Abstract
Cell vaccines engineered to express immunomodulators have shown feasibility in eliminating leukemia in murine models. Vectors for efficient gene delivery to primary human leukemia cells are required to translate this approach to clinical trials. In this study, second-generation lentiviral vectors derived from human immunodeficiency virus 1 were evaluated, with the cytomegalovirus (CMV) promoter driving expression of granulocyte-macrophage–colony-stimulating factor (GM-CSF) and CD80 in separate vectors or in a bicistronic vector. The vectors were pseudotyped with vesicular stomatitis virus G glycoprotein and concentrated to high titers (108-109 infective particles/mL). Human acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and chronic myeloid leukemia cell lines transduced with the monocistronic pHR-CD80 vector or the bicistronic pHR-GM/CD vector became 75% to 95% CD80 positive (CD80+). More important, transduction of primary human ALL and AML blasts with high-titer lentiviral vectors was consistently successful (40%-95% CD80+). The average amount of GM-CSF secretion by the leukemia cell lines transduced with the pHR-GM-CSF monocistronic vector was 2182.9 pg/106 cells per 24 hours. Secretion was markedly lower with the bicistronic pHR-GM/CD vector (average, 225.7 pg/106 cells per 24 hours). Lower amounts of CMV-driven messenger RNA were detected with the bicistronic vector, which may account for its poor expression of GM-CSF. Primary ALL cells transduced to express CD80 stimulated T-cell proliferation in an autologous mixed lymphocyte reaction. This stimulation was specifically blocked with monoclonal antibodies reactive against CD80 or by recombinant cytotoxic T-lymphocyte antigen 4–immunoglobulin fusion protein. These results show the feasibility of efficiently transducing primary leukemia cells with lentiviral vectors to express immunomodulators to elicit antileukemic immune responses.
Introduction
Two signals are necessary to activate T lymphocytes and elicit a response against antigens, including those expressed by tumor cells. The first is the tumor-associated or specific antigen, which is delivered in the context of the major histocompatibility complex to the T-cell receptor.1 The second signal is the costimulation of the T cell by means of one or more membrane-bound ligands on the antigen-presenting cells (APC) or tumor cell. Antigen presentation in the absence of costimulation is likely to induce anergy. For example, lack of expression of costimulatory molecules by tumor cells may allow escape from immune recognition. Once a productive immune response has been generated, cytotoxic T lymphocytes can detect and destroy circulating cells presenting the antigen or antigens, whereas memory T cells can be reactivated by re-exposure to the antigen or antigens.2
CD80 (B7.1), a member of the B7 family, is a membrane-bound costimulatory ligand that triggers strong T-cell activation through its interaction with CD28.3 Human pre-B acute lymphoblastic leukemia (ALL) cells usually lack expression of CD80,4which may be sufficient for ALL cells to induce alloantigen-specific T-cell unresponsiveness in vitro.5 However, this anergy can be prevented by stimulation of the ALL cells by means of CD40, which up-regulates CD80 expression of the leukemia cell.5
Granulocyte-macrophage–colony-stimulating factor (GM-CSF) has been shown to stimulate in vitro growth, maturation, and function of APC such as dendritic cells and macrophages.6 The increased antitumor immune response observed when transduced tumor cells producing GM-CSF were injected into mice is presumed to be mediated by the role of GM-CSF in inducing the maturation and function of APC such as dendritic cells.7
We previously examined the in vivo effects of coexpression of the immune stimulatory molecules interleukin (IL)–2, GM-CSF, and CD80 by BM185, a murine transplantable ALL model.8 We found CD80 to be the most potent at inducing antileukemic responses. Furthermore, we observed that BM185 cells expressing both CD80 and GM-CSF were consistently more effective at inducing rejection of a high dose of BM185 cells injected intravenously after vaccination and could induce rejection of pre-established subcutaneous leukemia.9 This strong immune reaction is probably the result of the interaction among CD4+ T-helper, CD8+ T-cytotoxic, and dendritic cells.9 Therefore, transduction of a patient's leukemia cells to express the combination of CD80 and GM-CSF may be considered for immunotherapy.
Different types of gene-delivery vectors have been tested in human leukemia cells, including those based on Moloney murine leukemia viruses,10,11 herpesviruses,12adeno-associated viruses,13 and plasmids.10,14 We showed that lentiviral vectors efficiently transduce human leukemia cells10 and hematopoietic progenitor cells.15
Lentiviruses are complex retroviruses that infect macrophages and lymphocytes. The human immunodeficiency virus 1 (HIV-1) is one of these viruses and is effective in infecting nonproliferating or growth-arrested cells in vitro.16 One possible mechanism leading to infection of nonreplicating cells is the presence of nuclear localization signals in the lentiviral preintegration complex that mediate its active transport through the nucleopores during interphase.17 Primary leukemia cells show poor proliferation in vitro,18 which makes them good candidates for lentiviral vector transduction.
Here, we describe lentiviral constructs expressing CD80 and GM-CSF as monocistronic and bicistronic vectors. The viruses were packaged after a tripartite transient transfection procedure and pseudotyped with the vesicular stomatitis virus G (VSV-G) envelope, which allowed the virus to be concentrated to high titers.19-21 This report describes the potential use of these vectors for the delivery and coexpression of CD80 and GM-CSF genes in primary human leukemia cells and the autologous T-cell responses stimulated by transduced CD80-positive (CD80+) ALL cells.
Materials and methods
Cell lines
The 293T cell line was obtained from the American Type Culture Collection (Rockville, MD), and the 293 line was provided by Dr Nori Kasahara (University of Southern California, Los Angeles, CA). The human pre-B ALL cell lines used in this study were Nalm-6 (provided by Dr Dario Campana, St Jude Children's Research Hospital, Memphis, TN), Reh, and Sup-B15 (both from ATCC). The human myeloid leukemia cell lines used were the acute myeloid leukemia (AML) line 5 (provided by Dr Hal Broxmeyer, Walther Oncology Center, Indianapolis, IN); the AML line ML-1 (provided by Dr Donald Small, The Johns Hopkins Oncology Center, Baltimore, MD), the monocyte-like, histiocytic lymphoma line U-937; and the chronic myelogenous leukemia (CML) line K562 (ATCC).
Cell culture
293T and 293 fibroblast lines were cultured in Dulbecco modified Eagle medium with l-glutamine (BioWittaker, Walkersville, MD), 10% fetal bovine serum (FBS), and penicillin/streptomycin (50 U/mL). The leukemia cell lines were cultured in RPMI 1640 medium withl-glutamine (BioWhittaker), 10% FBS, and penicillin/streptomycin (50 U/mL). For the AML-5 cell line, IL-6 was added to the medium to produce a final concentration of 10 ng/mL. Cells were incubated at 37°C in 5% carbon dioxide (CO2). Experiments were done with cells in exponential growth phase, and all transductions were performed in AIM-V serum-free medium (Gibco BRL, Grand Island, NY).
Primary leukemia cells
Cryopreserved primary leukemia cells were obtained from cells remaining from diagnostic bone marrow aspirates (with > 70% leukemic blasts) obtained from pediatric patients. The samples were obtained and studies performed in accordance with protocols approved by the Committee on Clinical Investigation of Childrens Hospital Los Angeles and the Dana Farber Cancer Institute. The cells were cryopreserved in 10% dimethyl sulfoxide and 90% FBS. The cells were thawed at 37°C for 1 hour by using a thawing medium containing AIM-V, 30% FBS, 20 U/mL heparin, and 0.2 U/mL DNAase (Roche, Indianapolis, IN). After thawing, primary cells were washed twice with AIM-V medium. Primary ALL cells were cultured in AIM-V medium containing 100 μg/mL CD40L (Immunex, Seattle, WA), and primary AML cells were cultured in AIM-V medium containing 10 ng/mL IL-3 and 50 ng/mL stem cell factor (SCF) (SCF; R&D Systems, Minneapolis, MN).
Autologous T cells
Peripheral blood mononuclear cells from patients with leukemia were separated by density centrifugation. Cells were then stained with an anti-CD3 monoclonal antibody (mAb; clone UCHT1; Pharmingen, San Diego, CA), and T cells were sorted with a high-speed sorting apparatus (MoFlow; Cytomotion, Denver, CO). The T cells were more than 95% pure. Sorted T cells were used immediately for the mixed lymphocyte reaction (MLR).
Construction of pHR-CD80 vector
The CD80 complementary DNA (cDNA) was excised from the plasmid LL17722 with EcoRI, and the 900 base-pair fragment was subcloned into the EcoRI site of pIC20H23 to generate the plasmid CD80-pIC20H. The CD80 cDNA was then excised as a BamHI/XhoI fragment and introduced into the backbone of pHR cytomegalovirus (CMV)-neo,19 which had previously been digested withBamHI/XhoI to delete the neoropen-reading frame.
Construction of pHR-GM-CSF vector
The GM-CSF cDNA was excised from plasmid pCSF-124as an EcoRI fragment and subcloned into the EcoRI site of pIC20H23 generating GM-CSF-20H. This construct was digested with EcoRI and SalI, and the resulting fragment encoding GM-CSF was inserted into the pHR-CD80 backbone previously digested with EcoRI and XhoI to delete the CD80 cDNA.
Construction of PHR-GM/CD vector
The internal ribosome entry site (IRES) was obtained from plasmid E5m (provided by Dr Kathy Ponder, Washington University, St Louis, MO), which was subcloned as an EcoRI/NcoI fragment into pGEM-7 (Promega, Madison, WI) to generate pGEM-IRES. ANcoI/BamHI fragment encoding CD80 was obtained from plasmid CD80-pIC20H and subcloned into pGEM-IRES generating pGEM-IRES-CD80. The IRES-CD80 cassette was then excised withEcoRI and introduced into the backbone of pHR-CD80 (previously digested with EcoRI to delete CD80), resulting in the construct pHR-IRES-CD80. The GM-CSF gene was obtained as aBamHI/BglII fragment from GM-CSF-20H-2 (a modified version of GM-CSF-20H containing additional cloning sites) and introduced into the BamHI site of pHR-IRES-CD80. All constructs were rechecked by sequencing.
Production of lentivirus
The packaging construct pCMV-ΔR8.9 encoding HIV-1gag, pol, tat, andrev20 was provided by Dr Didier Trono and Dr Romain Zufferey (University of Geneva, Switzerland). The plasmid construct driving expression of the VSV-G envelope (pMD.G) and the vector containing the epidermal growth-factor receptor (EGFR) gene under control of the CMV promoter (pHR-CMV-EGFP)25 were provided by Dr Luigi Naldini (University of Torino, Italy). Lentivirus vectors were produced by transient cotransfection of 293T cells with the backbone vector plasmid derived from the pHR series, the packaging plasmid pCMV-ΔR8.9, and the plasmid pMD.G encoding the VSV-G envelope, as described previously.10,19 Viral titer was determined by assessing expression of the transgene (EGFP or CD80) in transduced 293 cells by flow cytometry as described previously.10 Viral p24 antigen concentration was determined by immunocapture (Coulter Immunotech, Miami, FL). The minimum detectable p24 concentration for this assay is 3 pg/mL.
Lentivirus-mediated gene transfer
Leukemia cells were washed twice with AIM-V and resuspended to obtain a cell density of 1 to 2 × 106 cells/mL. Then, 1 mL of the cell suspension was seeded in each well of 6-well plates and the viral supernatant was added to provide the desired concentration (0.5-1.0 × 107 infective particles/mL). Protamine sulfate was added at the final concentration of 6 μg/mL, and the transduction plates were incubated at 37°C in 5% CO2 for 5 hours. Subsequently, 3 mL of AIM-V medium (plus the corresponding cytokines) was added to each well and the plates were incubated for an additional 16 hours. Leukemia cells were washed twice with AIM-V medium and seeded at a density of 106 cells/mL in AIM-V and cytokines. Twenty-four hours later, the supernatant was collected for enzyme-linked immunosorbent assay (ELISA) and the cells were harvested for fluorescence-activated cell-sorter (FACS) analysis or kept in culture for additional time-point analyses.
FACS analysis
For analysis of EGFP expression, cells were washed once with phosphate-buffered saline (PBS) and resuspended in 100 μL of 1% paraformaldehyde for fixation. For analysis of expression of CD80, CD19 (B lymphoid marker), and CD33 (myeloid marker), cells were washed once with PBS, blocked with PBS containing mouse IgG (50 μg/mL) for 15 minutes on ice, stained with the corresponding mAb for 20 minutes, washed, and resuspended in 100 μL of 1% paraformaldehyde for fixation. The mAb to CD80 was conjugated with phycoerythrin (Pharmingen), and the mAbs against CD19 and CD33 were conjugated with fluorescein isothiocyanate, conjugated (FITC; Becton Dickinson, San Jose, CA). Detection of CD80, CD19, CD10, or EGFP was accomplished with a FACScan cytometer equipped with a 488-nm argon laser for excitation of the reporter proteins. To establish the background for fluorescence and to set gates for data acquisition, mock-transduced cells were used. Care was taken to analyze cells that were in the lymphoblast gate as indicated by forward- and side-scatter characteristics.
Analysis of GM-CSF
Expression of GM-CSF was analyzed by ELISA (R&D Systems) conducted according to the manufacturer's instructions. The minimum detectable concentration of GM-CSF after this assay is typically less than 3 pg/mL.
Southern and Northern blot analysis
Nalm-6 cells were transduced with pHR-GM-CSF, pHR-CD80, or pHR-GM/CD and maintained in culture for 8 days before harvest. Control (mock-transduced) cells were maintained under the same conditions. Cells were washed twice with PBS, and half of the cells were used for extracting DNA and the other half for RNA.
Genomic DNA was digested simultaneously with EcoRI andNcoI and separated on a 0.7% agarose gel in Tris-acetate-EDTA buffer. The blot was hybridized first to a phosphorus 32 (32P)–labeled DNA fragment encoding human CD80. The blot was then stripped and subsequently hybridized to a32P-labeled DNA fragment encoding human GM-CSF. Filters were washed to a stringency of 0.3 × SSC at 65°C and exposed to Kodak AR 5 films at −70°C.
Total cellular RNA was extracted with RNA STAT-60 (Tel-test, Friendswood, TX). Ten-microgram samples of total RNA were separated on a 1.2% agarose-formaldehyde gel in 3-[N-morpholino] propane sulfonic acid (MOPS) buffer and transferred to a nylon membrane. The RNA was transferred to nylon filters and blots were probed with a32P-labeled 0.9-kilobase (kb) DNA fragment encoding CD80 or with a 0.6-kb DNA fragment encoding human GM-CSF. Filters were washed to a stringency of 0.3 × SSC and 0.1% sodium dodecyl sulfate at 65°C and exposed to Kodak AR 5 films at −70°C.
Primary and secondary autologous MLR
For primary MLR, irradiated (3200 cGy) and nontransduced or transduced primary ALL cells were used as stimulators. For these experiments, ALL cells were transduced without adding exogenous CD40 ligand. ALL cells were cocultured at a 1 to 2 ratio with sorted autologous T cells in 96-well round-bottomed plates containing 105 ALL cells and 2 × 105 T cells in 200 μL of medium (small-scale MLR) or in 6-well plates containing 106 ALL cells and 2 × 106 T cells in 2 mL of medium (large-scale MLR) and incubated for 6 days at 37°C in a 5% CO2 incubator. All MLR cultures used RPMI-1640 supplemented with 4% heat-inactivated human AB serum (NABI, Miami, FL) and 10 U/mL recombinant human IL-2 (National Institutes of Health, Bethesda, MD) for the last 3 days of culture. These stimulator–responder cell ratios and times of incubation were found to represent the optimal culture conditions. All microcultures were performed in triplicate. Cells were pulsed with 0.037 MBq tritium-thymidine (Du Pont, Boston, MA) for the last 18 hours of the culture period and then harvested onto glass-fiber filters. Tritium-thymidine incorporation was measured by liquid scintillation spectrophotometry. Stimulation indexes (SI) were calculated for each individual experiment as SI = cpm (T cells + ALL cells)/cpm (T cells).
For the secondary MLR, T cells that were initially primed in the large-scale MLR with either nontransduced or transduced irradiated primary ALL cells were isolated by Ficoll-Hypaque density centrifugation and placed for 12 hours in RPMI-1640 with 4% heat-inactivated human AB serum. Rested, primed T cells were then rechallenged in triplicate with irradiated (3200 cGy) primary nontransduced or transduced ALL cells at stimulator-responder ratios of 1 to 2 in 96-well round-bottomed plates and incubated for 3 days at 37°C in a 5% CO2 incubator. Tritium-thymidine incorporation and stimulation indexes were determined as described above.
Blocking assays
Blocking experiments were performed by using an anti-CD80 mAb (4B2.C4) at 5 μg/mL or the fusion protein cytotoxic T-lymphocyte antigen 4–immunoglobulin (CTLA4-Ig) at 10 μg/mL.5 A control fusion protein and irrelevant mouse IgG were used as negative controls. Antibodies were premixed with T cells before the leukemia cells were added.
Results
Production and titer of lentiviral vectors expressing CD80 and GM-CSF
Lentiviral vectors were produced by transient cotransfection of 293T cells with the packaging vector pCMV-ΔD8.9,20 the VSV-G envelope encoding vector pMD.G,19 and the corresponding backbone vector pHR-CMV-EGFP,25 pHR-CD80, pHR-GM-CSF, or PHR-GM/CD (Figure 1). Supernatant viral titer analysis was done on 293 cells and followed by FACS analysis as previously described.10 The viral titers for the crude pHR-CD80 vector ranged from 1.0 to 3.0 × 107 infective particles/mL, whereas the pHR-GM/CD viral supernatants showed lower titers overall: 0.5 to 3.0 × 106 infective particles/mL. Concentration of the lentiviral particles by ultracentrifugation increased the titer levels by 10 fold to 100 fold.
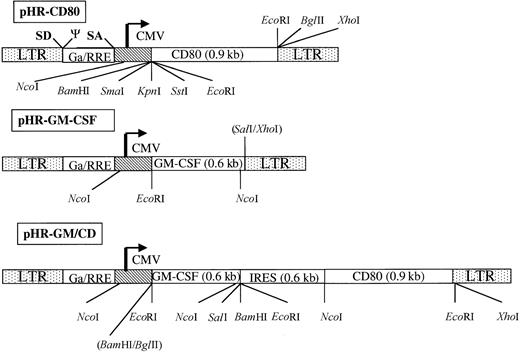
Schematic representation of the gene-transfer vectors pHR-CD80, pHR-GM-CSF, and PHR-GM/CD.
The long terminal repeats (LTR), the splice donor site (SD), the splice acceptor site (SA), the packaging signal, the cytomegalovirus (CMV) enhancer-promoter element, and the truncated and out-of-framegag gene (Ga) upstream of the Rev responsive element (RRE) are indicated (not in scale). The complementary DNA encoding human CD80 and human granulocyte-macrophage colony-stimulating factor (GM-CSF) are shown. To construct pHR-GM/CD, a bicistronic message was generated; this contained an internal ribosome entry site (IRES) upstream of the human CD80 open-reading frame. Some of the cleavage sites for restriction enzymes used for the design or verification of the constructs are shown.
Schematic representation of the gene-transfer vectors pHR-CD80, pHR-GM-CSF, and PHR-GM/CD.
The long terminal repeats (LTR), the splice donor site (SD), the splice acceptor site (SA), the packaging signal, the cytomegalovirus (CMV) enhancer-promoter element, and the truncated and out-of-framegag gene (Ga) upstream of the Rev responsive element (RRE) are indicated (not in scale). The complementary DNA encoding human CD80 and human granulocyte-macrophage colony-stimulating factor (GM-CSF) are shown. To construct pHR-GM/CD, a bicistronic message was generated; this contained an internal ribosome entry site (IRES) upstream of the human CD80 open-reading frame. Some of the cleavage sites for restriction enzymes used for the design or verification of the constructs are shown.
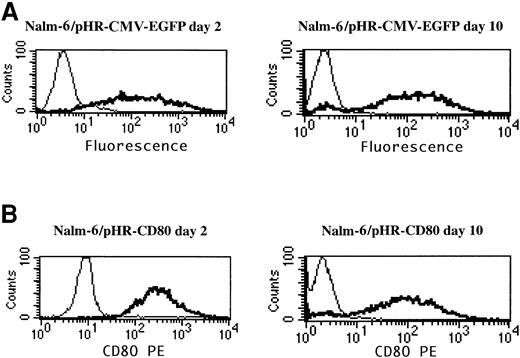
To determine whether the analysis of the CD80 transgene could be used to assess gene delivery, as was previously done with EGFP,10 Nalm-6 leukemia cells were transduced with pHR-CMV-EGFP and pHR-CD80 lentiviral vectors, and EGFP expression and CD80 expression were compared by using flow cytometry analysis (Figure2). The pHR-CD80 vector produced more than 90% CD80+ Nalm-6 cells (Figure 2B), comparable to the number of EGFP-positive cells resulting from the pHR-CMV-EGFP transduction (Figure 2A). To assess the stability of CD80 expression, transduced cells were maintained in culture for 10 days and FACS analysis was repeated. Expression of CD80 remained stable during this period, thus excluding the possibility that a pseudotransduction artifact was present. Therefore, CD80 detection in leukemia cells was used as a marker for gene-delivery efficiency.
CD80 expression is a suitable marker for gene-delivery assessment.
Nalm-6 cells were transduced for 24 hours with the pHR-CMV-EGFP or the pHR-CD80 lentiviral vector packaged in the vesicular stomatitis virus G envelope. Immunostaining of the CD80 molecule and flow cytometry analysis was performed 2 days and 10 days after transduction. Thin lines indicate mock transduction; and boldface lines, transduction with lentiviral vectors.
CD80 expression is a suitable marker for gene-delivery assessment.
Nalm-6 cells were transduced for 24 hours with the pHR-CMV-EGFP or the pHR-CD80 lentiviral vector packaged in the vesicular stomatitis virus G envelope. Immunostaining of the CD80 molecule and flow cytometry analysis was performed 2 days and 10 days after transduction. Thin lines indicate mock transduction; and boldface lines, transduction with lentiviral vectors.
Transduction of leukemia cell lines with lentiviral vectors expressing CD80 and GM-CSF
The correlation between viral titer and gene delivery was evaluated by titration of pHR-GM/CD lentiviral suspensions (ranging from 105-107 infective particles/mL) in transduction of the pre-B ALL cell line Nalm-6 and the AML cell line ML-1 (Table 1). Transduction efficiency and the level of expression of the transgenes depended on viral titer. At the highest titer evaluated (107 infective particles/mL, equivalent to a multiplicity of infection [MOI] of 10), more than 95% of the ALL and AML cells were transduced. At the 106titer (MOI of 1), 64.9% of Nalm-6 and 32.6% of ML-1 cells were transduced, whereas at the 105 titer (MOI of 0.1), CD80 expression was at baseline levels. CD80 expression (measured as the mean relative fluorescence of CD80+ cells) and GM-CSF coexpression (measured by ELISA) were also titer dependent. At the highest titer (107 infective particles/mL), GM-CSF production was 862 pg/106 cells per 24 hours and 419 pg/106 cells per 24 hours, respectively, for the Nalm-6 and ML-1 cell lines.
Expression of CD80 and GM-CSF by Nalm-6 and ML-1 cells transduced with pHR-GM/CD at various concentrations
| Infective particles/mL . | MOI . | Nalm-6 . | ML-1 . | ||||
|---|---|---|---|---|---|---|---|
| % CD80+ . | RMF . | GM-CSF* . | % CD80+ . | RMF . | GM-CSF* . | ||
| None (mock transduced) | 0.0 | 0.0 | 9.9 | 0 | 0.0 | 7.0 | 0 |
| 105 | 0.1 | 1.4 | 17.3 | 5 | 0.5 | 8.3 | 0 |
| 106 | 1.0 | 64.9 | 127.0 | 341 | 32.6 | 24.5 | 26 |
| 107 | 10.0 | 99.6 | 1147.4 | 862 | 98.3 | 131.2 | 419 |
| Infective particles/mL . | MOI . | Nalm-6 . | ML-1 . | ||||
|---|---|---|---|---|---|---|---|
| % CD80+ . | RMF . | GM-CSF* . | % CD80+ . | RMF . | GM-CSF* . | ||
| None (mock transduced) | 0.0 | 0.0 | 9.9 | 0 | 0.0 | 7.0 | 0 |
| 105 | 0.1 | 1.4 | 17.3 | 5 | 0.5 | 8.3 | 0 |
| 106 | 1.0 | 64.9 | 127.0 | 341 | 32.6 | 24.5 | 26 |
| 107 | 10.0 | 99.6 | 1147.4 | 862 | 98.3 | 131.2 | 419 |
GM-CSF indicates granulocyte-macrophage colony-stimulating factor; MOI, multiplicity of infection; and RMF, relative mean fluorescence for all cells analyzed.
Picograms per 106 cells per 24 hours.
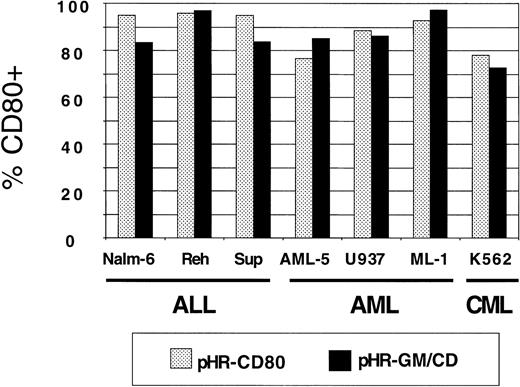
Panels of human lymphoid and myeloid leukemia lines were used for transduction with pHR-GM-CSF, pHR-CD80, and pHR-GM/CD vectors. Background CD80 and GM-CSF expression values from nontransduced (mock-transduced) cells were subtracted. The CD80 expression by transduced ALL (Nalm-6, Reh, and Sup-B15), AML (AML-5, U937, and ML-1), and CML (K562) cell lines is shown in Figure3. We observed high transduction efficiencies, ranging from 75% to 95%, for both the pHR-CD80 and the pHR-GM/CD vector.
CD80 expression in a panel of 7 human leukemia cell lines transduced with pHR-CD80 or pHR-GM/CD.
The percentage of CD80-positive (CD80+) cells in the fluorescence-activated cell-sorter analysis was calculated by subtracting the frequency of the transduced cells from the background (mock-transduced) controls.
CD80 expression in a panel of 7 human leukemia cell lines transduced with pHR-CD80 or pHR-GM/CD.
The percentage of CD80-positive (CD80+) cells in the fluorescence-activated cell-sorter analysis was calculated by subtracting the frequency of the transduced cells from the background (mock-transduced) controls.
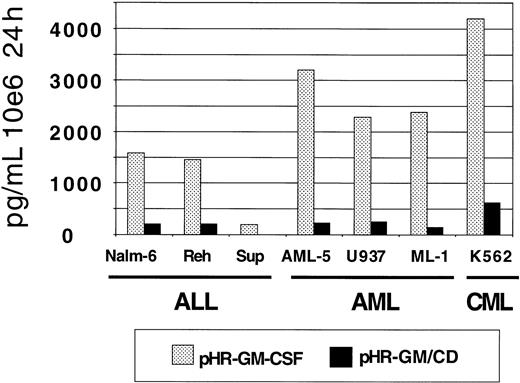
GM-CSF production by the transduced leukemia cell lines was measured by ELISA (Figure 4). The pHR-GM-CSF vector produced an average (± SD) of 2182.9 ± 1287.4 pg/106cells per 24 hours, whereas the pHR-GM/CD vector produced an average of 225.7 ± 193.1 pg/106 cells per 24 hours. A comparison between the human pre-B leukemia cell line (Nalm-6) and the human embryonic kidney fibroblast cell line 293 transduced with the pHR-GM/CD vector showed that the fibroblasts had GM-CSF production levels that were 5 to 10 times higher (data not shown).
GM-CSF expression in a panel of 7 human leukemia cell lines transduced with pHR-GM-CSF or pHR-GM/CD.
Final values were calculated by subtracting the enzyme-linked immunosorbent assay (ELISA) results for the mock-transduced controls from those for the transduced cells.
GM-CSF expression in a panel of 7 human leukemia cell lines transduced with pHR-GM-CSF or pHR-GM/CD.
Final values were calculated by subtracting the enzyme-linked immunosorbent assay (ELISA) results for the mock-transduced controls from those for the transduced cells.
Molecular analysis of lentivirus integration and messenger RNA (mRNA) expression
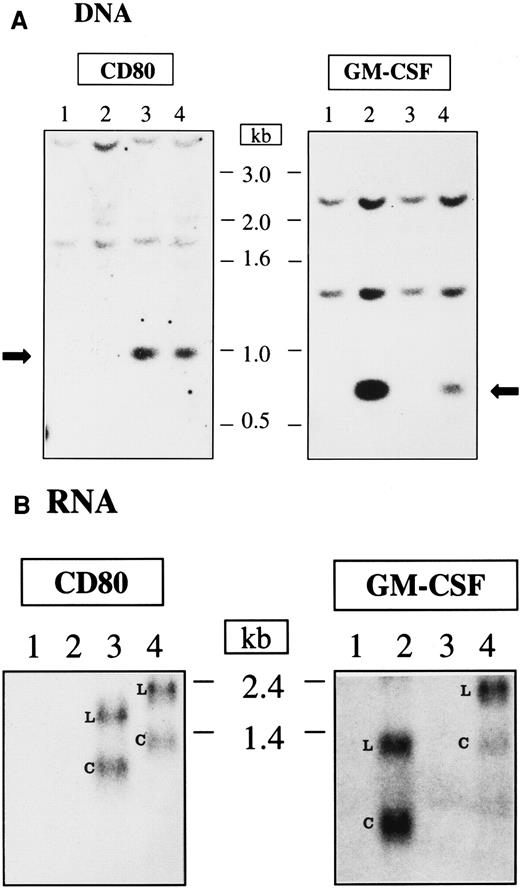
The integration and mRNA expression of CD80 and GM-CSF genes in transduced Nalm-6 cells were evaluated by Southern and Northern blot analysis, respectively (Figure 5A-B). Nontransduced Nalm-6 cells and Nalm-6 cells transduced with pHR-GM-CSF, pHR-CD80, or pHR-GM/CD vectors were maintained in culture for 8 days after transduction and harvested for DNA and RNA extraction. Genomic DNA was digested with restriction enzymes that cleave the DNA at the flanks of the CD80 and GM-CSF transgenes so that the cDNA integrity could be assessed by Southern blot analysis. The CD80 cDNA (approximately 0.9 kb) and the GM-CSF cDNA (approximately 0.6 kb) were readily detected in the cells transduced with the lentiviral vectors carrying the corresponding genes (Figure 5A). Higher-molecular-weight bands corresponding to fragments of genomic DNA that hybridized with the probes were also detected (Figure 5A).
DNA integration and RNA expression after lentiviral vector transduction of Nalm-6 cells.
(A) Southern blot analysis. The arrows indicate the integrated DNA originated from the transduction with lentiviral vectors. (B) Northern blot analysis; L indicates the LTR promoter–transcribed RNA; C, CMV promoter–transcribed RNA; 1, nontransduced cells; 2, cells transduced with pHR-GM-CSF; 3, cells transduced with pHR-CD80; and 4, cells transduced with pHR-GM/CD. The length of the DNA and RNA molecules is indicated in kilobases.
DNA integration and RNA expression after lentiviral vector transduction of Nalm-6 cells.
(A) Southern blot analysis. The arrows indicate the integrated DNA originated from the transduction with lentiviral vectors. (B) Northern blot analysis; L indicates the LTR promoter–transcribed RNA; C, CMV promoter–transcribed RNA; 1, nontransduced cells; 2, cells transduced with pHR-GM-CSF; 3, cells transduced with pHR-CD80; and 4, cells transduced with pHR-GM/CD. The length of the DNA and RNA molecules is indicated in kilobases.
The mRNA transcripts produced after Nalm-6 transduction with the lentiviral vectors were detected after Northern blotting and hybridization against CD80 or GM-CSF probes (Figure 5B). Two major RNA species were observed for each vector: longer transcripts (generated by transcription from the long terminal repeat [LTR] promoter) and shorter transcripts (generated by transcription from the internal CMV promoter). For the monocistronic mRNAs expressed from the pHR-CD80 and pHR-GM-CSF vectors, the transcripts derived from the CMV promoter were the most abundant RNA species (calculated ratios of CMV-derived transcripts to LTR-derived transcripts were 1.1 and 1.3 fold, respectively). However, reduced levels of mRNA transcribed from the CMV promoter were observed for the bicistronic mRNA expressed from the pHR-GM/CD vector (calculated ratios of CMV-derived transcripts to LTR-derived transcripts resulted in 0.7 fold for the hybridization against CD80 and 0.4 fold for the hybridization against GM-CSF), indicating lower processing, transport, or stability of this transcript.
Transduction of fresh primary leukemia cells with pHR-GM/CD promotes high CD80 expression and low GM-CSF secretion
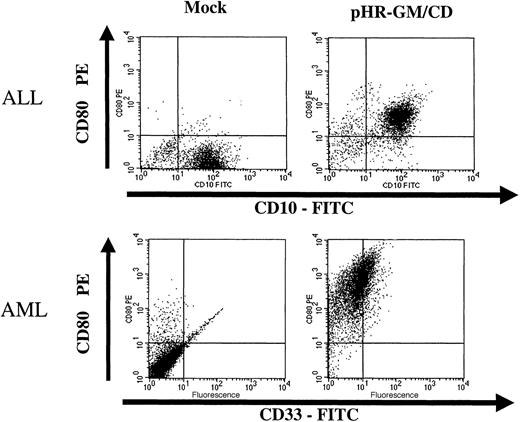
Fresh leukemia cells obtained from bone marrow aspirates were transduced with lentiviral vectors (Figure6). After cell-density separation, the mononuclear fraction was kept overnight in culture. The next day, the nonadherent cells were collected and transduced overnight with the pHR-GM/CD lentiviral vector. CD80 expression and GM-CSF expression were evaluated 48 hours after transduction. During the procedure, ALL and AML cells were maintained in AIM-V medium containing soluble CD40L (ALL cells) or IL-3 and SCF (AML cells). Under these conditions, we obtained good cell viability and more than 90% CD80+ marking in the transduced ALL and AML cells (Figure 6). Secreted GM-CSF levels were low, however, reaching only 11 pg/106 cells per 24 hours for ALL cells, and 137 pg/106 cells per 24 hours for AML cells.
Transduction of fresh acute lymphoblastic leukemia (ALL) cells and acute myeloid leukemia (AML) cells with pHR-GM/CD.
The panels on the left represent the nontransduced (mock-transduced) cells, and the panels on the right show the cells transduced with the pHR-GM/CD vector. Dot-plot analysis of the double staining for lineage markers (fluorescein isothiocyanate–conjugated anti-CD10 and anti-CD33) and transgene expression (phosphatidylethanolamine-conjugated anti-CD80) is shown. Isotype nonspecific control antibodies were used to set up the background levels.
Transduction of fresh acute lymphoblastic leukemia (ALL) cells and acute myeloid leukemia (AML) cells with pHR-GM/CD.
The panels on the left represent the nontransduced (mock-transduced) cells, and the panels on the right show the cells transduced with the pHR-GM/CD vector. Dot-plot analysis of the double staining for lineage markers (fluorescein isothiocyanate–conjugated anti-CD10 and anti-CD33) and transgene expression (phosphatidylethanolamine-conjugated anti-CD80) is shown. Isotype nonspecific control antibodies were used to set up the background levels.
pHR-GM/CD lentiviral vectors efficiently transduce cryopreserved primary ALL and AML cells
To evaluate a clinically applicable approach that uses cryopreserved leukemia cells for immunotherapy, transduction of frozen-thawed samples of leukemia cells was performed. We used cryopreserved bone marrow aspirates with a 70% or higher percentage of blasts that were obtained from pediatric patients with ALL or AML at diagnosis or relapse. The phenotypic features of each leukemia sample are shown in Table 2. After thawing, the ALL and AML samples were maintained in AIM-V medium and soluble CD40L (ALL sample) or IL-3 and SCF (AML sample). Under these conditions, we usually obtained 10% to 20% viability for ALL cells and more than 80% viability for AML cells, as assessed by trypan blue exclusion after 24 hours, with or without transduction. Cells were transduced with the pHR-GM/CD vector at a final viral concentration of 1 × 107 infective particles/mL (MOI of 10). CD80 expression and GM-CSF expression were assessed 48 hours after transduction by flow cytometry and ELISA, respectively. The reference cell lines Nalm-6 and ML-1 showed greater than 97% transduction (Figures 7 and8). The average (± SD) transduction efficiencies for 5 ALL and 5 AML samples from patients were 64.3% ± 23.1% and 77.6% ± 10.0%, respectively (Figures 7 and 8). GM-CSF expression was undetectable in transduced primary ALL cells, reflecting the low GM-CSF expression profile of the pHR-GM/CD vector and the poor viability of the cryopreserved ALL cells in culture. The average production of GM-CSF by transduced primary AML cells was 39.3 ± 36.2 pg/106 cells per 24 hours. Transduction with the lentiviral vectors did not produce signs of cytotoxicity or decreased cell viability during the 48 hours of culture after transduction.
Characteristics of ALL and AML samples and transduction efficiency
| Sample no. . | FAB . | Immunophenotype . | Cytogenetic findings . | % transduction . |
|---|---|---|---|---|
| ALL 1 | L2 | 45,19,10,20,13,34,38,TdT-positive | −14,+12p | 40.3 |
| ALL 2 | L2 | 45,19,10,20,HLA-DR,33,TdT-positive | bcr/abl-p210 | 90.3 |
| ALL 3 | L2 | 45,19,10,20,38,TdT-positive | t(1:19) | 63.9 |
| ALL 4 | L1 | 19,10,20,HLA-DR,34,38,TdT-positive | Normal | 84.5 |
| ALL 5 | L2 | 19,10,HLA-DR,34,38,TdT-positive | Not available | 42.6 |
| AML 1 | M4 | 13,33,34,TdT-negative | Not available | 75.4 |
| AML 2 | M2 | 13,33,34,HLA-DR,TdT-negative | t(6:9) | 61.7 |
| AML 3 | M4 | 13,33,HLA-DR,TdT-negative | t(8:21) | 87.6 |
| AML 4 | M4 | 33,4,TdT-negative | Not available | 84.2 |
| AML 5 | M4 | 33,65,TdT-negative | t(11:19) | 79.1 |
| Sample no. . | FAB . | Immunophenotype . | Cytogenetic findings . | % transduction . |
|---|---|---|---|---|
| ALL 1 | L2 | 45,19,10,20,13,34,38,TdT-positive | −14,+12p | 40.3 |
| ALL 2 | L2 | 45,19,10,20,HLA-DR,33,TdT-positive | bcr/abl-p210 | 90.3 |
| ALL 3 | L2 | 45,19,10,20,38,TdT-positive | t(1:19) | 63.9 |
| ALL 4 | L1 | 19,10,20,HLA-DR,34,38,TdT-positive | Normal | 84.5 |
| ALL 5 | L2 | 19,10,HLA-DR,34,38,TdT-positive | Not available | 42.6 |
| AML 1 | M4 | 13,33,34,TdT-negative | Not available | 75.4 |
| AML 2 | M2 | 13,33,34,HLA-DR,TdT-negative | t(6:9) | 61.7 |
| AML 3 | M4 | 13,33,HLA-DR,TdT-negative | t(8:21) | 87.6 |
| AML 4 | M4 | 33,4,TdT-negative | Not available | 84.2 |
| AML 5 | M4 | 33,65,TdT-negative | t(11:19) | 79.1 |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; and FAB, French-American-British leukemia classification.
pHR-GM/CD vector expression in transduced cryopreserved ALL cells.
(A) Nalm-6 reference cell line. (B-F) Cryopreserved primary ALL cells. The percentage of CD80+ cells was calculated by subtracting the transduced cells (boldface line) from the mock-transduced (thin line) control cells in the marker region.
pHR-GM/CD vector expression in transduced cryopreserved ALL cells.
(A) Nalm-6 reference cell line. (B-F) Cryopreserved primary ALL cells. The percentage of CD80+ cells was calculated by subtracting the transduced cells (boldface line) from the mock-transduced (thin line) control cells in the marker region.
pHR-GM/CD vector expression in transduced cryopreserved AML cells.
(A) ML-1 reference cell line. (B-F) Cryopreserved primary AML cells. The percentage of CD80+ cells was calculated by subtracting the transduced cells (boldface line) from the mock-transduced (thin line) control cells in the marker region.
pHR-GM/CD vector expression in transduced cryopreserved AML cells.
(A) ML-1 reference cell line. (B-F) Cryopreserved primary AML cells. The percentage of CD80+ cells was calculated by subtracting the transduced cells (boldface line) from the mock-transduced (thin line) control cells in the marker region.
Cotransduction of ALL and AML cells with the pHR-CD80 and pHR-GM-CSF vectors leads to higher levels of GM-CSF production than transduction with the bicistronic pHR-GM/CD vector
The low GM-CSF expression levels obtained after transduction of primary leukemia cells with the bicistronic pHR-GM/CD vector prompted us to investigate whether cotransduction of these cells with 2 separate vectors (pHR-CD80 and pHR-GM-CSF) would produce greater GM-CSF production. We therefore produced batches of pHR-CD80, pHR-GM-CSF, and pHR-GM/CD vectors and concentrated the supernatants by using ultracentrifugation. The HIV-1 gag p24 concentrations in the viral preparations were used as a biochemical reference to standardize the amounts of vector used for transductions. Thus, 100 ng/mL of p24 of the pHR-GM/CD bicistronic vector (approximately equivalent to 106 infective particles/mL, a MOI of 1) or a mixture containing 100 ng/mL of p24 of the pHR-CD80 and 100 ng/mL of p24 of the pHR-GM-CSF vectors (MOI of 1 for each) were used for transduction of leukemia cells (Figure 9). Forty-eight hours after transduction, transfer of the CD80 gene was evaluated by FACS.
Comparison of results of cotransduction of ALL (Nalm-6, ALL 1) and AML (ML-1, AML 1) leukemia cells with the separate pHR-CD80 and pHR-GM-CSF vectors and results with the bicistronic pHR-GM/CD vector.
(A) Frequency of CD80+ nontransduced (mock-transduced [M]) cells, cells transduced with pHR-GM-CSF and pHR-CD80 at a multiplicity of infection (MOI) of 1 for each (G+C), and cells transduced with the pHR-GM/CD vector at an MOI of 1. (B) Relative fluorescence of cells in the CD80+ gate. (C) GM-CSF expression measured as picograms per 106 cells per 24 hours.
Comparison of results of cotransduction of ALL (Nalm-6, ALL 1) and AML (ML-1, AML 1) leukemia cells with the separate pHR-CD80 and pHR-GM-CSF vectors and results with the bicistronic pHR-GM/CD vector.
(A) Frequency of CD80+ nontransduced (mock-transduced [M]) cells, cells transduced with pHR-GM-CSF and pHR-CD80 at a multiplicity of infection (MOI) of 1 for each (G+C), and cells transduced with the pHR-GM/CD vector at an MOI of 1. (B) Relative fluorescence of cells in the CD80+ gate. (C) GM-CSF expression measured as picograms per 106 cells per 24 hours.
The presence of CD80+ cells in the transduced ALL cell line (Nalm-6), cryopreserved primary ALL cells (ALL 1), AML cell line (ML-1), and cryopreserved primary AML cells (AML 1) did not differ significantly after cotransduction with pHR-GM-CSF and pHR-CD80 or with pHR-GM/CD (Figure 9A). The relative fluorescence emitted by the CD80+ cells was compared (Figure 9B). In ALL cells, the expression of CD80 after the cotransduction procedure was approximately 2-fold higher than after transduction with the bicistronic vector. The level of CD80 expression by transduced AML cells was lower overall than that by ALL cells, but there was no significant difference between transduction with pHR-CD80 and pHR-GM-CSF and transduction with pHR-GM/CD. Comparison of the GM-CSF production in the 4 cell types studied consistently showed at least a 6-fold higher expression for cotransduced cells than for cells transduced with the bicistronic vector (Figure 9C).
Autologous MLR and T-cell proliferation assay
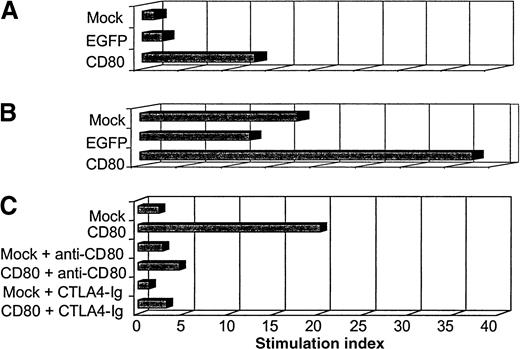
We performed functional experiments to evaluate the autologous T-cell response to transduced ALL cells expressing CD80. In the first experiment, we compared the T-cell stimulatory activity of ALL cells that were either nontransduced (mock), transduced to express a control transgene (EGFP), or transduced to express the immunomodulator CD80. Two days after transduction, irradiated (3200 cGy) primary ALL cells were coincubated with sorted autologous T cells in a primary MLR (small- and large-scale MLR). Five days later, the cells incubated in the small-scale MLR were labeled with tritium-thymidine and harvested a day later. The incorporated radioactivity was then measured.
The primary MLR showed that ALL-CD80 cells (but not ALL-mock or ALL-EGFP cells) stimulated significant T-cell proliferation (Figure10A). The larger primary MLR cultures were then restimulated with ALL-mock, ALL-EGFP, or ALL-CD80 cells. Five days after the secondary MLR, the effector cells were labeled with tritium-thymidine and harvested the next day. T cells stimulated with ALL-CD80 in the first MLR and restimulated with ALL-mock or ALL-EGFP cells maintained their proliferative potential, whereas T cells reprimed with ALL-CD80 cells showed a pronounced increase in their proliferation (Figure 10B).
Autologous mixed lymphocyte reaction (MLR) with primary ALL nontransduced (mock-transduced) cells, cells transduced with control pHR-CMV-EGFP (EGFP), or cells transduced with pHR-CD80 (CD80).
(A) Primary MLR. (B) Secondary MLR. (C) MLR performed in the presence of blocking antibody to CD80 or the fusion protein cytotoxic T-lymphocyte antigen 4–immunoglobulin.
Autologous mixed lymphocyte reaction (MLR) with primary ALL nontransduced (mock-transduced) cells, cells transduced with control pHR-CMV-EGFP (EGFP), or cells transduced with pHR-CD80 (CD80).
(A) Primary MLR. (B) Secondary MLR. (C) MLR performed in the presence of blocking antibody to CD80 or the fusion protein cytotoxic T-lymphocyte antigen 4–immunoglobulin.
In another experiment, which used paired autologous ALL and T cells from a second patient, we evaluated the function of CD80 by performing the MLR in the presence of anti-CD80 blocking antibody or the fusion protein CTLA4-Ig (which binds to CD80, thereby preventing its binding to CD28 on T cells). The findings of the previous MLR were reproduced: autologous T-cell proliferation was promoted by CD80+ ALL cells but not by nontransduced ALL cells (Figure 10C). This stimulatory effect was dramatically blocked by the anti-CD80 antibody or the CTLA4-Ig fusion protein (Figure 10C).
Discussion
In a murine model, we previously showed that coexpression of CD80 and GM-CSF by a leukemia cell vaccine elicits potent immunoreactivity against pre-established leukemia.9 To translate this finding to a clinical approach, we evaluated various vector systems for reporter gene delivery into human leukemia cells, and we found lentiviral vectors to be the best candidates among those studied.10 Here, we report the use of lentiviral vectors for the efficient delivery of 2 therapeutic genes, CD80 and GM-CSF, into human leukemia cell lines and primary leukemic blasts.
As shown in Figure 1, VSV-G–pseudotyped lentiviral vectors expressing CD80 (pHR-CD80) or GM-CSF (pHR-GM-CSF) and a bicistronic vector coexpressing CD80 and GM-CSF (pHR-GM/CD) were efficiently produced by transient cotransfection of 293T cells (106-107infective particles/mL). After transduction, CD80 gene delivery was followed by immunostaining and flow cytometry. ALL cells showed stable expression of CD80 for at least 10 days after transduction (Figure 2). Transduction of ALL and AML cell lines with different concentrations of pHR-GM/CD vector showed that the expression levels of CD80 and GM-CSF were dose dependent (Table 1).
Seven human leukemia cell lines (Nalm-6, Reh, Sup-B15, ML-1, U937, AML-5, and K562) were transduced with the 3 lentiviral vectors, and CD80 expression and GM-CSF expression were assessed by flow cytometry and ELISA, respectively. We observed comparable frequencies of CD80+ cells after transduction with the pHR-CD80 and pHR-GM/CD vectors (70%-95%; Figure 3). However, when we compared GM-CSF production from pHR-GM-CSF–transduced cells with that from pHR-GM/CD–transduced cells, we observed approximately 10-fold lower levels of GM-CSF production by the bicistronic vector (Figure 4).
To study this discrepancy, we investigated the integration pattern of the CD80 and GM-CSF cDNAs after transduction of Nalm-6 cells with the different vectors. Southern blot analysis showed that the full-length integrated cDNAs were detectable for all 3 vectors (Figure 5A), excluding the possibility of vector rearrangement. On the other hand, Northern blot analysis of the RNA transcripts expressed by the pHR-GM/CD vector showed lower amounts of the mRNA derived from the internal CMV promoter than of the mRNA derived from the LTR promoter (Figure 5B). This indicated that the bicistronic mRNA transcribed from the CMV promoter was less stable or less efficiently processed and transported to the cytoplasm.
The finding of low levels of GM-CSF production by leukemia cells transduced with the bicistronic pHR-GM/CD vector led us to evaluate whether cotransduction with 2 separate vectors, pHR-CD80 and pHR-GM-CSF, would lead to higher GM-CSF levels. This approach showed that the GM-CSF produced by the cotransduced cells reached moderate levels (120-4000 pg/106 cells per 24 hours) that were, on average, 6 times higher than the levels obtained after transduction with the bicistronic vector. The frequency of CD80+ cells and the levels of CD80 expression after the cotransduction approach were comparable or slightly superior than those after transduction with the pHR-GM/CD vector at equal MOIs.
It is questionable whether the amount of GM-CSF produced by the cotransduction approach would be sufficient to elicit an antileukemia effect in vivo. To address this issue, a systematic study of GM-CSF dosage as a variable in tumor cell vaccination in a murine melanoma M-3 model was conducted.26 Interestingly, immunity against tumor development was already high at moderate secretion levels (500 pg/106 cells per 24 hours to 10 ng/106cells per 24 hours) and reached a plateau at the highest obtainable GM-CSF production level (100-2000 ng/106 cells per 24 hours). These results indicate that high doses (> 10 ng/106 cells per 24 hours) of GM-CSF are not critical for generation of antitumor immune responses. On the other hand, dilution experiments that mixed varying numbers of transduced and nontransduced B16-F10 tumor cells showed that GM-CSF secretion below 36 ng/106 cells per 24 hours failed to generate the potent antitumor immunity observed at levels of secretion above this threshold.27 Dilution experiments with our murine BM185–CD80–GM-CSF leukemia model (expressing GM-CSF at the level of 80 ng/106 cells per 24 hours) also showed dose dependence on the ratio of transduced to nontransduced cells used for vaccination.9 Despite the lack of definitive information on this issue, the expression of GM-CSF or GM-CSF in combination with CD80 by the leukemia cells could possibly be optimized by using third-generation self-inactivating vectors (driving mRNA transcription solely from an internal promoter)28 that contain the woodchuck hepatitis virus post-transcriptional regulatory element to enhance RNA stability.29
Despite the low GM-CSF expression observed, we found that the lentiviral vectors consistently led to efficient gene transfer to primary ALL and AML cells without cytotoxic effects (Figures 6-8). These results are in agreement with findings of studies in which lentiviral vectors expressing the reporter gene EGFP were used to transduce ALL leukemia lines and primary ALL cells.10 Our initial reasons for evaluating lentiviral vectors were based on their previous success in transducing nonreplicating hematopoietic progenitor cells.15 Because primary leukemia cells replicate poorly in vitro, they are good candidates for lentiviral transduction. However, as we previously showed, other factors may also be involved, because leukemia cell lines that actively replicate in vitro are also preferentially transduced by lentiviral vectors rather than retroviral vectors.10 11
Vectors derived from herpes simplex viruses have shown a high efficiency of LacZ gene transfer into primary AML and ALL blasts (80%-100%).12 However, these vectors caused some degree of cytotoxicity, and expression declined 1 to 2 days after transduction.12 Transfection of AML blasts with a nonintegrating plasmid vector encoding CD80 showed only transient expression of CD80 on a small fraction of AML cells.14Thus, lentiviral vectors provide various advantages for production of a leukemia cell vaccine, including (1) consistent gene-delivery efficiency; (2) high levels of transgene expression (as shown here for CD80); (3) persistent expression; (4) no cytotoxic effects; and (5) ease of use (only one transduction cycle is required).
Studies in different animal models showed that the induction of CD80 expression on tumor cells is an efficient mechanism to increase their immunogenicity, leading to the rejection of established tumors and eventually conferring protection against tumor rechallenge.30-40 Here, we found that CD80-transduced leukemia cells induced proliferation of autologous leukemia-reactive T cells, which are not activated by nontransduced leukemia cells. The blockade of stimulation by either an anti-CD80 antibody or the fusion protein CTLA4-Ig showed clearly that this effect is mediated by CD80.
Clinical protocols for phase I studies of vaccination with autologous irradiated tumor cells engineered to secrete human GM-CSF have been developed.41-44 A phase I clinical trial using vaccination with melanoma cells expressing GM-CSF showed an intense infiltration of T lymphocytes, dendritic cells, macrophages, and eosinophils at the vaccination site and dense infiltration of T lymphocytes and plasma cells in metastatic lesions, associated with cytotoxic T cells and antibody responses.44 Protocols aimed at evaluating the antitumor responses generated from vaccination with autologous tumor cells engineered to express CD80 have also been described.45
To advance to clinical application of lentiviral vectors, more efficient and safer vectors will be needed.20,46,47Zufferey and colleagues28 produced a self-inactivating lentivirus vector with a 400-nucleotide deletion in the 3′ LTR that abolishes the LTR promoter activity and hampers recombination with wild-type HIV-1 in an infected host. This self-inactivating vector diminishes the concern regarding oncogenesis caused by promoter insertion and substantially alleviates the risk of vector mobilization with the wild-type virus, 2 important clinical considerations.
In conclusion, lentiviral vectors may be useful tools for immunotherapy of hematologic malignant diseases. Studies to optimize the expression of these vectors, improve their biosafety, and investigate the occurrence of replication-competent lentivirus after transduction will determine their potential use in future clinical trials.
Acknowledgments
We thank Dr Luigi Naldini, Dr Romain Zufferey, Dr Didier Trono, and Dr Inder M. Verma for providing us with the lentiviral vector system and useful technical advice and Lora W. Barsky and Monika Smogorzewska for technical assistance.
Supported by a translational grant from the Leukemia & Lymphoma Society to D.B.K. (LSA 6211-98) and a grant from the National Institutes of Health (PO1-CA68484) to L.M.N. D.B.K. is a recipient of an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation. R.S. is a recipient of a career development fellowship from Childrens Hospital Los Angeles and of a Special Fellow Award from the Leukemia & Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald B. Kohn, Childrens Hospital Los Angeles, Mailstop 62, 4650 Sunset Blvd, Los Angeles CA 90027; e-mail:dkohn@chla.usc.edu.



![Fig. 9. Comparison of results of cotransduction of ALL (Nalm-6, ALL 1) and AML (ML-1, AML 1) leukemia cells with the separate pHR-CD80 and pHR-GM-CSF vectors and results with the bicistronic pHR-GM/CD vector. / (A) Frequency of CD80+ nontransduced (mock-transduced [M]) cells, cells transduced with pHR-GM-CSF and pHR-CD80 at a multiplicity of infection (MOI) of 1 for each (G+C), and cells transduced with the pHR-GM/CD vector at an MOI of 1. (B) Relative fluorescence of cells in the CD80+ gate. (C) GM-CSF expression measured as picograms per 106 cells per 24 hours.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1317/4/m_h81600033009.jpeg?Expires=1769418222&Signature=Zak-T5HLkmgAQCccRx7L7iJDiQq~4RMyZVLTMfWwmIZ~nHhkqIFxgFBXRFlvUnPkncY2gqKWYFyAwiWtKq3~qquPIj~O3nTeraElxiikm-qNPGeRLyCaK8xVLcBeW0tWbTmwe~LZPTob49YXnkQc4yz50t3M4TidDuhRwfw1FmT0OpyR5E3O6UFYKhiEvcBkCD5ZhO~Q2EhfyVW4GsaV2vJB3ywLDGDRrZYRNdRuUpgpOmM6FrMmtZMukuacZ-qr0gYM91mqvsSGJXBn~AxdMs2ftqipeBiOUQcwf3DQKGUXo5n0Tnj11gkZa-Ip42zdCqqpQE-UO8EqfQzDR1AaUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal