Abstract
Engraftment potential of hematopoietic stem cells (HSCs) is likely to be dependent on several factors including expression of certain adhesion molecules (AMs) and degree of mitotic quiescence. The authors investigated the functional properties and engraftment potential of Sca-1+lin− cells subfractionated on the basis of expression, or lack thereof, of CD11a, CD43, CD49d, CD49e, or CD62L and correlated that expression with cell cycle status and proliferative potential of engrafting fractions. Donor-derived chimerism in mice receiving CD49e+ or CD43+ Sca-1+lin− cells was greater than that in mice receiving cells lacking these 2 markers, while Sca-1+lin− cells positive for CD11a and CD62L and bright for CD49d expression mediated minimal engraftment. AM phenotypes enriched for engraftment potential contained the majority of high proliferative potential–colony forming cells, low proliferative potential–colony forming cells, and cells providing rapid in vitro expansion. Cell cycle analysis of AM subpopulations revealed that, regardless of their bone marrow repopulating potential, Sca-1+lin− AM− cells contained a higher percentage of cells in G0/G1 than their AM+ counterparts. Interestingly, engrafting phenotypes, regardless of the status of their AM expression, were quicker to exit G0/G1 following in vitro cytokine stimulation than their opposing phenotypes. When engrafting phenotypes of Sca-1+lin− AM+ or AM−cells were further fractionated by Hoechst 33342 into G0/G1 or S/G2+M, cells providing long-term engraftment were predominantly contained within the quiescent fraction. These results define a theoretical phenotype of a Sca-1+lin− engrafting cell as one that is mitotically quiescent, CD43+, CD49e+, CD11a−, CD49ddim, and CD62L−. Furthermore, these data suggest that kinetics of in vitro proliferation may be a good predictor of engraftment potential of candidate populations of HSCs.
Introduction
The ability of hematopoietic stem cells (HSCs) to reconstitute normal bone marrow (BM) hematopoiesis following transplantation into suitable recipients relies on the potential of these cells to home to and anchor within the BM microenvironment. Homing is an intricate process by which HSCs, through interactions between adhesion molecules (AMs) and their counter-receptors expressed on BM endothelium, migrate through endothelial cells and into the stromal cell microenvironment. Within the BM microenvironment, the homing process continues as HSCs, again through interactions between AMs and cognant ligands, specifically anchor within appropriate BM niches and begin the process of hematopoiesis. Mobilization of HSCs from their BM niches into the periphery following administration of growth factors or chemotherapeutic agents is likely to involve sequential loss or alterations in adhesive interactions between HSCs and stromal cells, and then endothelium, with final release into the periphery. Although data implicating certain AMs in various stages of stem cell homing and egress from the BM are beginning to accumulate,1-7 much remains to be learned regarding the relationship between AMs and stem cell trafficking, and how this process affects stem cell engraftment and long-term hematopoiesis in transplanted recipients.
While AMs are likely to direct the trafficking of HSCs within the BM microenvironment and periphery, the position of these cells in specific phases of cell cycle is believed to dictate the hematopoietic potential of HSCs.8 A large body of evidence in both in vitro and in vivo systems supports the notion that mitotic quiescence is a fundamental characteristic of HSCs and is essential for the preservation of primitive hematopoietic function. Recent reports in the murine,9 feline,10 and human11systems suggest that the cell cycle of HSCs may be relatively long and that these cells may display reduced engraftment potential at the time of active cell division.12-14 On the other hand, few reports seem to indicate that cells capable of long-term engraftment cycle quickly following transplantation.15These data pose the question of whether changes in AM status, concurrent with entry of these cells into active cell division, influence the engraftment potential of cycling HSCs. We and others have begun to investigate relationships between cell cycle progression and expression or function of AMs on primitive hematopoietic progenitor cells (HPCs). Some reports recently demonstrated an increased expression of certain AMs16-19 and, in some cases, increased adhesion17 of primitive HPCs in active phases of cell cycle. These data, which clearly establish a link between cell cycle status and AM repertoire, suggest the possible existence of regulatory control mechanisms between expression or function of AMs and cell cycle position of HSCs, which may in turn have an impact on the engraftment potential of these cells.
In the present study, we examined the contribution of 6 AMs to the homing and long-term engraftment potential of primitive HPCs using an in vivo murine BM transplantation model. Results of these studies imply a theoretical phenotype of a Sca-1+lin−engrafting cell as expressing high levels of CD49e and CD43, and low levels of CD11a, CD49d, and CD62L. Cells within this AM-defined phenotype were determined to reside in G0/G1; however, their proliferative response to in vitro cytokine stimulation was rapid and proved to be a good predictor of long-term engraftment potential, as did the content of high proliferative potential–colony forming cells (HPP-CFCs) and low proliferative potential-colony forming cells (LPP-CFCs). Whether this AM phenotype imparts to Sca-1+lin− cells more efficient homing and/or anchorage within the BM microenvironment, or enriches for HSCs within Sca-1+lin− cells, remains to be determined.
Materials and methods
Mice
C57BL/6 female mice (Jackson Laboratories, Bar Harbor, ME) were purchased at 8 to 10 weeks of age and allowed to acclimate for 1 to 2 weeks prior to being used in these studies. C57BL/6 mice are Hbbs/Hbbs (hemoglobin single), are glucose phosphatase isoenzyme type Gpi-1b/Gpi-1b, and express the CD45.2 allotype. Congenic BM donors were of 2 different strains: (1) B6 mice, which are Hbbd/Hbbd(hemoglobin diffuse) and Gpi-1a/Gpi-1a(B6.Gpi-1a) and (2) B6.SJL-PtrcaPep3b/BoyJ mice (B6.BoyJ), which express the CD45.1 allotype. Congenic donor mice were maintained in our breeding colony and used between 8 to 12 weeks of age. These studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Flow cytometric cell sorting and analysis of Sca-1+lin− cells expressing one or more AMs
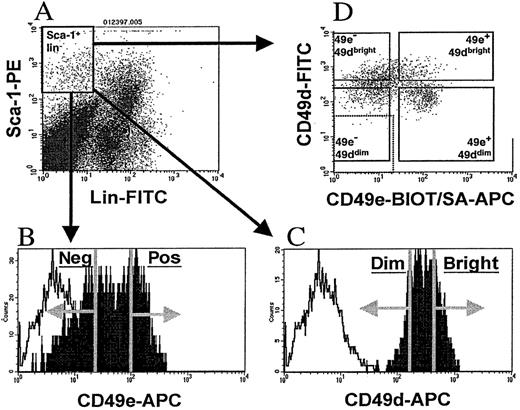
Sca-1+lin− cells were isolated from C57BL/6 mice as previously described.20 Briefly, low-density (1.077 g/mL or less) BM mononuclear cells were stained with phycoerythrin (PE)–conjugated Sca-1 and fluorescein isothiocyanate (FITC)–conjugated CD3 and CD45R/B220, and sorted by means of a FACStarplus flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) to yield Sca-1+lin− cells. To isolate AM subfractions of Sca-1+lin− cells, mononuclear cells were stained with Sca-1 and lineage monoclonal antibodies as above, along with the biotinylated antibody recognizing one of the following: CD11a (leukocyte-function–associated antigen 1 [LFA-1], clone 2D7), CD43 (Leukosialin, clone S7), CD44 (homing-associated cell AM[HCAM], clone IM7), CD49d (very late antigen 4 [VLA-4], clone 9C10), CD49e (VLA-5, clone 5H10-27; MFR5), or CD62L (L-selectin, clone MEL-14). All antibodies used in this study were obtained from Pharmingen (San Diego, CA). AM antibodies were visualized by streptavidin-APC (allophycocyanin) (Molecular Probes, Eugene, OR). Cells were sorted on a FACStarplus flow cytometer as follows (Figure1): A primary light scatter gate was constructed and used to visualize FITC and PE fluorescence of stained cells. A second gate was constructed to contain PE+ and FITC− cells (Sca-1+lin−cells, Figure 1A), and the APC fluorescence of these gated cells was examined in a single-parameter histogram (Figure 1B-C). The fluorescence of a sample stained with Sca-1–PE, CD3-FITC, B220-FITC, and nonspecific biotinylated antibodies followed by streptavidin-APC was used to determine background APC fluorescence of Sca-1+lin− cells. On the basis of this determination, sort regions were created within the APC histogram to isolate Sca-1+lin− cells that were positive or negative for CD11a, CD43, CD49e, or CD62L (referred to as Sca-1+lin− AM+ or Sca-1+lin− AM− cells hereafter) (Figure 1B). To ensure adequate separation, cells were gated to include the upper and lower 30% to 40% of the APC histogram, with the middle 30% to 40% of the cells discarded. In the cases of CD44 and CD49d, where nearly 100% of Sca-1+lin− cells expressed these 2 markers (Table 1), the brightest and dimmest 30% to 40% of Sca-1+lin− cells were sorted and referred to as “bright” or “dim,” respectively (Figure 1C). In some cases, Sca-1+lin− cells were sorted while ignoring the signal from the AM antibody, to obtain a control group of Sca-1+lin− cells. These cells were used to test for in vivo blocking activity of the AM antibody used for cell sorting. Cells were maintained at 4°C throughout cell sorting and were collected into Iscove's Modified Dulbecco's Medium (IMDM) containing 20% fetal bovine serum (FBS) (Hyclone, Logan, UT).
Flow cytometric cell sorting of Sca-1+lin− AM+ or AM−cells.
Low-density murine BM cells were stained with Sca-1–PE, FITC-conjugated CD3 and B220 (lin), and biotinylated antibodies recognizing either CD11a, CD43, CD44, CD49d, CD49e, or CD62L, followed by the addition of streptavidin-APC. A primary light scatter gate was constructed and used to visualize PE and FITC fluorescence (panel A). A second gate containing PE+ and FITC− cells (Sca-1+lin− cells, panel A) was drawn, and the APC fluorescence of these gated Sca-1+lin− cells were visualized in a single-parameter histogram (filled histograms, panels B and C). Background APC fluorescence of Sca-1+lin−cells (open histogram, panels B and C) was determined by staining a sample with Sca-1–PE, CD3-FITC, B220-FITC, and nonspecific biotinylated antibodies followed by streptavidin-APC. Sort regions, depicted by the vertical lines and arrows in panels B and C, were created to isolate the upper and lower 30% to 40% of Sca-1+lin− cells expressing the particular AM. For CD11a, CD43, CD49e, and CD62L, a distinct “positive” and “negative” AM fraction could be isolated (panel B), but in the cases of CD44 and CD49d, where nearly 100% of Sca-1+lin− cells expressed these markers, the brightest and dimmest 30% to 40% of Sca-1+lin− cells were sorted and referred to as “bright” and “dim,” respectively. To test for in vivo blocking activity of AM antibodies, a group of Sca-1+lin− cells were sorted from each AM group while the APC signal from the AM antibody was ignored. Sort regions are shown for CD49e and CD49d as representative examples. To isolate Sca-1+lin− cells on the basis of their expression of both CD49d and CD49e, Sca-1+lin− cells were sorted to purity, panel A. Since these cells were FITC−, they were stained with FITC-labeled CD49d and biotinylated CD49e, followed by streptavidin-APC (panel D). Based on nonspecific background fluorescence, denoted by dotted box in lower left of panel D, 4 sort regions were created as follows: (1) APC− FITC+ cells were gated and sorted as CD49e− CD49dbright; (2) APC+ FITC+ cells were sorted as CD49e+ CD49dbright; (3) APC−FITC− cells were sorted as CD49e−CD49ddim; and (4) APC+ FITC− cells were sorted as CD49e+ CD49ddim (panel D).
Flow cytometric cell sorting of Sca-1+lin− AM+ or AM−cells.
Low-density murine BM cells were stained with Sca-1–PE, FITC-conjugated CD3 and B220 (lin), and biotinylated antibodies recognizing either CD11a, CD43, CD44, CD49d, CD49e, or CD62L, followed by the addition of streptavidin-APC. A primary light scatter gate was constructed and used to visualize PE and FITC fluorescence (panel A). A second gate containing PE+ and FITC− cells (Sca-1+lin− cells, panel A) was drawn, and the APC fluorescence of these gated Sca-1+lin− cells were visualized in a single-parameter histogram (filled histograms, panels B and C). Background APC fluorescence of Sca-1+lin−cells (open histogram, panels B and C) was determined by staining a sample with Sca-1–PE, CD3-FITC, B220-FITC, and nonspecific biotinylated antibodies followed by streptavidin-APC. Sort regions, depicted by the vertical lines and arrows in panels B and C, were created to isolate the upper and lower 30% to 40% of Sca-1+lin− cells expressing the particular AM. For CD11a, CD43, CD49e, and CD62L, a distinct “positive” and “negative” AM fraction could be isolated (panel B), but in the cases of CD44 and CD49d, where nearly 100% of Sca-1+lin− cells expressed these markers, the brightest and dimmest 30% to 40% of Sca-1+lin− cells were sorted and referred to as “bright” and “dim,” respectively. To test for in vivo blocking activity of AM antibodies, a group of Sca-1+lin− cells were sorted from each AM group while the APC signal from the AM antibody was ignored. Sort regions are shown for CD49e and CD49d as representative examples. To isolate Sca-1+lin− cells on the basis of their expression of both CD49d and CD49e, Sca-1+lin− cells were sorted to purity, panel A. Since these cells were FITC−, they were stained with FITC-labeled CD49d and biotinylated CD49e, followed by streptavidin-APC (panel D). Based on nonspecific background fluorescence, denoted by dotted box in lower left of panel D, 4 sort regions were created as follows: (1) APC− FITC+ cells were gated and sorted as CD49e− CD49dbright; (2) APC+ FITC+ cells were sorted as CD49e+ CD49dbright; (3) APC−FITC− cells were sorted as CD49e−CD49ddim; and (4) APC+ FITC− cells were sorted as CD49e+ CD49ddim (panel D).
Expression of AMs on Sca-1+lin−cells
| Adhesion molecule CD designation . | CD11a . | CD43 . | CD44 . | CD49d . | CD49e . | CD62L . |
|---|---|---|---|---|---|---|
| Common name | LFA-1 | Leukosialin | HCAM | VLA-4 | VLA-5 | L-selectin |
| Percentage of positive Sca-1+lin−cells* | 77 ± 7.4 | 83 ± 6.6 | 99 ± 0.6 | 94 ± 2.8 | 63 ± 9.0 | 46 ± 5.2 |
| Adhesion molecule CD designation . | CD11a . | CD43 . | CD44 . | CD49d . | CD49e . | CD62L . |
|---|---|---|---|---|---|---|
| Common name | LFA-1 | Leukosialin | HCAM | VLA-4 | VLA-5 | L-selectin |
| Percentage of positive Sca-1+lin−cells* | 77 ± 7.4 | 83 ± 6.6 | 99 ± 0.6 | 94 ± 2.8 | 63 ± 9.0 | 46 ± 5.2 |
AM indicates adhesion molecule; LFA-1, leukocyte-function–associated antigen 1, HCAM, homing-associated cell AM, and VLA, very late antigen
Individual expression of 6 AMs on fresh Sca-1+lin− cells was determined by flow cytometry as described in “Materials and methods.” Data represent the mean percentage of positive Sca-1+lin−cells ± SEM; n = 5 to 8 for each AM.
To isolate Sca-1±lin− cells on the basis of their expression of both CD49d and CD49e, Sca-1±lin− cells were isolated with the use of Sca-1–PE, CD3-FITC, and B220-FITC as described above. These cells, which are PE+ and FITC−, were then stained with biotinylated CD49e and FITC-labeled CD49d (Figure 1D). CD49e was developed with streptavidin-APC, and the cells were washed and resuspended for flow cytometric cell sorting on a FACStarplus flow cytometer. Stained Sca-1±lin− cells contained within a primary light scatter gate were examined for their FITC and APC fluorescence in a dual parameter dot plot, and 4 sort regions (Figure 1D), each containing 10% to 20% of the Sca-1±lin−cells, were created as follows: (1) APC−FITC± cells were gated and sorted as CD49e−CD49dbright; (2) APC± FITC± cells were sorted as CD49e± CD49dbright; (3) APC− FITC− cells were sorted as CD49e− CD49ddim; and (4) APC±FITC− cells were sorted as CD49e±CD49ddim. Cells were maintained at 4°C throughout cell sorting and were collected into IMDM containing 20% FBS.
To analyze the dual expression of AM on Sca-1+lin− cells, groups of Sca-1+lin− AM+ or AM−cells were isolated as described above with the use of Sca-1–FITC, CD3-PE, B220-PE, and biotinylated-AM antibodies developed with streptavidin-APC. The resulting groups of Sca-1+lin− cells, which are negative for PE fluorescence, were then stained with the PE-conjugate of the remaining 5 AM antibodies not used in the primary sort (ie, Sca-1+lin− CD49e+ and Sca-1+ lin− CD49e− cells would be subsequently stained with CD11a, CD43, CD44, CD49d, and CD62L). The PE fluorescence of these stained cells was examined on a FACScan (Becton Dickinson), and the percentage of each primary adhesion phenotype expressing each of the other AMs was calculated on the basis of background fluorescence of cells stained with PE-conjugated nonspecific myeloma proteins. In every case, background PE fluorescence of Sca-1+lin− AM+ or AM− cells was less than 5% of that observed after staining these cells with 1 of the 5 PE-conjugated AM antibodies.
Separation of cell cycle subfractions of Sca-1+lin− AM+ or AM− cells
Sca-1+lin−AM+ or AM− cells enriched for engraftment potential were isolated as described above, resuspended in Hoechst buffer (Hanks' balanced salt solution [Biowhittaker, Walkersville, MD], 20 mmol/L HEPES [Biowhittaker], 1 g/L glucose, and 10% fetal calf serum [FCS]), and stained with Hoechst 33342 (Molecular Probes) at 10 μmol/L for 45 minutes at 37°C as previously described.21 To limit dye efflux via the MDR-1 pump, 100μmol/L verapamil (Sigma Chemical Co, St Louis, MO) was added to the staining buffer.22 Cells were washed and resuspended in Hoechst buffer plus verapamil for cell sorting. Cells falling within a primary light scatter gate and containing 2n DNA were gated and sorted as G0/G1. Cells containing between 2n and 4n DNA were gated and sorted as S/G2+M. Hypodiploid events, when present, were excluded from sort windows. When quantities were sufficient, a small portion of sorted cells were subjected to postsort analyses, either by examining Hoechst fluorescence or by propidium iodide staining, as previously described.23
HPP-CFC and LPP-CFC assay
Between 0.8 × 103 and 1.5 × 103Sca-1+lin− cells or AM subfractions were suspended in triplicate in 1 mL double-layer agar cultures and assayed for HPP-CFCs and LPP-CFCs as previously described.24Cultures were incubated in a 100%-humidified 5% O2, 10% CO2, and 85% N2 environment. Recombinant hematopoietic growth factors were used as follows: 200 U/mL murine interleukin-3 (mIL3), 1000 U/mL mIL1-α, 50 ng/mL murine stem cell factor (mSCF), 25 ng/mL murine granulocyte macrophage–colony stimulating factor (mGM-CSF) (all from PeproTech, Rocky Hill, NJ), and 1600 U/mL human macrophage–CSF-1 (hM-CSF, Genetics Institute, Camden, MA). On day 14, colonies larger than 0.5 mm were scored as HPP-CFC and those smaller than 0.5mm as LPP-CFC.
Transplantation protocol
C57BL/6 female recipients between 10 and 12 weeks of age were lethally irradiated (split dose of 700 centrigrays [cGy] followed by 350 cGy 3 to 4 hours later) from a 137Cs gamma irradiator (GammaCell 40; Nordion International, Kanata, Ontario, Canada). Mice were transplanted via tail vein injections 3 to 6 hours later with 0.25 × 103 to 1.0 × 103 donor B6.Gpi-1a or B6.BoyJ cells, along with 0.3 × 105 to 1.0 × 105 competitor cells (low-density BM cells of C57BL/6 origin), as described in Figures. Recipient mice were bled from the tail vein monthly until 6 or 8 months posttransplantation for analysis of donor-derived hematopoiesis.
Determination of donor chimerism in transplanted recipients
Chimerism in C57BL/6 mice, when B6.Gpi-1amice were used as donors, was determined by analyzing the percentage of donor-derived hemoglobin or Gpi-1 in peripheral blood obtained from tail veins as previously described.25 To examine multilineage engraftment in these mice, whole-blood samples obtained from the retro-orbital sinus were stained separately with FITC-conjugated CD45R/B220, or FITC-conjugated CD3, and PE-conjugated Gr-1, lysed, and then isolated by flow cytometric cell sorting to yield B lymphocytes, T lymphocytes, and granulocytes, respectively. These purified lineages were then analyzed for Gpi-1 content.25
Chimerism in C57BL/6 mice receiving donor cells of B6 BoyJ origin was determined by means of flow cytometry to calculate the percentage of CD45.2− peripheral blood cells. Whole-blood samples obtained from tail veins were lysed and stained with PE-conjugated CD45 and FITC-conjugated CD45.2. CD45+ cells were gated and examined for the percentage of CD45.2−cells (cells of B6.BoyJ origin). Multilineage engraftment in these mice was determined by staining with each of the 3 following combinations of antibodies, CD45.2-FITC and Gr-1–PE, CD45.1-PE and CD3-FITC, and CD45.1-PE and B220-FITC, and analyzing the percentages of donor-derived granulocytes, T lymphocytes, and B lymphocytes, respectively.
Short-term culture
Cultures of 0.2 × 103 to 4 × 103Sca-1+lin− AM+ or AM−cells, or cell cycle subfractions of these cells, were initiated in IMDM supplemented with 20% FBS and 2 × 10−5 mol/L 2-mercaptoethanol in an atmosphere of 5% CO2 in 100%-humidified air. Cytokines were delivered on day 0 as follows: 100 ng/mL mSCF (PeproTech), 500 U/mL mIL1α (Genzyme, Cambridge, MA), 100 U/mL mIL3 (Genzyme), 100 ng/mL hIL6, and 50 ng/mL human Flt3 ligand (hFlt3-L). The hIL6 and hFlt3-L were kind gifts from Amgen (Thousand Oaks, CA) and Immunex (Seattle, WA), respectively. Care was taken to keep the cell concentration below 1 × 106cells/mL. Fresh cells or aliquots of cultured cells were removed on days 1 and 2 and stained with propidium iodide for cell cycle analysis23 or incubated for an additional 11 to 14 days, at which time cells were harvested, enumerated, and analyzed by flow cytometry for the percentage of cells expressing Sca-1 with the use of anti–Sca-1–PE.
Statistical analysis
Data are expressed as the mean ± SEM where applicable. Differences between groups were analyzed by means of an unpaired 2-sided t test. A probability value of less than .05 was considered significant. Regression analysis was used to analyze the rate of exit from G0/G1 phases of the cell cycle.
Results
Expression of AMs on Sca-1+lin− cells
As a first step in defining AMs important for engraftment of primitive HPCs, we examined the pattern of expression of CD11a, CD43, CD44, CD49d, CD49e, and CD62L on fresh Sca-1+lin− cells. As seen in Table 1, expression of these 6 AMs was varied, with nearly 100% of Sca-1+lin− cells expressing CD44 and CD49d, and approximately 50% expressing CD49e and CD62L. Expression of CD11a and CD43 was observed on approximately 80% of Sca-1+lin− cells.
Primitive and mature progenitor cell content of Sca-1+lin−AM+ or AM− cells
Since HPP-CFCs likely contain some of the most primitive HPCs,26 the HPP-CFC frequency of our 12 groups of Sca-1+lin− AM+ or AM−cells was determined. Data in Table 2show that CD43 and CD49e were expressed on the majority of HPP-CFCs residing in the Sca-1+lin− cell fraction, while Sca-1+lin− cells lacking expression of either CD11a or CD62L contained a higher fraction of HPP-CFCs than Sca-1+lin− CD11a+ or Sca-1+lin− CD62L+ cells (Table 2). LPP-CFCs, progenitors more committed to lineage differentiation than HPP-CFCs, were mostly enriched among the same AM profile as HPP-CFCs (Table 2). To ensure that the AM antibody used for cell sorting did not induce any negative influences on primitive HPC clonogenic activity, Sca-1+lin− cells treated with each AM antibody but not sorted on adhesion phenotype were also assayed for HPP- and LPP-CFC content. HPP- and LPP-CFC activity of these antibody-treated cells was not different from that of untreated Sca-1+lin− cells (data not shown), indicating that the AM antibodies used for cell sorting did not inhibit in vitro activity of progenitor cells. Fractions enriched for HPP- and LPP-CFC activity also exhibited greater cellular expansion during 11 days of in vitro cytokine-stimulated cell culture (Table 2).
HPP-CFC and LPP-CFC content and proliferative potential of Sca-1+lin−AM+ or AM−cells
| . | Sca-1+Lin− . | CD11a . | CD43 . | CD44 . | CD49d . | CD49e . | CD62L . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos . | Neg . | Pos . | Neg . | Bright . | Dim . | Bright . | Dim . | Pos . | Neg . | Pos . | Neg . | ||
| HPP-CFC* | 100 | 6 | 126 | 58 | 0 | 45 | 86 | 71 | 88 | 186 | 4 | 59 | 106 |
| LPP-CFC* | 100 | 10 | 115 | 47 | 1 | 35 | 55 | 57 | 58 | 148 | 9 | 37 | 69 |
| Proliferative potential† | Nd | 440 | 650 | 480 | 360 | 290 | 190 | 200 | 650 | 230 | 15 | 110 | 570 |
| . | Sca-1+Lin− . | CD11a . | CD43 . | CD44 . | CD49d . | CD49e . | CD62L . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos . | Neg . | Pos . | Neg . | Bright . | Dim . | Bright . | Dim . | Pos . | Neg . | Pos . | Neg . | ||
| HPP-CFC* | 100 | 6 | 126 | 58 | 0 | 45 | 86 | 71 | 88 | 186 | 4 | 59 | 106 |
| LPP-CFC* | 100 | 10 | 115 | 47 | 1 | 35 | 55 | 57 | 58 | 148 | 9 | 37 | 69 |
| Proliferative potential† | Nd | 440 | 650 | 480 | 360 | 290 | 190 | 200 | 650 | 230 | 15 | 110 | 570 |
HPP-CFC indicates high proliferative potential–colony forming cells; and LPP-CFC, low proliferative potential-colony forming cells.
HPP-CFC and LPP-CFC data are normalized by assigning a value of 100% to the HPP-CFC and LPP-CFC content of Sca-1+lin− cells in each experiment, and adjusting remaining values accordingly. Data are means from 3 separate experiments. For ease of presentation, SEM values are excluded from the table, but range from 0 to 35 for HPP-CFC data, and from 0 to 27 for LPP-CFC data.
Proliferative potential is expressed as fold-increase in total cells from day 0 to day 11. AM+ and AM−Sca-1+lin− cells were cultured in vitro with mSCF, mIL1α, mIL3, hIL6, and hFlt3-L for 11 days. Fold-increase was calculated by dividing the cell number on day 11 by the input number on day 0. Data shown are from 1 of 2 experiments with similar results.
Chimerism in mice transplanted with Sca-1+lin− AM+ or AM− cells
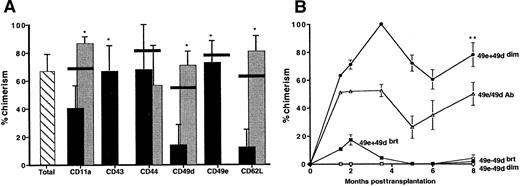
To determine the engraftment potential of the 12 groups of Sca-1+lin− AM+ or AM−cells, a competitive repopulation assay in lethally irradiated recipient mice was performed. Chimerism in recipient mice transplanted with either total Sca-1+lin− cells or Sca-1+lin− AM+ or AM−cells was evident 4 weeks posttransplantation and continued to increase until 2 months posttransplantation, at which time chimerism stabilized (data not shown). Figure 2A shows that Sca-1+lin− cells expressing CD43 and CD49e appeared to be highly enriched for long-term engraftment potential, as Sca-1+lin− cells lacking expression of either of these 2 molecules failed to provide measurable chimerism in recipients at 6 months posttransplantation. Sca-1+lin− cells expressing low levels of CD11a, CD49d, and CD62L were superior competitors compared with their counterparts expressing higher levels (Figure 2A). Expression of CD44 did not appear to significantly correlate with enhanced or diminished engraftment potential of Sca-1+lin− cells. As indicated by the horizontal bars in Figure 2A, anti-AM antibodies used for cell sorting did not interfere with engraftment of Sca-1+lin− cells, as mice transplanted with Sca-1+lin− cells treated with each anti-AM antibody exhibited chimerism similar to that of mice receiving untreated Sca-1+lin− cells. All groups of Sca-1+lin−AM+ or AM− cells contributed equally to lineage-specific hematopoiesis, as indicated by similar levels of donor-derived chimerism in myeloid (Gr-1+) and lymphoid (CD3+ or B220+) cells (data not shown).
Percentage of donor-derived chimerism in transplanted mice.
Percentage of donor-derived chimerism in mice transplanted with Sca-1+lin− cells, Sca-1+lin− AM+ or AM−cells (panel A), or Sca-1+lin− cells fractionated with the use of CD49d and CD49e simultaneously (panel B). Lethally irradiated C57BL/6 mice were transplanted with 1 × 103 total Sca-1+lin− cells (hatched bar), Sca-1+lin−AM+ (black bars), or Sca-1+lin−AM− (gray bars), all of B6.Gpi-1a origin, along with 3 × 104 C57BL/6 competitor cells. Chimerism was monitored monthly by hemoglobin analysis of peripheral blood cells. Horizontal black lines represent mean engraftment in mice transplanted with “antibody control” cells (Sca-1+lin−cells treated with the AM antibody but not sorted for the AM phenotype). Data are expressed in panel A as the mean ± SEM of 3 to 12 mice per group from 1 to 5 separate experiments (analyzed at 6 months posttransplantation), and in panel B as the mean ± SEM of 3 to 5 mice per group in 1 experiment. Trends in engraftment similar to that seen in panel A were obtained in experiments in which B6.BoyJ donor cells were used along with 1 × 105 C57BL/6 competitor cells. *The presence of statistical significance between the AM+ and AM− fraction of Sca-1+lin− cells; P < .05. **The presence of statistical significance between Sca-1+lin− CD49e+CD49ddim cells and each of the other 3 phenotypes at 8 months posttransplantation.
Percentage of donor-derived chimerism in transplanted mice.
Percentage of donor-derived chimerism in mice transplanted with Sca-1+lin− cells, Sca-1+lin− AM+ or AM−cells (panel A), or Sca-1+lin− cells fractionated with the use of CD49d and CD49e simultaneously (panel B). Lethally irradiated C57BL/6 mice were transplanted with 1 × 103 total Sca-1+lin− cells (hatched bar), Sca-1+lin−AM+ (black bars), or Sca-1+lin−AM− (gray bars), all of B6.Gpi-1a origin, along with 3 × 104 C57BL/6 competitor cells. Chimerism was monitored monthly by hemoglobin analysis of peripheral blood cells. Horizontal black lines represent mean engraftment in mice transplanted with “antibody control” cells (Sca-1+lin−cells treated with the AM antibody but not sorted for the AM phenotype). Data are expressed in panel A as the mean ± SEM of 3 to 12 mice per group from 1 to 5 separate experiments (analyzed at 6 months posttransplantation), and in panel B as the mean ± SEM of 3 to 5 mice per group in 1 experiment. Trends in engraftment similar to that seen in panel A were obtained in experiments in which B6.BoyJ donor cells were used along with 1 × 105 C57BL/6 competitor cells. *The presence of statistical significance between the AM+ and AM− fraction of Sca-1+lin− cells; P < .05. **The presence of statistical significance between Sca-1+lin− CD49e+CD49ddim cells and each of the other 3 phenotypes at 8 months posttransplantation.
The importance of CD49e expression in fractionating Sca-1+lin− cells into engrafting phenotypes is illustrated in Figure 2B. With the use of both CD49e and CD49d, Sca-1+lin− cells were further fractionated to yield 4 groups of cells (each being either positive or negative for either marker). All 4 groups of cells were analyzed for their engraftment potential in the same competitive repopulation assay described above. Figure 2B shows that chimerism in mice transplanted with Sca-1+lin− cells expressing a CD49e+ CD49ddim phenotype was higher than that in mice transplanted with similar cells exhibiting a CD49e+CD49dbright phenotype, while mice receiving either group of Sca-1+lin− cells devoid of CD49e expression had undetectable engraftment. These results are consistent with data in Figure 2A, in that Sca-1+lin− cells that were CD49e+ or CD49ddim were better competitors than their CD49e− or CD49dbright counterparts, respectively. Mice receiving Sca-1+lin−CD49e+ CD49dbright cells exhibited transient chimerism at 2 months posttransplantation that diminished after several months, suggesting that this phenotype consisted of cells capable of short-term engraftment only. Sca-1+lin− cells exposed to CD49e and CD49d antibodies provided 50% ± 8% chimerism in recipients, a figure not statistically different from that provided by untreated Sca-1+lin− cells (67.8% ± 11% chimerism, P > 0.1) (Figure 1A). Taken together, these results suggest a theoretical phenotype of a Sca-1+lin− engrafting cell as being positive for expression of CD43 and CD49e, but negative or low for expression of CD11a, CD49d, and CD62L.
Analysis of AM expression on engrafting vs nonengrafting phenotypes of Sca-1+lin− cells
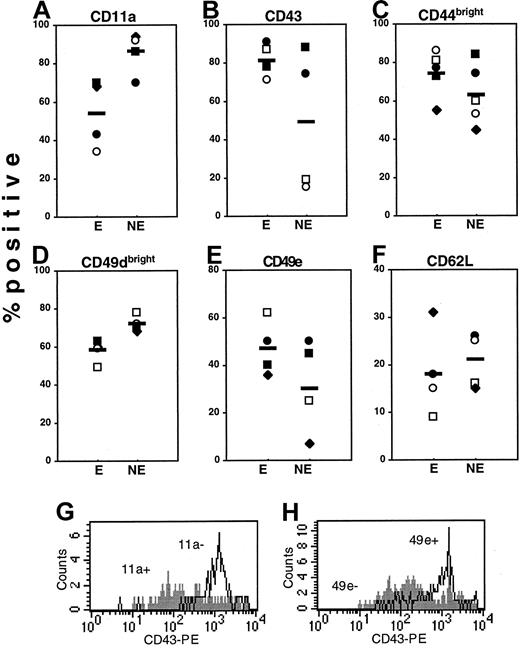
To better identify AMs potentially important in engraftment, subfractions of Sca-1+lin− cells demonstrated as having enriched engraftment potential were surveyed for their repertoire of AM expression in comparison with their nonengrafting counterparts. Figure 3shows that expression of CD11a (panel A), CD49dbright(panel D), and CD62L (panel F) was slightly lower on engrafting than on nonengrafting phenotypes, while expression of CD43 and CD49e was greater on engrafting cells. Interestingly, expression of CD43 (panel B) was more than 4 times greater on engrafting phenotypes defined by CD11a and CD49e, and expression of CD49e (Panel E) was more than 2- and 5-fold greater on engrafting phenotypes defined by CD11a and CD43, respectively; this illustrates once again the importance of CD43 and CD49e in engraftment. Examination of mean channel fluorescence and peak channel fluorescence also revealed high levels of expression of CD43 and CD49e on engrafting phenotypes of Sca-1±lin− cells (data not shown). Expression of CD44bright (Figure 3C) was slightly increased on engrafting phenotypes.
Expression of AMs on engrafting and nonengrafting phenotypes of Sca-1+lin− cells.
Sca-1+lin− cells were subfractionated by cell sorting into AM+/AMbright or AM−/AMdim groups with the use of CD11a (■), CD43 (♦), CD49d (●), CD49e (○), and CD62L (▪) to give engrafting or nonengrafting phenotypes, designated E and NE, respectively. CD44 was omitted in the primary sort since this marker was not useful in defining engrafting phenotypes (Figure 2). These resulting 10 groups of cells were phenotyped for their expression of other AMs not used in the primary sort (listed at the top of each scatter plot), and the percentage of each engrafting (E) and nonengrafting (NE) phenotype positive for expression of other AMs was calculated after subtracting nonspecific background fluorescence. Each individual data point represents the percentage of positive expression; horizontal bars are mean expression. For example, the open circles in Panel A represent the expression of CD11a on CD49e+ cells (E) and CD49e− cells (NE). In panel B, open squares represent expression of CD43 on CD11a− cells (E) and CD11a+ cells (NE), also shown in panel G. Since 100% of Sca-1+lin− cells express CD44 and CD49d (Table 1), only the proportion of engrafting and nonengrafting phenotypes positive for CD44bright and CD49dbright expression was calculated. The histogram in panel G illustrates the high level of expression of CD43 (x-axis) on Sca-1[+lin− CD11a− cells (open histogram) relative to CD11a+ cells (filled histogram), while panel H shows the high level of expression of CD43 on Sca-1+lin− CD49e+ cells (open histogram) relative to CD49e− cells (filled histogram). Data are from 1 of 2 experiments with similar results.
Expression of AMs on engrafting and nonengrafting phenotypes of Sca-1+lin− cells.
Sca-1+lin− cells were subfractionated by cell sorting into AM+/AMbright or AM−/AMdim groups with the use of CD11a (■), CD43 (♦), CD49d (●), CD49e (○), and CD62L (▪) to give engrafting or nonengrafting phenotypes, designated E and NE, respectively. CD44 was omitted in the primary sort since this marker was not useful in defining engrafting phenotypes (Figure 2). These resulting 10 groups of cells were phenotyped for their expression of other AMs not used in the primary sort (listed at the top of each scatter plot), and the percentage of each engrafting (E) and nonengrafting (NE) phenotype positive for expression of other AMs was calculated after subtracting nonspecific background fluorescence. Each individual data point represents the percentage of positive expression; horizontal bars are mean expression. For example, the open circles in Panel A represent the expression of CD11a on CD49e+ cells (E) and CD49e− cells (NE). In panel B, open squares represent expression of CD43 on CD11a− cells (E) and CD11a+ cells (NE), also shown in panel G. Since 100% of Sca-1+lin− cells express CD44 and CD49d (Table 1), only the proportion of engrafting and nonengrafting phenotypes positive for CD44bright and CD49dbright expression was calculated. The histogram in panel G illustrates the high level of expression of CD43 (x-axis) on Sca-1[+lin− CD11a− cells (open histogram) relative to CD11a+ cells (filled histogram), while panel H shows the high level of expression of CD43 on Sca-1+lin− CD49e+ cells (open histogram) relative to CD49e− cells (filled histogram). Data are from 1 of 2 experiments with similar results.
Cell cycle status
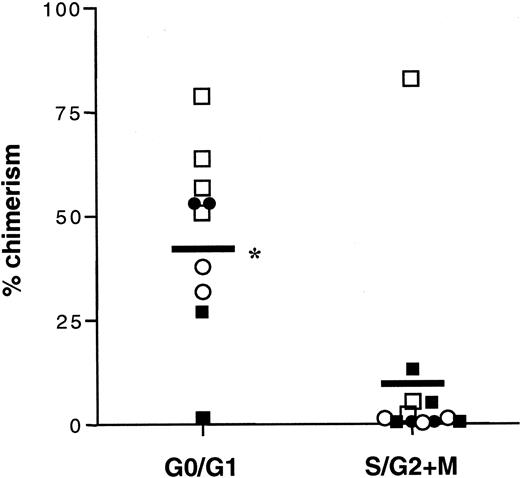
Since HSCs are believed to represent relatively quiescent cells, the cell cycle status of the 12 phenotypes of Sca-1+lin− AM+ or AM−cells was evaluated to determine whether each of the AM subfractions of Sca-1+lin− cells enriched for engraftment potential would also be more quiescent than the corresponding nonengrafting phenotype. However, 5 out of 6 Sca-1+lin− fractions that were negative or dim for expression of AMs were significantly more quiescent than the AM+ fraction (Figure 4). In the cases of CD49d and CD62L, the engrafting phenotype was more quiescent than the nonengrafting, but in the cases of CD43 and CD49e, the engrafting phenotype contained the higher percentage of cycling cells (Figure 4).
Cell cycle status of fresh Sca-1+lin− AM+ or AM−cells or total Sca-1+lin− cells.
Fractions of Sca-1+lin− AM+ (black bars), Sca-1+lin− AM− (gray bars), or total Sca-1+lin− cells (horizontal line) were stained with propidium iodide and analyzed on a FACScan flow cytometer for cell cycle status as previously described.23Data are reported as the percentage of cells in G0/G1 phase of the cell cycle (mean ± SEM; n = 6 to 7 for every group). *The presence of statistical significance when the AM− fraction is compared with its AM+ counterpart; P < .05.
Cell cycle status of fresh Sca-1+lin− AM+ or AM−cells or total Sca-1+lin− cells.
Fractions of Sca-1+lin− AM+ (black bars), Sca-1+lin− AM− (gray bars), or total Sca-1+lin− cells (horizontal line) were stained with propidium iodide and analyzed on a FACScan flow cytometer for cell cycle status as previously described.23Data are reported as the percentage of cells in G0/G1 phase of the cell cycle (mean ± SEM; n = 6 to 7 for every group). *The presence of statistical significance when the AM− fraction is compared with its AM+ counterpart; P < .05.
The cell cycle status of Sca-1+lin− cells fractionated with the use of both CD49e and CD49d expression also correlated with the level of expression of these 2 AMs. Sca-1+lin− cells lacking expression of both markers were more quiescent (98.6% ± 0.4% in G0/G1) than Sca-1+lin−cells positive for the expression of both CD49e and CD49d (78.3% ± 2.8% in G0/G1). The degree of quiescence of Sca-1+lin− cells lacking expression of one but not both markers fell between that of double-positive and double-negative cells (CD49e+/CD49ddim = 93.2% ± 1.9% G0/G1; CD49e−/CD49dbright = 96.0% ± 2.0% G0/G1; n = 2 for all cell cycle analyses).
Cell cycle progression of AM subfractions of Sca-1+lin− cells
Rapid in vivo proliferation of HSCs following transplantation has been reported to correlate with long-term hematopoietic function of transplanted cells.27 We have already shown that cellular expansion in vitro (Table 2) correlates with long-term engraftment capability of Sca-1+lin− AM+ or AM− cells (Figure 2). Differences in proliferative potential between engrafting and nonengrafting phenotypes may be related to the rate at which these cells exit G0/G1 in vitro, as shown in Figure5. AM fractions enriched for engraftment potential, regardless of their relative degree of quiescence on day 0, exited G0/G1 more rapidly than nonengrafting phenotypes (Figure 5). Cell cycle progression of Sca-1+lin− cells subfractionated with the use of both CD49e and CD49d also correlated with the engraftment potential of these cells. Sca-1+lin− cells most enriched for engraftment potential among these 4 phenotypes (CD49e+/CD49ddim) exited G0/G1 more rapidly (slope = −25) than cells lacking expression of CD49e (slopes = −2 to −11), or Sca-1+lin− cells expressing both CD49e and CD49d cells (slope = −16).
Exit from G0/G1 phases of cell cycle.
Exit from G0/G1 phases of cell cycle of total Sca-1+lin− cells (▴), or engrafting phenotypes (dotted lines) and nonengrafting phenotypes (solid lines) of Sca-1+lin− AM+ (▪) and AM− (○) cells. Up to 4 × 103 cells/mL were supplemented with 20% FBS, 2 × 10−5 mol/L 2-mercaptoethanol, 100 ng/mL mSCF and hIL6, 500 U/mL mIL1α, 100 U/mL mIL3, and 50 ng/mL hFlt3-L. Fresh cells and aliquots of cultured cells removed on days 1 and 2 were stained with propidium iodide and analyzed for cell cycle status as previously described.23 Data are expressed as the mean ± SEM; n = 2 to 3 for each time point. Slopes of linear regression lines generated from these data are indicated next to the appropriate lines to illustrate relative rates of exit from G0/G1 of the different populations of cells. *The presence of statistical significance in slope of regression line of engrafting phenotype when compared with the slope generated from its nonengrafting counterpart; P < .05.
Exit from G0/G1 phases of cell cycle.
Exit from G0/G1 phases of cell cycle of total Sca-1+lin− cells (▴), or engrafting phenotypes (dotted lines) and nonengrafting phenotypes (solid lines) of Sca-1+lin− AM+ (▪) and AM− (○) cells. Up to 4 × 103 cells/mL were supplemented with 20% FBS, 2 × 10−5 mol/L 2-mercaptoethanol, 100 ng/mL mSCF and hIL6, 500 U/mL mIL1α, 100 U/mL mIL3, and 50 ng/mL hFlt3-L. Fresh cells and aliquots of cultured cells removed on days 1 and 2 were stained with propidium iodide and analyzed for cell cycle status as previously described.23 Data are expressed as the mean ± SEM; n = 2 to 3 for each time point. Slopes of linear regression lines generated from these data are indicated next to the appropriate lines to illustrate relative rates of exit from G0/G1 of the different populations of cells. *The presence of statistical significance in slope of regression line of engrafting phenotype when compared with the slope generated from its nonengrafting counterpart; P < .05.
Engraftment potential of Sca-1+lin−AM+ or AM− cells subfractionated on the basis of position in cell cycle
The relatively high percentage of cycling cells in engrafting phenotypes defined as CD43+ and CD49e+ of Sca-1+lin− cells (Figure 4) led us to question whether quiescent or cycling cells within the AM-defined engrafting phenotypes were contributing to engraftment potential and in vitro hematopoiesis of these cells. To this end, engrafting phenotypes of Sca-1+lin− AM+ or AM−cells were subfractionated into G0/G1 and S/G2+M fractions with the use of Hoechst 33342 and examined for their engraftment potential. Figure 6shows chimerism in mice transplanted with G0/G1or S/G2 + M fractions of total Sca-1+lin− cells, Sca-1+lin− CD11a−, Sca-1+lin− CD49ddim, and Sca-1+lin− CD49e+ cells. G0/G1 cells, regardless of which AM was used in fractionation, provided greater levels of chimerism than equal numbers of S/G2+M cells (P < .005), illustrating the enhanced engraftment potential of quiescent cells. G0/G1 cells also provided higher levels of chimerism than equal numbers of total Sca-1+lin− AM subfraction (data not shown), negating any suggestion that the Hoechst dye may negatively influence the function of primitive HPCs in active phases of cell cycle. Of interest in these analyses is that, although a relatively large percentage of Sca-1+lin− CD49e+cells were in active phases of cell cycle (Figure 4), only those in G0/G1 accounted for the majority of the engraftment potential of this phenotype. All groups of cells contributed equally to lineage-specific hematopoiesis (myeloid and lymphoid; data not shown). Table 3 shows that the quiescent fractions of engrafting Sca-1+lin− cells possessed greater proliferative potential and gave rise to greater numbers of Sca-1+ cells during 14 days of in vitro cytokine-stimulated cell culture than engrafting cells in active phases of cell cycle.
Percentage of donor-derived chimerism of cell cycle subfractions.
Percentage of donor-derived chimerism at 6 months posttransplantation in mice receiving G0/G1 or S/G2+M subfractions of total Sca-1+lin− cells (▪), Sca-1+lin− CD11a− cells (■), Sca-1+lin− CD49ddim cells (●), or Sca-1+lin− CD49e+ cells (○). Total Sca-1+lin− cells or CD11a−, CD49ddim, or CD49e+ subfractions of Sca-1+lin− cells were isolated by cell sorting and then subfractionated by means of Hoechst 33342 into G0/G1 or S/G2+M fractions. Between 0.4 to 1.0 × 103 sorted donor cells from B6.BoyJ mice were transplanted into lethally irradiated C57BL/6 mice along with 1.0 × 105 C57BL/6 competitor cells. Chimerism was monitored monthly by CD45.2 analysis of peripheral white blood cells. Purity of sorted donor G0/G1 cells was greater than 97%; purity of donor S/G2+M cells was greater than 82%. Data points are values for the percentage of chimerism for individual mice; horizontal bars are mean chimerism levels. n = 10 for G0/G1 mice, n = 12 for S/G2+M mice. *The presence of statistical significance between mice transplanted with G0/G1 and S/G2+M fractions of engrafting phenotypes; P < .005.
Percentage of donor-derived chimerism of cell cycle subfractions.
Percentage of donor-derived chimerism at 6 months posttransplantation in mice receiving G0/G1 or S/G2+M subfractions of total Sca-1+lin− cells (▪), Sca-1+lin− CD11a− cells (■), Sca-1+lin− CD49ddim cells (●), or Sca-1+lin− CD49e+ cells (○). Total Sca-1+lin− cells or CD11a−, CD49ddim, or CD49e+ subfractions of Sca-1+lin− cells were isolated by cell sorting and then subfractionated by means of Hoechst 33342 into G0/G1 or S/G2+M fractions. Between 0.4 to 1.0 × 103 sorted donor cells from B6.BoyJ mice were transplanted into lethally irradiated C57BL/6 mice along with 1.0 × 105 C57BL/6 competitor cells. Chimerism was monitored monthly by CD45.2 analysis of peripheral white blood cells. Purity of sorted donor G0/G1 cells was greater than 97%; purity of donor S/G2+M cells was greater than 82%. Data points are values for the percentage of chimerism for individual mice; horizontal bars are mean chimerism levels. n = 10 for G0/G1 mice, n = 12 for S/G2+M mice. *The presence of statistical significance between mice transplanted with G0/G1 and S/G2+M fractions of engrafting phenotypes; P < .005.
Fold-increase in total cells and Sca-1+ cells in cultures initiated with engrafting phenotypes of Sca-1+lin−AM+ or AM−cells fractionated on the basis of position in cell cycle
| Phenotype . | Sca-1+lin− . | CD11a− . | CD43+ . | CD49ddim . | CD49e+ . | CD62L− . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | |
| Total cells3-150 | 1200 | 70 | 1900 | 1400 | 4100 | 100 | 840 | 10 | 3900 | 240 | 4900 | 50 |
| Sca1+cells3-151 | 1030 | 35 | 1800 | 1200 | 3970 | 10 | 330 | 0 | 2200 | 33 | 2340 | 0 |
| Phenotype . | Sca-1+lin− . | CD11a− . | CD43+ . | CD49ddim . | CD49e+ . | CD62L− . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | G0/G1 . | S/G2+M . | |
| Total cells3-150 | 1200 | 70 | 1900 | 1400 | 4100 | 100 | 840 | 10 | 3900 | 240 | 4900 | 50 |
| Sca1+cells3-151 | 1030 | 35 | 1800 | 1200 | 3970 | 10 | 330 | 0 | 2200 | 33 | 2340 | 0 |
AM indicates adhesion molecule.
Fold-increase in total cells was calculated by dividing the cell number on day 14 of cytokine supplemented in vitro culture by the input number on day 0.
Fold-increase in Sca-1+ cells was calculated by multiplying the percentage of Sca-1+ cells on day 14, determined by flow cytometry, by the total number of cells, and then dividing this number by the input number on day 0.
Discussion
In this report, we define a theoretical phenotype of a Sca-1+lin− long-term engrafting cell as a quiescent cell that expresses high levels of CD43 and CD49e, expresses low or negligible levels of CD11a, CD49d, and CD62L, and proliferates rapidly in vitro following cytokine stimulation. These data are a first step in defining AMs on primitive quiescent HPCs potentially important in the homing and/or anchorage of long-term engrafting cells within the BM microenvironment.
To the best of our knowledge, antibodies used in these studies were nonblocking. In mice where long-term engraftment potential was predominantly among AM−/AMdim phenotypes (CD11a, CD49d, CD62L), testing of blocking activity was crucial since low chimerism in AM+ mice may have resulted from blockade of homing or engraftment. While other investigators showed variable inhibition using these same clones but with different cell types in different assays,28-30 we found similar chimerism and HPP-CFC frequency in untreated and antibody-treated Sca-1+lin− cells, indicating that these clones were not inhibitory to the in vivo or in vitro activity of Sca-1+lin− cells.
Although much work has been performed illustrating the importance of several AMs in various aspects of stem cell function, AMs involved in the sequential movement of transplanted or mobilized HSCs through BM endothelium to or from the periphery are not yet defined. It is likely that different AMs are involved at different stages of stem cell homing, from initial tethering of HSCs on BM endothelium, to rolling, attachment, passage through endothelial barriers, movement within BM tissues, and final anchorage within specialized BM niches, similar to that described for lymphocyte trafficking and leukocyte emigration.31 Mobilization could conceivably involve similar AMs but in reverse order. We found that a sizable fraction of Sca-1+lin− CD49e+ cells co-expressed CD43, suggesting that either or both AMs could be involved at some step of the homing process. A role for CD49e in murine long-term and nonobese diabetic/severe combined immunodeficiency (SCID) repopulating cell function has been previously suggested by van der Loo et al,3 while other investigators32 showed no involvement of CD49e in homing or mobilization of murine clonogenic HPCs. Expression of CD43 on primitive human33 and murine HPCs,34 and on cells capable of secondary SCID repopulation,35 has been previously documented. These observations do not exclude the involvement, either synergistically or in a redundant fashion, of other, yet unidentified, AMs in homing of HSCs to the BM. Of the 6 AMs examined in our studies, only CD44 was inefficient in fractionating Sca-1+lin− cells into engrafting versus nonengrafting phenotypes. Given the cooperation between CD44 and other AMs,36 as well as the high expression of CD44 on Sca-1+lin− cells, it is possible that CD44 may not be directly involved in homing, but may regulate the function of other AMs involved in this process.
While a role of CD49d in homing to and egress from the BM of clonogenic progenitors28,37,38 and malignant cells39,40 has been well defined, to date no one has directly examined CD49d in homing of long-term engrafting cells. The laboratory of Dr Papayannopoulou has shown the ability of function-blocking anti-CD49d antibody to mobilize murine long-term engrafting cells7 and, more recently, to possess a role in homing and mobilization of human CD34+ cells in fetal sheep.1 Given the complexity of homing, it is possible that CD49d may not be involved in the initial steps of HSC homing, but may be critical for the lodgment of HSCs within BM microenvironment following homing via different AMs. This notion is supported by our findings demonstrating that Sca-1+lin− cells expressing low levels of CD49d were enriched for long-term engrafting cells (Figure 2). It is also possible that cell cycle status16-19 or rapid changes in functional status5 41-43 may be involved in the control of CD49d function.
CD62L expression on human mobilized peripheral blood (MPB) CD34+ cells has been shown to correlate with rapid platelet engraftment,4,44 but was found in our studies to be detrimental to engraftment potential, presenting the possibility that short-term engrafting cells may express CD62L but long-term engrafting cells may not. Such a concept, however, could be in direct conflict with that recently presented by Ziljmans et al,45 who found primitive HPCs to be responsible for both short- and long-term engraftment. Additionally, since the AM profile is reportedly different in human BM and MPB CD34+cells,4,5,44 46-49 it should not be surprising to find differences in the AM profiles of long-term reconstituting cells from either BM or MPB, within or outside species barriers.
A surprising finding in our studies was the rapid in vitro proliferation and cycling of long-term engrafting cells, which are generally believed to be quiescent.8 While it is possible that cytokine-responsive cells in vitro were not the same cells contributing to long-term engraftment, several lines of evidence support the notion that murine long-term engrafting cells cycle rapidly, at least following transplantation.15,27,50 The rate of in vivo proliferation of colony-forming unit–spleen has been demonstrated to predict long-term engraftment potential of transplanted HPC.27,50 In addition, Nilsson et al15 demonstrated cycling of engrafting murine HSCs within the first 12 hours posttransplantation. Collectively, these data suggest that rapid proliferative responses to cytokine stimulation, either in vivo or in vitro, may be predictive of engraftment potential of selected groups of murine primitive HPCs.
The relationship between cell cycle position and engraftment capability of HSCs has been the focus of much investigation.12,13,15,51Sca-1+lin− CD43+ and CD49e+ cells were found to contain a relatively large percentage of cycling cells, posing the question of whether engrafting cells were contained within quiescent or cycling compartments. Despite possessing an apparently crucial repertoire of AMs, cycling cells were deficient in engraftment potential, similar to what has been previously reported in human12 and murine51 systems. Since HPCs express some AMs16-19 and primitive markers52,53 in a cell cycle–related fashion, it is possible that changes in AMs on cycling cells lead to an engraftment defect. Alternatively, as HSCs exit G0/G1 and begin to cycle, hematopoietic potential of these cells may be compromised, as has been previously suggested.12-14Characteristics of putative murine HSCs defined in these studies may be applicable for the selection and in vitro manipulation of analogous human HSCs as targets for gene therapy and ex vivo expansion protocols.
Supported by National Institutes of Health grants RO1 HL55716 and RO1 HL62200 (to E.F.S.); Herman B Wells Center for Pediatric Research is a Center of Excellence in Molecular Hematology (NIDDK P30 DK49218).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christie M. Orschell-Traycoff, Indiana University School of Medicine, 1044 West Walnut St, R4-202, Indianapolis, IN 46202-5254; e-mail: ctraycof@iupui.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal