and has been shown to dephosphorylate PIP3 both in vitro and in vivo.47-51 Thus, the FcγRIIB1/SHIP-mediated destruction of the PIP3, and in turn, termination of multiple PI-3 kinase dependent events, has been attributed to the negative regulation due to BCR+FcγRIIB1 coengagement. We have previously demonstrated that BCR-induced activation of Akt is inhibited by co–cross-linking of BCR+FcγRIIB1.4 Subsequently, others have also reported an FcγRIIB1/SHIP-dependent inhibition of Akt in B cells.3,5,6 B cells made deficient in SHIP fail to show this Akt inhibition, and reconstitution of this cell line with SHIP restores the FcγRIIB1-mediated Akt inhibition (data not shown). Mast cells derived from SHIP-deficient mice, compared with normal mice, have increased and prolonged Akt activation and are less susceptible to apoptotic stimuli.52 This has been linked to myeloid hyperplasia and splenomegaly in the SHIP-deficient mice.52Moreover, overexpression of SHIP in growth factor–dependent cell lines leads to increased apoptosis.49,53 A similar mechanism may be responsible for FcγRIIB1-mediated induction of B-cell apoptosis.54
Although the above studies clearly demonstrated a role for SHIP in inhibiting Akt activation, the precise mechanism by which this inhibition is achieved remains unclear. Several possibilities exist. Destruction of PIP3 by enzymatic activity of SHIP could make PIP3 limiting for Akt PH domain binding, and thereby inhibit the membrane localization of Akt. Alternatively, SHIP could negatively regulate PDK1 and indirectly mediate Akt inhibition. Both mechanisms may also be operational concurrently. To better understand the SHIP-mediated inhibition of Akt, we used an Akt that has been engineered to be constitutively localized on the membrane through a myristoylation motif, and fused to the hormone-binding domain of the murine estrogen receptor (Myr Akt-ER).20 Despite being membrane bound, activation of Myr Akt-ER is inducible only after the addition of the drug 4-hydroxytamoxifen (4-HT). Because we observed that this Akt-ER requires phosphorylation by PDKs, this allowed us to address whether SHIP regulates Akt activation at the level of Akt, PDK or both. With the use of stable B-cell lines expressing this Myr Akt-ER and by the cross-linking of BCR with or without FcγRIIB1 in the presence or absence of tamoxifen, we demonstrate that SHIP inhibits Akt primarily at the level of Akt membrane localization, and not through inhibition of PDKs.
Materials and methods
Plasmids, cell lines, and antibodies
The HA-tagged myristoylated Δ4-129Akt-ER construct and the control construct with an A2 mutation in the myristoylation sequence subcloned into the PWZLneo plasmid were generously provided by Dr Richard Roth (Stanford, CA).20 The BALB/c murine B-cell lymphoma line, A20, was grown in RPMI-1640 media, supplemented with 10% fetal bovine serum, penicillin, streptomycin, 2 mmol/L L-glutamine (Life Technologies, Grand Island, NY), and 50 μmol/L β-mercaptoethanol (Sigma, St Louis, MO). The CTLL-2 line was grown in the same media with 5 U/mL of recombinant interleukin-2 (IL-2) (Hoffman-LaRoche, Nutley, NJ). Rabbit antimouse whole Ig antibody and the F(ab′)2 fragment were obtained from Jackson Immunoresearch Labs (West Grove, PA). The anti-HA 12CA5 murine monoclonal antibody and the 2.4G2 rat monoclonal antibody against FcγRIIB1 were purified from hybridoma supernatants. The rabbit polyclonal antibody to the carboxy-terminus of ERα, the donkey antimouse IgG, FITC-labeled antidonkey secondary antibody, and the mouse monoclonal antibody specific for the carboxy-terminus of p70S6k were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phosphospecific antibodies to Thr308 or Ser473 of murine Akt and the antibody to phopsho-p44 MAPK were from New England Biolabs (Beverly, MA).
Transfections
The A20 cells (107 in 0.5 mL of growth medium) were transfected with 25 μg linearized plasmid DNA encoding Myr Akt-ER and non-Myr Akt-ER, by electroporation at 250V and 960 μF with an electroporator from BTX (San Diego, CA). Cells were cultured for 24 hours, then selected in 96-well plates in media containing 2 mg/mL G418 (Gibco, Gaithersburg, MD). The A20 clones expressing HA-tagged Akt-ER were identified by immunoprecipitation and Western blotting using anti-HA or anti-ER antibodies. Expression of BCR and FcγRIIB1 were analyzed by flow cytometry, as described previously.4
Stimulations, immunoprecipitations, and immunoblotting
Parental A20 cells or transfectants were washed and resuspended at 15 × 106 cells/mL in RPMI-1640. As indicated, cells were preincubated at 37°C with 1 μmol/L 4-hydroxy tamoxifen (4-HT) (Sigma) for 20 minutes to 3 hours.20 The cells were washed, resuspended in RPMI medium, and incubated for 5 minutes each with either 1.4 μg/mL antimouse F(ab′)2 fragment to stimulate the BCR alone, or 2.5 μg/mL intact antimouse IgG for BCR+FcγRIIB1 co–cross-linking. Cells were quickly pelleted, and lysed at 4°C for 15 minutes with lysis buffer, containing 50 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, 1% NP-40, 10 mmol/L Na pyrophosphate, 10 mmol/L NaF, 1 mmol/L Na3VO4, 2 mmol/L PMSF, and 10 μg/mL each of aprotinin, leupeptin, pepstatin, and AEBSF. After centrifugation at 14 000 rpm for 10 minutes at 4°C, the supernatant was used directly for immunoblotting, or, specific proteins were precipitated using the relevant antibody and protein A–conjugated beads for 2 hours at 4°C. Beads were washed, and bound proteins were analyzed using standard SDS-PAGE, and the immunoblots were developed using chemiluminescence.
Kinase assays
After 4-HT incubation and/or stimulation of BCR alone or BCR+FcγRIIB1, 15 × 106 A20 lymphocytes were lysed (same lysis buffer as above with addition of 1 μmol/L microcystin), and Akt-ER was immunoprecipitated using anti-HA and protein A beads. The Akt kinase assay using a GSK-3–derived substrate peptide was performed using the Akt immunoprecipitation kinase assay kit purchased from Upstate Biotechnology (Lake Placid, NY).4 The p70S6k activity was measured under the same conditions using 0.1 mmol/L Rsk substrate peptide (Santa Cruz Biotechnology).
Intracellular calcium measurements
The 2 × 106 A20 lymphocytes were washed and resuspended in 1 mL RPMI-1640, and incubated in 1 μg/mL Indo 1 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C. The cells were washed 3 times and resuspended in a buffer containing 10 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 0.1% glucose, and 1% fetal calf serum. The calcium flux was recorded on excitation at 340 nm as the ratio of fluorescence emissions at 398 and 480 nm with an Hitachi 2000 spectrofluorimeter (Hitachi Instruments, Naperville, IL). The background was recorded for 30 seconds before addition of either 13 μg/mL of the antimouse F(ab′)2 fragment for BCR cross-linking or 23 μg/mL of intact antimouse IgG for BCR+FcγRIIB1 co–cross-linking. The data are presented as Indo-1 fluorescence ratio. To determine the effect of Akt-ER activation on calcium flux, similar recordings were performed after addition of 1 μmol/L 4-HT for 2 hours to A20 cells before Indo-1 loading.
CTLL proliferation assay
The 5 × 104 A20 cells in 200 μL/well in a 96-well plate were stimulated in triplicate with F(ab′)2 or intact antimouse IgG antibody for 24 hours at different concentrations from 7.5 to 15 μg/mL. Where indicated, 1 μmol/L 4-HT was added and the drug was present throughout the 24-hour stimulation period. A 125 μL sample of the supernatants was collected from each well, frozen, and thawed once. IL-2-dependent CTLL cells (washed 4 times in phosphate-buffered saline [PBS] to remove any remaining IL-2 in the growth medium) were plated in 96-well plates at 104 per well in 50 μL fresh growth medium without IL-2. Supernatants from A20 cells (50 μL) was added to each well and incubated for 16 hours.3H-thymidine was then added at 0.037 MBq (1 μCi) per well for an additional 5 hours, and the incorporated radioactivity was measured.55
Results
Expression of a membrane-targeted, regulative Akt in B cells
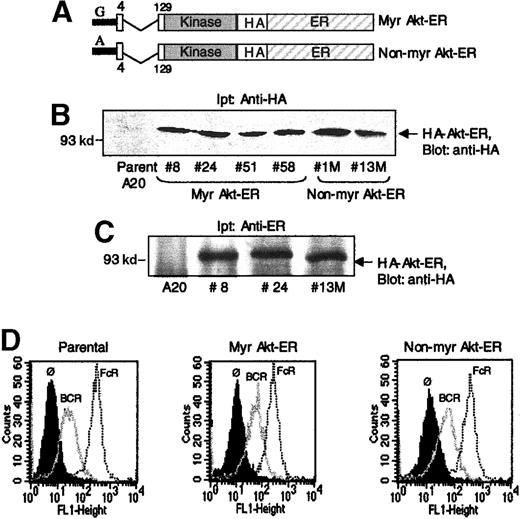
To study the regulation of Akt during BCR and FcγRIIB1 signaling, murine A20 B lymphocytes were stably transfected with an inducible Akt construct previously used by Kohn et al20 to study Akt activation in NIH3T3 cells and adipocytes. This modified Akt contains the catalytically active portion of the Akt kinase, but lacks the PH domain necessary for membrane localization. Instead it is targeted to the membrane through a myristoylation sequence added to its amino terminus and was fused at the C-terminus to the hormone-binding domain of murine estrogen receptor (ER) (Figure1A). This myristoylated Akt-ER fusion protein (Myr Akt-ER) remains inactive until the addition of the estrogen analogue, 4-hydroxytamoxifen (4-HT),20 which is thought to relieve steric inhibition of Akt by the ER domain. In the presence of 4-HT, the Akt-ER protein becomes phosphorylated and this has been correlated with Akt-ER activation.20 This inducible Akt-ER provided a tool to address whether Akt inhibition by FcγRIIB1/SHIP is regulated at the level of membrane localization and/or through the subsequent phosphorylation of Akt by PDKs. As a control, we used an Akt-ER fusion protein that carried a mutation of the critical +2 glycine within the myristoylation sequence and therefore not targeted to the membrane (non-Myr Akt-ER) (Figure1A).20
Expression of Myr Akt-ER and non-Myr Akt-ER constructs in B lymphocytes.
(A) Schematic depiction of the Myr Akt-ER and non-Myr Akt-ER constructs. Myristoylation sequence from Src, or a G-→ A mutation within this sequence (in the non-Myr Akt-ER version) was added to the N-terminus of PH-domain deleted (region between aa 4-129) Akt. This Akt, followed by a hemagglutinin (HA) tag, was fused to the estrogen receptor hormone-binding domain (ER). (B) A20 cells were transfected with Akt-ER constructs and the expression in different clones of Myr Akt-ER (clones 8, 24, 51, 58) or non-Myr Akt-ER (clones 1M, 13M) was analyzed by anti-HA immunoprecipitation, followed by anti-HA immunoblotting. (C) Lysates from some of the clones were immunoprecipitated with anti-ER antibodies and immunoblotted with anti-HA. Molecular mass standards (in kilodaltons) are indicated to the left. (D) Comparable expression of BCR and FcγRIIB1 in the clones. Parental A20 cells and those transfected with the Myr Akt-ER or the non-Myr Akt-ER were incubated with antibodies specific for BCR (thin trace) or FcγRIIB1 (dotted line trace) and their expression was analyzed by flow cytometry. The background staining on the cells is shown as a solid histogram (Ø).
Expression of Myr Akt-ER and non-Myr Akt-ER constructs in B lymphocytes.
(A) Schematic depiction of the Myr Akt-ER and non-Myr Akt-ER constructs. Myristoylation sequence from Src, or a G-→ A mutation within this sequence (in the non-Myr Akt-ER version) was added to the N-terminus of PH-domain deleted (region between aa 4-129) Akt. This Akt, followed by a hemagglutinin (HA) tag, was fused to the estrogen receptor hormone-binding domain (ER). (B) A20 cells were transfected with Akt-ER constructs and the expression in different clones of Myr Akt-ER (clones 8, 24, 51, 58) or non-Myr Akt-ER (clones 1M, 13M) was analyzed by anti-HA immunoprecipitation, followed by anti-HA immunoblotting. (C) Lysates from some of the clones were immunoprecipitated with anti-ER antibodies and immunoblotted with anti-HA. Molecular mass standards (in kilodaltons) are indicated to the left. (D) Comparable expression of BCR and FcγRIIB1 in the clones. Parental A20 cells and those transfected with the Myr Akt-ER or the non-Myr Akt-ER were incubated with antibodies specific for BCR (thin trace) or FcγRIIB1 (dotted line trace) and their expression was analyzed by flow cytometry. The background staining on the cells is shown as a solid histogram (Ø).
Multiple murine A20 clones stably expressing Myr Akt-ER and the Non-myr Akt-ER were identified by anti-HA or anti-ER immunoprecipitations, followed by anti-HA immunoblotting (Figure 1B-C, respectively). The functional data presented later in this manuscript were obtained from several of these stable clones. BCR and FcγRIIB1 expression on the different clones were comparable to parental A20 cells (shown for one clone of each of Myr Akt-ER and non-Myr Akt-ER) (Figure 1D).
The Akt-ER can be activated in B cells by 4-HT
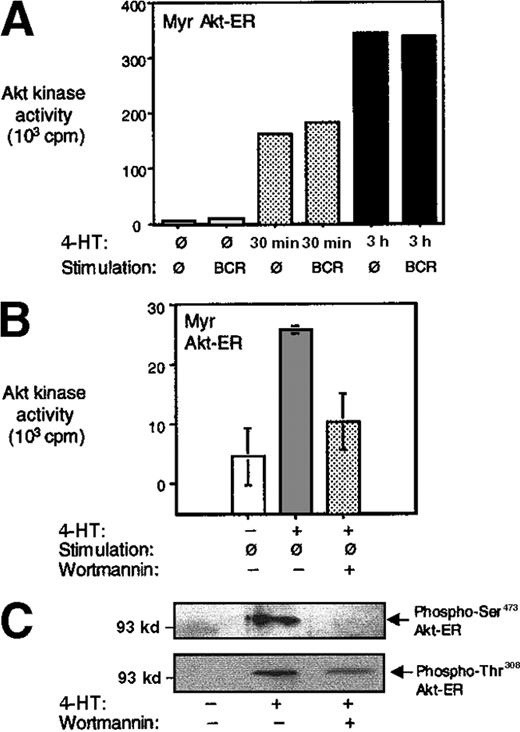
Myr Akt-ER–expressing A20 B cells were incubated with 4-HT for varying periods to test the inducible activation. The cells were lysed and the Akt-ER was immunoprecipitated with anti-HA antibodies. The in vitro Akt kinase activity was determined using a glycogen synthase kinase-3 (GSK3)-derived peptide as a substrate (see “Materials and methods”).4 In the absence of 4-HT, there was little Akt activity above background. On 4-HT addition, we observed a 20-fold increase in Akt activity within 30 minutes, and a continued linear increase in kinase activity after 3 hours (Figure2A). This is consistent with the findings of Kohn et al.20 The influence of BCR on Akt-ER kinase activity was determined by cross-linking the cells with anti-BCR antibodies. Consistently, BCR cross-linking did not further enhance the Akt-ER kinase activity above the effect of 4-HT addition (Figures 2A and 3A). Although direct comparisons in kinase activities between the endogenous Akt and Akt-ER is not feasible because of 2 different precipitation antibodies being used, we consistently observed that the maximal Akt-ER activity (induced by 4-HT) is roughly comparable to the maximal endogenous Akt activity under similar kinase assay conditions (about a 30-fold increase in both cases). Non-Myr Akt-ER was not activated by tamoxifen addition above baseline in any of the conditions tested (Figure 3A and data not shown).
Activation of Akt-ER by addition of 4-HT.
(A) Myr Akt-ER–expressing cells were incubated with 4-HT for the indicated times and left unstimulated or stimulated by BCR cross-linking for 5 minutes. The cells were lysed, and the kinase activity of Akt-ER was analyzed in anti-HA immunoprecipitates by phosphorylation of a GSK-3–derived substrate peptide (see “Materials and methods”). The small increase seen with BCR cross-linking after 30 minutes is not a reproducible result. The non-Myr Akt-ER did not show any activity above background before or after 4-HT addition in a similar experiment (data not shown). The data are representative of at least 3 independent experiments. (B) In an experiment similar to A, one set of cells were pretreated with 100 nmol/L wortmannin for 15 minutes before addition of 4-HT for 1 hour. HA-immunoprecipitates were tested for Akt-ER kinase activity as described above. (C) Untreated, 4-HT–treated or 4-HT+wortmannin–treated cells were lysed and phosphorylation of Akt-ER was assessed by immunoblotting using phosphospecific Akt antibodies reactive against Ser473 or Thr308. Akt-ER protein was present in all lanes as assessed by anti-HA immunoblotting (data not shown).
Activation of Akt-ER by addition of 4-HT.
(A) Myr Akt-ER–expressing cells were incubated with 4-HT for the indicated times and left unstimulated or stimulated by BCR cross-linking for 5 minutes. The cells were lysed, and the kinase activity of Akt-ER was analyzed in anti-HA immunoprecipitates by phosphorylation of a GSK-3–derived substrate peptide (see “Materials and methods”). The small increase seen with BCR cross-linking after 30 minutes is not a reproducible result. The non-Myr Akt-ER did not show any activity above background before or after 4-HT addition in a similar experiment (data not shown). The data are representative of at least 3 independent experiments. (B) In an experiment similar to A, one set of cells were pretreated with 100 nmol/L wortmannin for 15 minutes before addition of 4-HT for 1 hour. HA-immunoprecipitates were tested for Akt-ER kinase activity as described above. (C) Untreated, 4-HT–treated or 4-HT+wortmannin–treated cells were lysed and phosphorylation of Akt-ER was assessed by immunoblotting using phosphospecific Akt antibodies reactive against Ser473 or Thr308. Akt-ER protein was present in all lanes as assessed by anti-HA immunoblotting (data not shown).
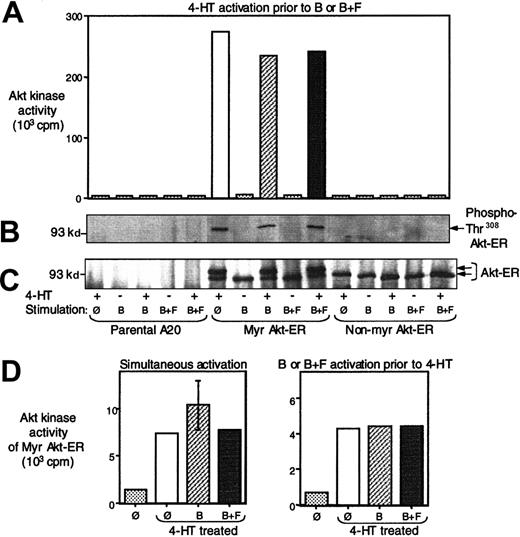
FcγRIIB/SHIP does not inhibit Myr Akt-ER phosphorylation and activation.
Parental A20 cells, or those transfected with Myr Akt-ER or the non-Myr Akt-ER were pretreated with 4-HT and then either left unstimulated, stimulated by cross-linking the BCR (B), or stimulated by co–cross-linking the BCR with the FcgRIIB1 (B+F). Cell lysates were then precipitated with anti-HA antibody and analyzed as described below. (A) In vitro kinase activity toward a GSK3 peptide and the amount of radioactive 32P incorporation was measured as described in Figure 2. The data are representative of 5 independent experiments using 4 different clones. (B) The cell lysates from the same experiment was also immunoprecipitated with anti-ER antibody and the phosphorylation on Thr308 of Akt construct was assessed by immunoblotting with phosphospecific anti-Akt antibody. (C) The membrane from B was stripped and reprobed with an anti-HA antibody to determine protein levels. The phosphorylated Akt migrated with slower mobility and was seen as a second band only in lanes where phospho-Akt was detectable (seen in B) and correlated with the in vitro Akt kinase activity (seen in A). (D) The left panel represents Myr Akt-ER activity after 10 minutes of simultaneous cell treatment with 4-HT and BCR cross-linking (B) or BCR+FcgRIIB1 (B+F) co–cross-linking. The right panel represents Myr Akt-ER kinase activity after 2 minutes of prestimulation of cells with BCR cross-linking or BCR+FcgRIIB1 co–cross-linking (B+F), followed by 8 minutes of treatment with 4-HT. Baseline radioactivity seen with beads alone without anti-Akt antibody was subtracted from all measurements.
FcγRIIB/SHIP does not inhibit Myr Akt-ER phosphorylation and activation.
Parental A20 cells, or those transfected with Myr Akt-ER or the non-Myr Akt-ER were pretreated with 4-HT and then either left unstimulated, stimulated by cross-linking the BCR (B), or stimulated by co–cross-linking the BCR with the FcgRIIB1 (B+F). Cell lysates were then precipitated with anti-HA antibody and analyzed as described below. (A) In vitro kinase activity toward a GSK3 peptide and the amount of radioactive 32P incorporation was measured as described in Figure 2. The data are representative of 5 independent experiments using 4 different clones. (B) The cell lysates from the same experiment was also immunoprecipitated with anti-ER antibody and the phosphorylation on Thr308 of Akt construct was assessed by immunoblotting with phosphospecific anti-Akt antibody. (C) The membrane from B was stripped and reprobed with an anti-HA antibody to determine protein levels. The phosphorylated Akt migrated with slower mobility and was seen as a second band only in lanes where phospho-Akt was detectable (seen in B) and correlated with the in vitro Akt kinase activity (seen in A). (D) The left panel represents Myr Akt-ER activity after 10 minutes of simultaneous cell treatment with 4-HT and BCR cross-linking (B) or BCR+FcgRIIB1 (B+F) co–cross-linking. The right panel represents Myr Akt-ER kinase activity after 2 minutes of prestimulation of cells with BCR cross-linking or BCR+FcgRIIB1 co–cross-linking (B+F), followed by 8 minutes of treatment with 4-HT. Baseline radioactivity seen with beads alone without anti-Akt antibody was subtracted from all measurements.
PDK1, the kinase that phosphorylates Akt, also contains a PH domain that binds PIP3.37-40 PDK1 and PDK2 (PDK1 in complex with another protein) have been shown to phosphorylate Akt on Thr308 and Ser473, respectively, in a PIP3-dependent manner. We tested whether Akt-ER activation would also be PIP3 dependent. Addition of wortmannin, a drug that inhibits PIP3 generation, significantly diminished the 4-HT–dependent Akt-ER kinase activation and this correlated with the inhibition of phosphorylation of Akt-ER on Ser473 and Thr308 (Figure 2B-C). We observed less inhibition of Thr308 phosphorylation, compared with Ser473, after wortmannin addition, although significance of this remains unclear.
Membrane targeted Akt-ER is not inhibited by FcγRIIB1/SHIP
We and others have previously demonstrated that activation of endogenous Akt is inhibited by co–cross-linking of BCR with FcγRIIB1 and that this inhibition is dependent on SHIP. Here, we addressed whether the Myr Akt-ER is sensitive to inhibition by FcγRIIB1/SHIP. Parental A20 cells, Myr Akt-ER, and non-Myr Akt-ER expressing cells were pretreated with 4-HT and then stimulated by BCR cross-linking alone, or BCR+FcγRIIB1 co–cross-linking. The cells were lysed and the Akt-ER kinase activities were measured after anti-HA immunoprecipitation.
Treatment of cells with 4-HT led to a marked increase in Myr Akt-ER kinase activity (Figure 3A). This directly correlated with PDK-dependent phosphorylation of Akt-ER from the same experiment determined by immunoblotting with antiphospho-Akt (Thr308) antibody or the mobility shift in the Myr Akt-ER because of phosphorylation (Figure 3B-C). BCR cross-linking alone, in the absence of tamoxifen, did not lead to phosphorylation of Akt-ER or to an increase in kinase activity. Moreover, the kinase activity of Myr Akt-ER induced by 4-HT was not further enhanced by BCR cross-linking alone. Most importantly, BCR+FcγRIIB1 co–cross-linking did not inhibit the kinase activity of the Myr Akt-ER or its phosphorylation in the presence of tamoxifen (Figure 3A). The data presented in Figure 3was representative of 5 independent experiments and with multiple stable transfectants. These observations suggested that once Akt was membrane-localized, its phosphorylation and activation were no longer sensitive to inhibition by FcγRIIB1/SHIP. The non-Myr Akt-ER (which is not targeted to the membrane) showed no increase in kinase activity or phosphorylation after tamoxifen addition. As expected, no Akt-ER protein or kinase activity was precipitated from parental A20 cells.
Although the above experiments clearly indicated that BCR-FcγRIIB1 co–cross-linking failed to inhibit a membrane bound and activated Akt, tamoxifen pretreatment did not fully evaluate the possible inhibitory effects of FcγRIIB1/SHIP on PDK recruitment and its subsequent phosphorylation of Akt. To address this, tamoxifen was added either simultaneously or 2 minutes after BCR or BCR+FcγRIIB1 co–cross-linking. Because activation of Akt-ER by 4-HT was still dependent on phosphorylation by PDKs, we reasoned that a putative inhibitory effect of SHIP on PDK would be manifested as a diminished Akt-ER kinase activity. However, Akt-ER kinase activity observed with addition of tamoxifen either simultaneously, or immediately after BCR+FcγRIIB1 co–cross-linking was not different from data obtained with tamoxifen pretreatment (Figure 3D). It is noteworthy that a previous study has shown that inhibitory effect of FcγRIIB1/SHIP on Akt activity can be seen as early 15 seconds.5 Thus, if SHIP were to regulate PDKs, this would have been revealed by the addition of 4-HT concurrently or 2-minutes after BCR+FcγRIIB1 cross-linking. Additional experiments in which either the initial FcγRIIB1 cross-linking or the duration of HT treatment was modified (varied between 2 and 20 minutes) also failed to show inhibition of Akt-ER activity due to BCR+FcγRIIB1 co–cross-linking. Taken together, these data showed that SHIP inhibited Akt primarily at the level of Akt recruitment to the membrane and not at the level of membrane recruitment of PDKs.
Endogenous Akt, MAP kinase pathways and calcium flux are not affected in Akt-ER expressing cells
Several signaling events are downstream of BCR-mediated PI-3 kinase induction and include the initiation of calcium flux2,45 56 and activation of MAP kinase (MAPK) (our unpublished data). Although these signaling pathways are all inhibited by the PI3-kinase inhibitor, wortmannin, they have not been shown to be contingent on the activation of Akt, or each other in B cells. We therefore tested whether 4-HT treatment of the cells containing the transfected Myr Akt-ER could influence these other PIP3-mediated events in a manner different from that in parental B cells.
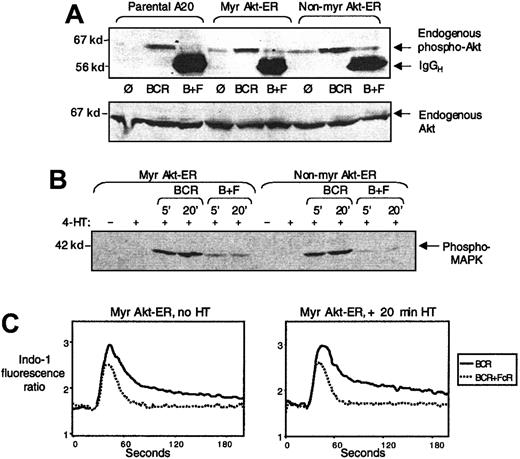
We first addressed the effect of Akt-ER expression on endogenous Akt activation by BCR cross-linking and the inhibition because of co–cross-linking with FcγRIIB1. Parental A20 cells and cells expressing Myr Akt-ER or non-Myr Akt-ER were stimulated for 5 minutes by BCR cross-linking alone or BCR+FcγRIIB1 co–cross-linking. Anti-phospho Akt (Thr308) immunoblotting of whole cell lysates showed that the endogenous Akt was activated comparably by BCR cross-linking in all 3 cell types and was inhibited by FcγRIIB1 co–cross-linking (Figure 4A). Anti-Akt immunoblotting (using a PH-domain specific anti-Akt antibody, which does not cross react with Akt-ER) showed comparable protein levels in all lanes. This indicated that FcγRIIB1/SHIP inhibited the endogenous Akt, even though the Myr Akt-ER protein in the same cells was not sensitive to such inhibition.
Activation of endogenous Akt and MAPK and intracellular calcium flux occur normally in Myr Akt-ER–expressing cells.
(A) Parental A20 cells (lanes 1-3), Myr Akt-ER expressing cells (lanes 4-6) or the non-Myr Akt-ER cells (lanes 7-9) were left unstimulated (lanes 1, 4, 7), stimulated by cross-linking the BCR alone (lanes 2, 5, 8), or co–cross-linking the BCR with FcγRIIB1 (lanes 3, 6, 9). Cell lysates were analyzed for Akt phosphorylation by immunoblotting with phosphospecific anti-Akt antibody (Thr308). The same gel was stripped and reprobed with anti-Akt antibody to demonstrate the protein level in all lanes. (B) Myr Akt-ER or non-Myr Akt-ER expressing cells were stimulated with BCR alone or BCR+FcγRIIB1 co–cross-linking for 5 or 20 minutes in the presence or absence of 4-HT. The lysates were run by SDS-PAGE and probed with anti-phospho MAPK antibody. The addition of 4-HT did not alter the MAPK phosphorylation in response to BCR stimulation or the inhibition of phosphorylation by BCR+FcγRIIB1 cross-linking. (C) Myr Akt-ER expressing cells were loaded with Indo-1 and the 398/480-fluorescence ratio of Indo-1 as a measure of rise in intracellular calcium, was analyzed after cross-linking of BCR or BCR+FcγRIIB1. There was no detectable difference in the calcium flux profiles because of tamoxifen addition.
Activation of endogenous Akt and MAPK and intracellular calcium flux occur normally in Myr Akt-ER–expressing cells.
(A) Parental A20 cells (lanes 1-3), Myr Akt-ER expressing cells (lanes 4-6) or the non-Myr Akt-ER cells (lanes 7-9) were left unstimulated (lanes 1, 4, 7), stimulated by cross-linking the BCR alone (lanes 2, 5, 8), or co–cross-linking the BCR with FcγRIIB1 (lanes 3, 6, 9). Cell lysates were analyzed for Akt phosphorylation by immunoblotting with phosphospecific anti-Akt antibody (Thr308). The same gel was stripped and reprobed with anti-Akt antibody to demonstrate the protein level in all lanes. (B) Myr Akt-ER or non-Myr Akt-ER expressing cells were stimulated with BCR alone or BCR+FcγRIIB1 co–cross-linking for 5 or 20 minutes in the presence or absence of 4-HT. The lysates were run by SDS-PAGE and probed with anti-phospho MAPK antibody. The addition of 4-HT did not alter the MAPK phosphorylation in response to BCR stimulation or the inhibition of phosphorylation by BCR+FcγRIIB1 cross-linking. (C) Myr Akt-ER expressing cells were loaded with Indo-1 and the 398/480-fluorescence ratio of Indo-1 as a measure of rise in intracellular calcium, was analyzed after cross-linking of BCR or BCR+FcγRIIB1. There was no detectable difference in the calcium flux profiles because of tamoxifen addition.
To examine the effect of BCR+FcγRIIB1 co–cross-linking on the inhibition of BCR-induced MAPK activation, we compared MAPK phosphorylation in Myr Akt-ER– and non-Myr Akt-ER–transfected cells. MAPK was phosphorylated in response to BCR cross-linking and this was inhibited by BCR+FcγRIIB1 coengagement (Figure 4B). Importantly, this was unaffected by tamoxifen treatment of these cells. Thus, FcγRIIB1-mediated inhibition of MAPK signaling was intact in the Akt-ER–expressing cells and the activation of MAPK and Akt in A20 B cells was not interdependent.
We also determined the pattern of BCR-induced intracellular calcium flux and the inhibition of this flux by BCR+FcγRIIB1 co–cross-linking, with or without tamoxifen pretreatment in Akt-ER–expressing cells. Addition of 4-HT did not affect the calcium flux profiles in the Myr Akt-ER–expressing cells (Figure 4C) and was essentially identical to that seen in parental A20 cells (data not shown). This is consistent with our earlier observation that Akt activation and calcium flux may represent 2 distinct signaling pathways activated downstream of the BCR and PI-3 kinase.4
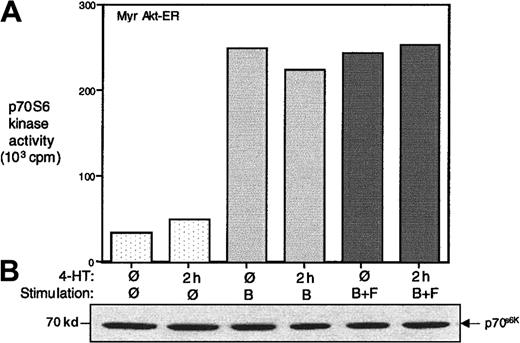
The p70S6 kinase activation is independent of Akt activity in B cells and is not inhibited by SHIP
The p70S6 kinase (p70S6k) has been recognized as a key downstream target of the PI-3 kinase signaling pathway.57It has recently been demonstrated that PDK1 functions as an upstream kinase that phosphorylates both p70S6k and Akt.58 In some cases, an Akt-dependent phosphorylation of p70S6k has also been demonstrated.20,59 60 We examined the activation p70S6k in Myr Akt-ER–expressing cells in response to BCR activation and the effect of BCR+FcγRIIB1 co–cross-linking. The cell lysates were immunoprecipitated with anti-p70S6k antibody and the in vitro kinase assay was carried out using a Rsk peptide substrate. As shown in Figure5A, BCR alone cross-linking (in the presence or absence of tamoxifen) led to a 5-fold increase in endogenous p70S6k activity. Interestingly, BCR+FcγRIIB1 co–cross-linking did not lead to an inhibition of p70S6k activation.
P70S6 kinase activation may be independent of Akt activation in B cells.
Parental A20 cells or those transfected with the Myr Akt-ER were serum starved for 16 hours and then left unstimulated, stimulated for 5 minutes by cross-linking the BCR alone (B), or co–cross-linking the BCR+FcγRIIB1 (B+F) in the absence or presence of 4-HT. Cell lysates were immunoprecipitated with anti-p70S6k antibody, and in vitro kinase activity was assessed using a Rsk substrate peptide. The protein levels in the precipitates were analyzed by anti-p70S6k immunoblotting. The data are representative of at least 3 independent experiments.
P70S6 kinase activation may be independent of Akt activation in B cells.
Parental A20 cells or those transfected with the Myr Akt-ER were serum starved for 16 hours and then left unstimulated, stimulated for 5 minutes by cross-linking the BCR alone (B), or co–cross-linking the BCR+FcγRIIB1 (B+F) in the absence or presence of 4-HT. Cell lysates were immunoprecipitated with anti-p70S6k antibody, and in vitro kinase activity was assessed using a Rsk substrate peptide. The protein levels in the precipitates were analyzed by anti-p70S6k immunoblotting. The data are representative of at least 3 independent experiments.
Also, addition of 4-HT to these cells in the absence of BCR cross-linking (ie, Akt activation alone), did not lead to a significant increase in p70S6k activity. Although this is in apparent contrast to previous studies in which constitutive activation of Akt led to an increase in p70S6k activity, this may be due to differences in cell type.20 59 Moreover, we had examined endogenous p70S6k, whereas the previous studies had analyzed overexpressed p70S6k. BCR-dependent p70S6k activation was still inhibited by wortmannin in these cells, indicating that p70S6k activation still occurred downstream of PI-3 kinase (data not shown). Consistent with our observation that SHIP does not seem to regulate PDK-dependent phosphorylation of Akt-ER, phosphorylation of p70S6k by PDKs and the subsequent p70S6k activation was insensitive to FcγRIIB1/SHIP (Figure 5).
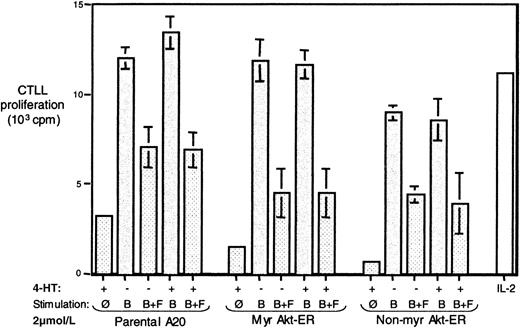
Activation of membrane targeted Akt alone is insufficient to overcome the FcγRIIB-mediated inhibition of IL-2 production
It has been observed that stimulation of the A20 cells by BCR cross-linking leads to secretion of IL-2 by this cell line and is inhibited by co–cross-linking BCR and the FcγRIIB1.2,46,56 Since Eder et al61 have demonstrated that Akt may play a role in IL-2 production in T cells, we tested whether activated membrane-targeted Akt can influence downstream events that lead to BCR-induced IL-2 production and whether it can partially or fully “rescue” the FcγRIIB1-mediated inhibition of this IL-2 response. Parental A20 cells and those transfected with Myr Akt-ER or non-Myr Akt-ER were stimulated by BCR cross-linking alone or by BCR+ FcγRIIB1, in the presence or absence of 4-HT, and the secretion of IL-2 in the supernatants was tested (see “Materials and methods”).
As seen in Figure 6, all 3 cell types produced IL-2 in response to BCR stimulation and this IL-2 secretion was inhibited by BCR+FcγRIIB1 co–cross-linking. Moreover, the addition of tamoxifen and the subsequent activation of Akt-ER had no detectable effect on the IL-2 response of these cells. The same pattern was observed in multiple experiments using lower serial dilutions of stimulating antibody (data not shown). Because we obtain roughly comparable in vitro kinase activity for 4-HT stimulated Akt-ER and BCR-activated endogenous Akt (data not shown), we conclude that rescue of the Akt activation alone is insufficient to overcome the down-regulation by FcγRIIB1/SHIP. Because BCR+FcγRIIB1 co–cross-linking does inhibit other PI-3 kinase-dependent pathways such as calcium flux and MAPK in these cells, it is likely that these other pathways are absolutely essential for the BCR-mediated regulation of IL-2 expression.
Activation of Akt-ER alone is not sufficient to overcome inhibition of BCR-induced IL-2 production by BCR+FcγRIIB1 co–cross-linking.
Parental A20 cells or those transfected with the Myr Akt-ER or non-Myr Akt-ER were left unstimulated or stimulated for 24 hours by cross-linking the BCR alone (B), or by co–cross-linking the BCR with the FcγRIIB1 (B+F), in the absence or presence of 4-HT. Each stimulation condition was performed in triplicate. The supernatants were harvested after 24 hours and the presence of IL-2 was determined by a bioassay using the proliferation of the IL-2–dependent cell line CTLL-2 (see “Materials and methods”). The background CTLL proliferation in the absence of B-cell supernatants has been subtracted from all samples; the proliferation with 2 U/mL of recombinant human IL-2 is shown for reference.
Activation of Akt-ER alone is not sufficient to overcome inhibition of BCR-induced IL-2 production by BCR+FcγRIIB1 co–cross-linking.
Parental A20 cells or those transfected with the Myr Akt-ER or non-Myr Akt-ER were left unstimulated or stimulated for 24 hours by cross-linking the BCR alone (B), or by co–cross-linking the BCR with the FcγRIIB1 (B+F), in the absence or presence of 4-HT. Each stimulation condition was performed in triplicate. The supernatants were harvested after 24 hours and the presence of IL-2 was determined by a bioassay using the proliferation of the IL-2–dependent cell line CTLL-2 (see “Materials and methods”). The background CTLL proliferation in the absence of B-cell supernatants has been subtracted from all samples; the proliferation with 2 U/mL of recombinant human IL-2 is shown for reference.
Discussion
Activation of the serine/threonine kinase Akt and the regulation of its activation are recognized as critical in controlling proliferative/survival signals via many hematopoietic receptors.8,33,34,62 Previous studies have demonstrated that the inositol phosphatase SHIP, through its dephosphorylation of the membrane phospholipid PI(3,4,5)P3, inhibits Akt activation.4-6 In this report, we addressed whether SHIP-mediated destruction of PIP3 in B cells inhibits Akt directly at the level of Akt recruitment to the membrane, at the level of PDK recruitment/phosphorylation of Akt, or both. We demonstrate that a constitutively membrane targeted, but inducible Akt construct is not susceptible to FcγRIIB1/SHIP-mediated inhibition, whereas under the same conditions, the endogenous Akt is inhibited by SHIP. Our data also suggest that SHIP does not inhibit the PDK-dependent phosphorylation or activation of Akt. Taken together, the data presented here demonstrate that SHIP inhibits Akt primarily through regulation of Akt membrane localization. Additionally, rescue of the Akt activation pathway alone was not sufficient to overcome the FcγRIIB1/SHIP-mediated inhibition of BCR-induced IL-2 production. This suggests that in addition to Akt, multiple pathways are simultaneously affected by SHIP action. Interestingly, we also observe that, although FcγRIIB1/SHIP down-modulated several PI-3 kinase–dependent events, it does not inhibit p70S6k activation.
A number of studies have used transient, constitutive activation of Akt through membrane targeting to demonstrate a role for Akt in protecting cells against apoptosis induced by factor deprivation or/DNA damage.16-18 However, one caveat with this approach is the presence of constitutively active protein throughout the time of the experiment. The inducible Akt-ER designed by Kohn et al,20and used in this report, remains completely inactive and allows the tamoxifen-dependent activation of Akt when desired. The myristoylated Akt-ER fusion protein still required PDK-dependent phosphorylation for activation, and required the prior addition of tamoxifen to “open up” the Akt for phosphorylation by PDKs. Thus, this Akt-ER fusion protein allowed us to delineate which step(s) of the Akt activation sequence is inhibited by SHIP, by adding tamoxifen before, after, or simultaneously with BCR stimulation or BCR+FcγRIIB1 co–cross-linking.
Because the endogenous Akt in cells is recruited to the membrane during activation and has been shown to move to the nucleus after activation,5 whether the myristoylated Akt can also move to the nucleus has been debated.8 However, the v-Akt, which is also myristoylated (through the gag fusion), appears to be located at the membrane, cytosol, and in the nucleus and can mediate tumor formation.42 Similarly, engineered myristoylated Akt proteins have been shown to be functional and protect cells against apoptosis.16-18 Recently, Klippel et al60have demonstrated that a similar myristoylated Akt-ER fusion protein can mediate cell cycle progression after tamoxifen addition. The rationale for our use of Myr Akt-ER was the specific advantage this construct provided in addressing SHIP-mediated regulation of Akt. The non-Myr Akt-ER was completely inactive even after tamoxifen addition and thus helped to rule out nonspecific effects due to stable expression of Akt-ER fusion proteins in the B cells.
Several lines of evidence shown here suggested that SHIP regulates Akt primarily at the level of Akt recruitment to the membrane. First, once activated by tamoxifen addition, the Akt-ER was resistant to inhibition by FcγRIIB1 co–cross-linking. Because the Akt-ER activation was dependent on Akt phosphorylation, but was unaffected by SHIP, this suggested that SHIP did not regulate Akt after it was phosphorylated and activated. Second, addition of 4-HT concurrent with BCR+FcγRIIB1 co–cross-linking or 2 to 5 minutes after BCR+FcγRIIB1 co–cross-linking did not reverse the Akt-ER activation by tamoxifen. In fact, a steady and progressive increase in Akt-ER activation on addition of 4-HT was completely unaffected by FcγRIIB1/SHIP. This again suggested that, once membrane-targeted and opened up by drug addition, the phosphorylation of Akt by PDKs and the subsequent Akt-ER activation were not the steps regulated by SHIP. It is noteworthy that the endogenous Akt was inhibited by SHIP under the same conditions and thus was not a failure of SHIP to function in these cells. Third, we have failed to see a significant increase in Akt-ER activation because of BCR cross-linking over and above the tamoxifen-mediated increase in Akt kinase activity tested under various conditions. If BCR cross-linking were to regulate/augment Akt activation after its membrane localization, in the presence of 4-HT, we would have expected to see an increase in Akt-ER kinase activity after BCR cross-linking. Thus, our data strongly suggest that the major checkpoint in BCR-induced Akt activation is its membrane recruitment and that FcγRIIB1/SHIP targets this step for inhibition of Akt activation. Consistent with this conclusion, during the preparation of this manuscript, Astoul et al5 have reported that Akt membrane localization was inhibited by BCR+FcγRIIB1 co–cross-linking as determined by confocal microscopy.
We examined the possibility that FcγRIIB1/SHIP could additionally regulate PDK1 or PDK2, whose membrane localization also depends on PIP3. PDK-1 has been shown by others to have increased membrane translocation after PDGF activation in fibroblasts,41 but it is not known if membrane translocation of PDK-1 is also increased with BCR stimulation in B cells. Were this the case, we might expect that BCR stimulation would further increase the activation of membrane bound Akt secondary to the increase in tamoxifen addition. As discussed above, we have failed to see such an increase. Moreover, FcγRIIB1 co–cross-linking with the BCR failed to inhibit Akt-ER activation even when the tamoxifen is added 2 to 5 minutes after antibody cross-linking. If SHIP were to influence PDK recruitment or its activaiton, this would have been manifested as decreased Akt-ER phosphorylation and, in turn, a diminished Akt-ER kinase activity. We failed to see this in our experiments under various conditions. Likely explanations are that either PDK-1 membrane translocation is not enhanced by BCR stimulation, or that the constitutive levels of membrane bound PDK-1 (due to basal levels of PIP3) are sufficient to phosphorylate a majority of the available membrane-bound Akt.
It is noteworthy that even though both calcium flux and Akt activation are PIP3-dependent events in B cells, BCR-induced calcium flux and its inhibition by BCR+FcγRIIB1 co–cross-linking were not influenced by the Myr Akt-ER activation through tamoxifen. Although a recent study has demonstrated that Akt activation is impaired in Btk-deficient cells (another PIP3-dependent kinase required for the initiation of calcium flux in B cells),63 we have detected comparable Akt activation in wild-type and Btk-deficient DT40 B cells (data not shown). MAPK activation also appeared to be independent of Akt activation in B cells, as the Akt-ER activation had no detectable effect on MAPK phosphorylation in response to BCR stimulation or its inhibition by BCR+FcγRIIB1 co–cross-linking.
Although not a normal feature of B cells, activation via the BCR in the A20 B cells leads to IL-2 gene transcription and the secretion of IL-2. It has been previously reported that in T cells, Akt may play a role in regulating IL-2 gene transcription.61 However, we failed to observe an increase in IL-2 production in Myr Akt-ER expressing cells after BCR activation in the presence of tamoxifen. Additionally, we did not see a rescue as a result of Akt-ER of the inhibition of IL-2 production by FcγRIIB1 cross-linking. It is likely that similar to the case in T cells, the IL-2 promoter in A20 B cells is also regulated by the Ras/MAPK pathway and calcium signals. Because the inhibition of these pathways by FcγRIIB1 still occurred in the Myr Akt-ER–expressing cells (in the presence or absence of tamoxifen), the Akt activation alone may not be sufficient to overcome this inhibition.
We also observed that a putative Akt cytosolic target, p70S6k, appeared to be independent of Akt membrane localization and activation. Although this is in contrast to previously published reports,20,59,60 the difference could be attributed to cell type or to the fact that we examined endogenous p70S6k activation, whereas the previous studies addressed activation of transiently overexpressed p70S6k. Interestingly, although p70S6k was activated 5-fold by BCR stimulation, co–cross-linking of BCR+FcγRIIB1 had no inhibitory effect on p70S6k activation. Wortmannin, however, completely inhibited the activation (data not shown). Thus, although 3 other PI-3 kinase–dependent signaling events, such as calcium flux, and Akt and MAPK activation, are potently inhibited by FcγRIIB1/SHIP, p70S6k activation was not inhibited. Recently, it has been shown that p70S6k requires phosphorylation by PDK-1 to become active.58 Because PDK-1 is not inhibited by FcγRIIB1/SHIP as seen in the context of Akt activation, this may be an explanation for the failure of FcγRIIB1/SHIP to inhibit p70S6k activation.
In summary, we have examined the regulation of Akt membrane translocation under inhibitory signaling conditions in B cells and demonstrate that Akt inhibition by FcγRIIB1/SHIP is mediated primarily through inhibition of Akt translocation to the membrane. Because current evidence suggests that cytokine-mediated Akt activation is prolonged and increased in strength in the absence of SHIP, and has been correlated with hyperproliferation in the myeloid lineage, our data have implications for abnormal proliferation of hematopoietic cells.51,52 64
Acknowledgments
We thank Dr Richard Roth for providing the plasmids encoding Myr Akt-ER and non-Myr Akt-ER, and we also thank the members of the Ravichandran laboratory for their helpful discussions.
Supported by a grant from the National Institutes of Health (AI 43425) to K.S.R.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kodimangalam S. Ravichandran, Beirne B. Carter Center, Bldg MR4, Rm 4012F, HSC, University of Virginia, Charlottesville, VA 22908; email: kr4h@virginia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal