Abstract
Resting lymphocytes are refractory to gene transfer using Moloney murine leukemia virus (MMLV)-based retroviral vectors because of their quiescent status. Recently, it has been shown that lentiviral vectors are capable of transferring genes into nondividing and terminally differentiated cells. We used human immunodeficiency virus type-1 (HIV-1)–based vectors expressing enhanced green fluorescent protein (EGFP) driven by different promoters (CMV, MPSV, or PGK) and investigated their ability to transduce human T- and B-cell lines, as well as resting or activated primary peripheral and umbilical cord blood lymphocytes. The effects of the presence or the absence of HIV-1 accessory proteins (Vif, Vpr, Vpu, and Nef) in the vector system were also assessed. Flow cytometry analysis showed no differences in the ability of these vectors of transferring the reporter gene into lymphocytic lines and mitogen-stimulated primary lymphocytes in the presence or the absence of HIV-1 accessory proteins (APs). Similarly, viral supernatants generated in the presence of accessory genes could efficiently transduce various subsets of resting lymphocytes and provide long-term expression of the transgene. No significant transduction-induced changes in cell activation or cycling status were observed and Alu-HIV-1 long terminal repeat polymerase chain reaction (LTR PCR) analysis demonstrated integration of the vector sequences at the molecular level. In contrast, in the absence of HIV-1 APs, lentiviral vectors failed to integrate and express the transgene in resting lymphocytes. These results show that transduction of primary resting lymphocytes with HIV-1–based vectors requires the presence of viral accessory proteins.
Introduction
Genetic alteration of peripheral blood T lymphocytes has been shown to have potential therapeutic applications for inherited and acquired immunodeficiencies.1-3 Similar strategies have also been proposed to deliver immunomodulatory cytokines for cancer therapy.4 Although Moloney murine leukemia virus (MMLV)-based retroviral vectors have been used successfully to transduce cells from various tissues, including cells of hematopoietic origin, transduction of primary lymphocytes has been limited primarily by the requirement that the cells be proliferating for stable retroviral integration. Potent induction of cell proliferation in vitro has been used to overcome this problem.1,5 In vitro culture, however, is known to alter the CD4+/CD8+ ratios and T-cell receptor (TCR) repertoire composition of T lymphocytes and may induce changes of their cytokine secretion profile.1,6 7Therefore, to preserve the functional integrity of the target lymphocytes, it would be desirable to avoid inducing cell division during the gene transfer procedures.
As an alternative approach to retroviral-mediated gene transfer, we explored the use of human immunodeficiency virus type-1 (HIV-1)–based lentiviral vectors. These vectors are attractive in that either proliferating or quiescent cells can be transduced and the sustained expression of the transgene can be achieved by the stable integration of vector into the host cell genome.8-11 These important advantages of lentiviral vectors derive from partial retention of wild-type HIV-1 biologic characteristics. However, whether HIV-1 is able to establish stable infection in primary quiescent lymphocytes remains controversial. Several in vitro reports have demonstrated that HIV-1 can readily enter quiescent T cells in culture and subsequently be induced to replicate after stimulation through antigenic or costimulatory processes.12,13 On the other hand, some investigators have shown that HIV-1 infection of quiescent T cells in vitro results in only partially reverse transcribed viral DNA that is labile and rapidly degraded,14,15 whereas others have reported the establishment of a complete infectious cycle with detectable production of early mRNA transcription.12 In support of these last findings, Ostrowski et al16 have recently demonstrated the presence of integrated HIV-1 provirus within naı̈ve CD45RA+/CD62L+/CD4+ T cells isolated from patients who are infected with HIV-1. In addition, replication-competent HIV-1 was isolated from the same cells after in vitro stimulation, suggesting that naı̈ve CD4+ T cells may be a reservoir for HIV-1. Similarly, Zhang et al17 have also recently shown productive infection and replication of SIV and HIV in resting T cells in rhesus monkeys and HIV-1–infected human subjects, respectively. Taken together, these studies suggest that, after HIV-1 entry, additional host factors must be provided concomitant with T-cell activation to permit productive replication and integration of HIV-1 DNA into the host genome.13,18 Part of the explanation for this complex virus-host interaction probably lies in the organization of the HIV-1 viral genome that encodes 6 regulatory proteins that are not found in other classes of retroviruses. Some of these products (Tat and Rev) are essential for HIV replication, whereas others (Vif, Vpr, Vpu, and Nef) appear to modulate the infection and replication processes in some nondividing cell types and have therefore been associated with HIV-1 virulence.19 20
Although wild-type HIV-1 infection of resting or activated primary lymphocytes has been studied extensively, the ability of replication-defective HIV-1–based gene transfer vectors to infecting these cell types has not been studied in detail. In this study, we have attempted to elucidate the importance of viral accessory proteins in HIV-1–based vector-mediated gene transfer into lymphocytic cells. As targets, we used immortalized T- and B-lymphoid cell lines, as well as well-characterized, highly purified resting and mitogen-stimulated human lymphocytes obtained from adult peripheral blood and umbilical cord blood samples. We have also examined the relative efficiencies of different promoters in driving long-term transgene expression in these cell types.
Materials and methods
Plasmid construction
The lentiviral vector plasmid pHR′CMV-LacZ8 was used as a gene transfer vector with the following modifications. The cytomegalovirus (CMV) early promoter was replaced with either the human phosphoglycerate kinase (PGK) promoter or the myeloproliferative sarcoma virus (MPSV) 3′ long terminal repeat (LTR) sequences isolated from the pMND-X-IRES-EGFP vector21 (gift of Dr D. B. Kohn) by polymerase chain reaction (PCR)-based amplification. The 3.1-kilobase (kb) LacZ complementary DNA (cDNA) was replaced with the cDNA of enhanced green fluorescent protein (EGFP) (Clonetech Laboratories, Palo Alto, CA). The packaging plasmids pCMVΔR8.2 (carrying all the HIV-1 accessory genes, vif, vpu, vpr,and nef)9 and pCMVΔR8.91 (devoid of all HIV-1 accessory genes)22 were used to express the HIV-1gag, pol, tat, and rev genes and thereby produce lentiviral structural and regulatory proteins. The plasmid pMD.G carrying the vesicular stomatitis virus envelope G protein (VSV-G) coding sequence driven by the CMV promoter and followed by the β-globin polyadenylation site8 was used to pseudotype the vector particles. The plasmids pHR′CMV-LacZ, pCMVΔR8.2, and pMD.G were kindly provided by Dr I. M. Verma of the Salk Institute, San Diego, CA, and the plasmid pCMVΔR8.91 was a gift from Dr D. Trono, Department of Genetics and Microbiology, CMU, Geneva, Switzerland.
Cell culture
The lymphoid cell lines (SupT1, Jurkat, and EBV-immortalized lymphoblastoid B cells) and the human embryonic kidney cell line, 293T were cultured at 37°C, 5% CO2 in RPMI 1640 and Dulbecco Modified Eagle's Medium (Gibco-BRL, Gaithersburg, MD), respectively, in the presence of 10% fetal bovine serum (FBS) (Gemini-Bioproducts, Inc, Calabasas, CA). Fresh peripheral blood (PB) samples were obtained from healthy, HIV-seronegative donors. Cord blood (CB) samples were obtained from placental and umbilical samples scheduled for discard at the Montgomery General Hospital. Mononuclear cells (MNCs) were obtained from blood samples by Ficoll gradient centrifugation (Organon Teknika Co, Durham, NC). Peripheral blood lymphocytes (PBLs) or cord blood lymphocytes (CBLs) were enriched from MNC fractions by a 2-step plastic adherence to remove contaminating adherent cells. Briefly, the MNCs were resuspended in RPMI 1640 containing 1% heat inactivated FBS, incubated on 30-mm diameter plastic dishes (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) at 37°C for 3 hours. The nonadherent cells were then collected and again incubated at 37°C for 12 to 20 hours to completely eliminate adherent cells. Purity of the isolated lymphocytes was more than 98% as determined by immunophenotyping and flow cytometry. To obtain mitogen-activated lymphocytes, PBLs or CBLs were cultured in the presence of 5 μg/mL of phytohemagglutinin (PHA) (Sigma Chemical Co, St Louis, MO) and 100 U/mL of recombinant human interleukin (rhIL)-2 (PeproTech, Norwood, MA) for 3 days and thereafter maintained in RPMI containing 10% FBS and 100 U/mL of rhIL-2.
Vector production and transductions
Replication-defective lentiviral particles pseudotyped with VSV-G envelope were produced by 3-plasmid transient transfection of 293T cells as previously described8 with 15 μg of one of the gene transfer constructs (pHR′CMV-EGFP, pHR′PGK-EGFP, or pHR′MPSV-EGFP), 10 μg of either pCMVΔR8.2 or pCMVΔR8.91, and 5 μg of pMD.G, using a calcium phosphate transfection kit (Gibco-BRL). The transfection medium was replaced after 12 hours with fresh culture medium. Viral supernatants were harvested at 65 hours after transfection and filtered through 0.45 μm filters (Nalgene, Rochester, NY). Supernatants were either used immediately for transductions or were aliquoted and stored frozen at −80°C until use. Titers of viral supernatants were determined by quantification of p24 gag by enzyme-linked immunosorbent assay (ELISA) (Coulter Diagnostics, Hialeah, FL), and also by transducing 293T cells with serial dilutions of viral supernatants, followed by flow cytometry analysis of EGFP-positive cells, 48 hours after transduction. It was assumed that 1 ng of p24 is approximately equivalent to 1000 to 5000 transducing units.23 All lentiviral vector preparations were tested for the presence of replication-competent lentivirus (RCL) by transducing 293T cells and assaying culture medium for the presence of p24 gag after at least 5 cell passages. No RCL was detectable in any of the vector preparations tested.
Transductions of lymphoid cells were performed on fibronectin-coated 6-well plates (Becton Dickinson, San Jose, CA) in the presence of 5 μg/mL polybrene (Sigma Chemical Co), at an multiplicity of infection (MOI) of approximately 5 to 10. Cells were exposed to viral supernatant at a density of 106 cells per well in 3 mL final volume. After 30 minutes of centrifugation at 800g at 32°C, transductions were allowed to proceed for additional 48 hours. After transduction, cells were washed 3 times with phosphate-buffered saline (PBS), trypsinized for 5 minutes to remove potential unadsorbed viral particles, and reincubated in fresh culture medium. In the case of resting lymphocytes, after transduction, cells were cultured either in the absence of stimulation for 10 days or stimulated with PHA (5 μg/mL) and rhIL-2 (100 U/mL) for 3 days, then cultured in the presence of rhIL-2 (100 U/mL) and stimulated with PHA every 2 weeks. Cell samples were harvested periodically for flow cytometry analysis of EGFP expression.
Flow cytometry
To assess the purity and activation status of the target lymphocyte population, 5 × 105 adherent cells-depleted, resting or mitogen-stimulated lymphocytes were stained with fluorescein isothiocyanate (FITC)- and/or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) (all from Becton Dickinson) for the lineage specific human markers CD3, CD4, CD8, CD19, CD13, CD14, and CD56, and the cell activation markers CD69, CD25, and CD71. Cells transduced under different conditions were stained with mAbs to the same cell surface markers on day 5 after transduction. Isotype FITC- and/or PE-conjugated control antibodies were used to assess specificity of immunostaining procedures and to set quadrant regions. After staining and washing, cells were fixed in 4% paraformaldehyde and 5000 to 10 000 events were acquired with a FACSCalibur and analyzed using the CellQuest software (Becton Dickinson).
Cell cycle analysis
Cell cycle status was monitored by measuring cellular DNA content using propidium iodide (PI) staining and flow cytometry analysis.24 Briefly, cell samples were obtained before and after transduction, washed in PBS, and resuspended in ice cold 80% ethanol. After additional washes with PBS, cells were treated with 1 mL of PBS containing 180 units of RNase A at 37°C for 30 minutes and subsequently stained with PBS containing 30 μg/mL of PI (Sigma Chemical). The cellular DNA content was then analyzed using a FACSCalibur. Data acquisition and analysis were performed with the CellQuest and ModFit LT softwares, respectively.
Assays for integrated HIV-1 vector in primary human lymphocytes
To demonstrate the integration of the HIV-1 vector in the transduced lymphocytes, genomic DNA was purified from both activated and resting primary lymphocytes 5 days after transduction and subjected to Alu-HIV-1 LTR PCR as described previously.25 Briefly, the first PCR was carried out using a sense oligomer specific for the conserved sequences of human Alu (5′-GGCACTTTGGGAGGCCAAGG-3′) and an antisense oligomer specific for the long terminal sequences (LTR) of HIV-1 (5′-TTTTCGCGAGCGGCCGCTGCTAGAGATTTTCCACACTG-3′). In all amplifications, 50 μL of reaction mixture containing 250 ng of genomic DNA, 10 mmol/L Tris-HCl, 50 mmol/L KCl, 0.2 mmol/L dNTPs, 1.5 mmol/L MgCl2, 25 pmol of each of the primers, and 2 units of platinum Taq DNA polymerase (Gibco-BRL) were used. After the first DNA denaturation and activation of the enzyme at 94°C for 3 minutes, 35 amplification cycles were performed consisting of denaturation for 1 minute at 94°C, annealing for 1 minute at 60°C, and extension for 2.5 minutes at 72°C. One aliquot (one-fiftieth) of the first PCR products was subjected to a second PCR amplification using the previously published nested primers, NI-2 5′ (5′-CACACACAAGGCTACTTCCCT-3′) and NI-2 3′ (5′-GCCACTCCCCAGTCCCGCCC-3′), resulting in the amplification of a 317-base pair (bp) portion of the HIV-1 LTR region. Twenty-nine amplification cycles were performed (30 seconds at 95°C, 30 seconds at 63°C, and 1 minute at 72°C) in a 50-μL PCR containing 10 mmol/L Tris-HCl, 50 mmol/L KCl, 0.2 mmol/L dNTPs, 1.25 mmol/L MgCl2, 25 pmol of each of the nested primers, and 2 units of Gold Taq DNA polymerase (Gibco-BRL). In control reactions, genomic DNA that had not been subjected to the first round of PCR was also amplified using the second PCR primers to exclude the presence of plasmid and/or residual nonintegrated vector DNA. PCR products were analyzed by gel electrophoresis, followed by Southern blot and hybridization by using 32P end-labeled oligonucleotide probes (5′-GGATGGTGCTTCAAGTTAGTACC-3′). To further confirm the specificity of the amplification product, direct automated sequencing of the PCR fragment was performed using the dideoxynucleotide method and the NI-2 5′ and NI-2 3′ primers.
Results
Multiply attenuated HIV-1 vectors efficiently transduce and stably express the transgene in lymphoid cell lines
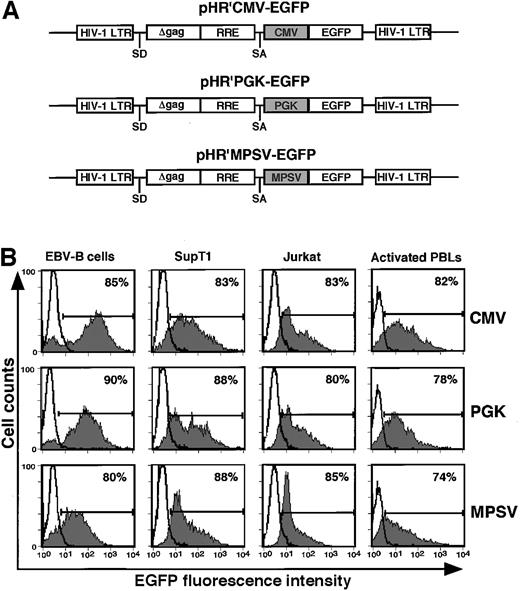
To investigate the role of HIV-1 accessory proteins (APs) in lentiviral-mediated gene transfer into actively proliferating transformed lymphoid cell lines, VSV-G pseudotyped lentiviral supernatants were generated using 2 different packaging constructs, pCMVΔR8.2 (providing expression of all APs)9 and pCMVΔR8.91 (lacking all accessory genes),22 and different gene transfer vectors carrying EGFP under the transcriptional control of 3 different promoters (Figure1A). Consistent with earlier reports,8 22 pseudotyping of the pHR′-based vector was highly efficient, even in the absence of accessory genes, with titers of the unconcentrated virus measured on 293T cells reaching 5 to 10 × 106 transducing units per milliliter. We used FACS analysis to test in parallel the transduction efficiency of these vectors on rapidly proliferating T-cell lines, SupT1 and Jurkat, as well as EBV-lymphoblastoid B cells. The stability of reporter gene expression in the transduced cells was followed for at least 6 months after transduction in in vitro culture. As shown in Table 1, lentiviral particles generated using the pCMVΔR8.91 packaging construct transduced lymphoid cell lines as efficiently as virus stocks that had been prepared using the pCMVΔR8.2 construct. EGFP expression was very stable, as determined by the high percentage (more than or equal to 70%) of cells showing sustained expression of EGFP for 4 months after transduction (Figure 1B) regardless of the promoters used (CMV, PGK, or MPSV). Taken together, these results indicate that APs are dispensable for transduction of actively proliferating T- and B-cell lines, and that all 3 promoters studied were similarly active in these cells.
Schematic representation of HIV-1–based EGFP expression vectors.
(A) pHR′CMV-EGFP, pHR′PGK-EGFP, and pHR′MPSV-EGFP are HIV-1–based gene transfer vectors generated from pHR'CMV-LacZ,8 in which the CMV promoter was replaced with either PGK or MPSV promoters, and the transgene LacZ was replaced with EGFP, as described in “Materials and methods.” (B) Efficacy of different promoters in driving long-term transgene expression in lymphoid cell lines and activated primary PBLs after HIV-1 vector-mediated gene transfer. Cells were transduced with VSV-G peudotyped, accessory proteins deleted pHR′ vectors carrying EGFP driven by 3 different promoters (CMV, PGK, and MPSV) at an MOI of 5 to 10. Transduced lymphoid cell lines and primary activated human PBLs were cultured in vitro for 4 months and 2 weeks, respectively, before FACS analysis. Fluorescence of untransduced cells is represented by open histograms, whereas the shaded gray areas represent EGFP fluorescence derived from transduced cells. Data shown are representative of similar results obtained from 2 independent experiments.
Schematic representation of HIV-1–based EGFP expression vectors.
(A) pHR′CMV-EGFP, pHR′PGK-EGFP, and pHR′MPSV-EGFP are HIV-1–based gene transfer vectors generated from pHR'CMV-LacZ,8 in which the CMV promoter was replaced with either PGK or MPSV promoters, and the transgene LacZ was replaced with EGFP, as described in “Materials and methods.” (B) Efficacy of different promoters in driving long-term transgene expression in lymphoid cell lines and activated primary PBLs after HIV-1 vector-mediated gene transfer. Cells were transduced with VSV-G peudotyped, accessory proteins deleted pHR′ vectors carrying EGFP driven by 3 different promoters (CMV, PGK, and MPSV) at an MOI of 5 to 10. Transduced lymphoid cell lines and primary activated human PBLs were cultured in vitro for 4 months and 2 weeks, respectively, before FACS analysis. Fluorescence of untransduced cells is represented by open histograms, whereas the shaded gray areas represent EGFP fluorescence derived from transduced cells. Data shown are representative of similar results obtained from 2 independent experiments.
HIV-1 vectors do not require accessory proteins for efficient transduction of lymphoid cell lines
| . | Percentage of EGFP-positive cells . | ||
|---|---|---|---|
| SupT-1 . | Jurkat . | EBV-B cells . | |
| +APs | 87.0 ± 4.3 | 81.7 ± 2.3 | 90.3 ± 3.2 |
| ΔAPs | 82.6 ± 4.4 | 76.2 ± 4.0 | 89.0 ± 4.2 |
| . | Percentage of EGFP-positive cells . | ||
|---|---|---|---|
| SupT-1 . | Jurkat . | EBV-B cells . | |
| +APs | 87.0 ± 4.3 | 81.7 ± 2.3 | 90.3 ± 3.2 |
| ΔAPs | 82.6 ± 4.4 | 76.2 ± 4.0 | 89.0 ± 4.2 |
Cells were transduced with VSV-G pseudotyped pHR′GK-EGFP vector generated in the presence (+) or the absence (Δ) of HIV-1 accessory proteins and at an MOI of 5 to 10. Stable transgene expression was determined by FACS analysis of EGFP positivity in cells cultured for at least 6 months. Data shown are mean ± SE obtained from 5 independent experiments for SupT-1 and EBV-B cells, and replicate assays using Jurkat cells.
Transduction of mitogen-stimulated primary lymphocytes does not require the presence of accessory proteins
To determine the relative efficiency of the HIV-1–vector pseudotypes to infect and stably express the transgene in mitogen-activated primary human lymphocytes in the presence or absence of APs, lymphocytes were prepared from 5 different PB and 2 CB samples obtained from healthy donors and stimulated with PHA and rhIL-2 for 3 days. Immunophenotyping and FACS analysis data were obtained from 3 PB samples and showed that 95.7% ± 3.8% (mean ± SE) of the cells were CD3+ of which 65.0% ± 2.9% and 35.0% ± 2.9% were CD4+ and CD8+ cells, respectively. The majority of cells were positive for the expression of cellular activation markers CD25 (96.8% ± 1.4%) and CD71 (89.0% ± 1.7%). Cell cycle analysis by PI staining showed that 30.0% ± 0.9% of the cells was in S-phase, demonstrating active proliferation, as expected. These cells were exposed to pHR′PGK-EGFP lentiviral vector produced in the presence or the absence of APs and analyzed for EGFP expression by FACS 3 days after transduction and periodically thereafter for up to 4 months. Similarly to the lymphoid cell lines, we observed no differences between the promoters CMV, PGK, and MPSV in driving long-term transgene expression in activated primary lymphocytes (Figure 1B). On average, the percentage of EGFP-positive cells at 3 days after transduction was more than 90%, regardless of the presence or the absence of APs in the vector preparation (Table 2). The average number of EGFP-positive cells decreased to approximately 70% during the following 2 weeks in culture (Table 2 and Figure 1B) and gradually declined to approximately 25% at 4 months after transduction. Similar results were obtained with activated CBLs transduced with the same vectors (Table 2).
Relative gene transfer efficiencies and long-term transgene expression in mitogen-stimulated and resting primary human lymphocytes transduced with HIV-1-based vectors
| Cell sample . | . | APs . | Percentage of EGFP-positive cells after culture . | ||||
|---|---|---|---|---|---|---|---|
| 3 days . | 2 wk . | 4 wk . | 8 wk . | 16 wk . | |||
| PBLs* | Stimulated | + | 96.4 ± 1.0 | 70.6 ± 1.8 | 53.8 ± 1.8 | 38.8 ± 2.4 | 26.7 ± 2.9† |
| Δ | 93.0 ± 1.3 | 71.2 ± 0.6 | 45.6 ± 2.0 | 33.0 ± 1.7 | 25.3 ± 2.7† | ||
| Resting | + | 90.2 ± 1.1 | 69.0 ± 3.2 | 49.8 ± 1.3 | 34.6 ± 1.9 | 22.3 ± 0.7† | |
| Δ | 4.8 ± 0.7 | 3.6 ± 0.7 | 3.2 ± 0.4 | 2.5 ± 0.4 | 2.3 ± 0.3† | ||
| CBLs‡ | Stimulated | + | 89.0 ± 2.0 | 71.0 ± 3.0 | 53.0 ± 4.5 | 35.0 ± 0.1 | 29.02-153 |
| Δ | 89.0 ± 1.5 | 71.0 ± 0.5 | 43.0 ± 5.0 | 26.0 ± 1.5 | 23.02-153 | ||
| Resting | + | 86.0 ± 6.5 | 65.0 ± 4.0 | 43.0 ± 7.0 | 33.0 ± 6.0 | 18.02-153 | |
| Δ | 5.0 ± 2.0 | 4.0 ± 2.0 | 3.5 ± 1.5 | 2.5 ± 1.5 | ND | ||
| Cell sample . | . | APs . | Percentage of EGFP-positive cells after culture . | ||||
|---|---|---|---|---|---|---|---|
| 3 days . | 2 wk . | 4 wk . | 8 wk . | 16 wk . | |||
| PBLs* | Stimulated | + | 96.4 ± 1.0 | 70.6 ± 1.8 | 53.8 ± 1.8 | 38.8 ± 2.4 | 26.7 ± 2.9† |
| Δ | 93.0 ± 1.3 | 71.2 ± 0.6 | 45.6 ± 2.0 | 33.0 ± 1.7 | 25.3 ± 2.7† | ||
| Resting | + | 90.2 ± 1.1 | 69.0 ± 3.2 | 49.8 ± 1.3 | 34.6 ± 1.9 | 22.3 ± 0.7† | |
| Δ | 4.8 ± 0.7 | 3.6 ± 0.7 | 3.2 ± 0.4 | 2.5 ± 0.4 | 2.3 ± 0.3† | ||
| CBLs‡ | Stimulated | + | 89.0 ± 2.0 | 71.0 ± 3.0 | 53.0 ± 4.5 | 35.0 ± 0.1 | 29.02-153 |
| Δ | 89.0 ± 1.5 | 71.0 ± 0.5 | 43.0 ± 5.0 | 26.0 ± 1.5 | 23.02-153 | ||
| Resting | + | 86.0 ± 6.5 | 65.0 ± 4.0 | 43.0 ± 7.0 | 33.0 ± 6.0 | 18.02-153 | |
| Δ | 5.0 ± 2.0 | 4.0 ± 2.0 | 3.5 ± 1.5 | 2.5 ± 1.5 | ND | ||
Primary human lymphocytes isolated from PB and CB samples were either left unstimulated or stimulated with PHA and rhIL-2 for 3 days and then transduced with VSV-G pseudotyped pHR′PGK-EGFP viral supernatants generated in the presence (+) or the absence (Δ) of HIV-1 accessory proteins (APs) at an MOI of 5 to 10. After transduction, the resting lymphocytes were stimulated with mitogens and cultured in vitro in medium containing rhIL-2 and periodically stimulated with PHA. The number of EGFP-positive cells (mean ± SE) was determined at indicated time points by FACS analysis.
Values obtained from 5 samples unless otherwise noted.
Values obtained from 3 samples.
Values obtained from 2 samples, unless otherwise noted.
Data obtained from one sample.
ND = not done.
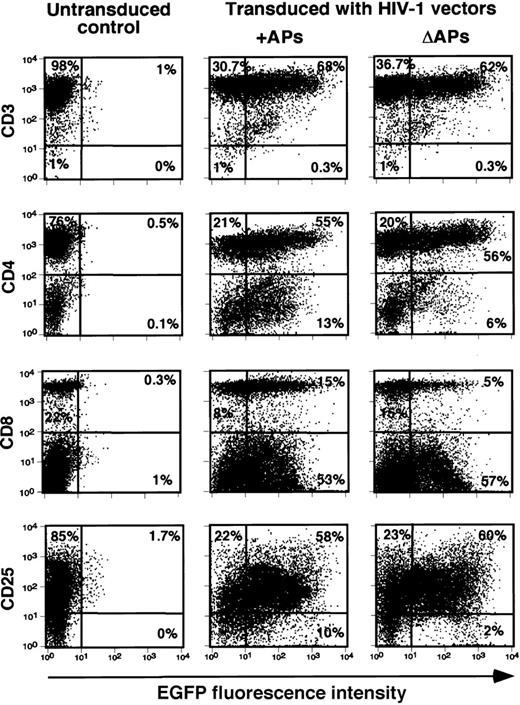
We next assessed the ability of the same vectors to transduce different subsets of mitogen-stimulated lymphocytes. Representative results with activated PBLs transduced with pHR′PGK-EGFP vectors are shown in Figure2. Analysis of EGFP expression 5 days after transduction consistently demonstrated more than or equal to 60% gene transfer efficiency in activated CD3+ lymphocytes, the majority of which were also highly positive for activation markers CD25. Interestingly, the number of EGFP-positive CD8+lymphocytes was reduced when cells were transduced by vectors lacking APs, whereas no clear differences were noted for CD4+cells. Similar results were observed in a second independent experiment with cells from different donors (data not shown). These results show that, with the possible exception of CD8+ T cells, vector stocks produced using packaging constructs devoid of APs encoding regions were as efficient as the corresponding stocks containing APs in transducing activated lymphocytes.
Efficient gene transfer and transgene expression by HIV-1 vectors in activated human lymphocyte subsets.
Transduction of PHA/rhIL-2–stimulated lymphocytes was carried out as described in “Materials and methods.” Relative gene transfer efficiency in T-cell subsets in the presence (+) or the absence (Δ) of APs were determined by analyzing the number of EGFP positive cells 5 days after transduction by FACS; cells were stained with the indicated PE-conjugated mAbs. Data shown are representative of similar data obtained from 2 independent experiments.
Efficient gene transfer and transgene expression by HIV-1 vectors in activated human lymphocyte subsets.
Transduction of PHA/rhIL-2–stimulated lymphocytes was carried out as described in “Materials and methods.” Relative gene transfer efficiency in T-cell subsets in the presence (+) or the absence (Δ) of APs were determined by analyzing the number of EGFP positive cells 5 days after transduction by FACS; cells were stained with the indicated PE-conjugated mAbs. Data shown are representative of similar data obtained from 2 independent experiments.
HIV-1 accessory proteins are required for lentiviral-mediated gene transfer into resting primary lymphocytes
Purified lymphocytes were prepared from 5 PB samples and 2 CB samples from different healthy donors. The immunophenotypes of unstimulated, adherent cells-depleted PBLs were assessed by immunostaining of 3 PB samples for the cell surface markers CD3, CD4, CD8, CD19, CD56, CD13, and CD14. The resting status of isolated cells was determined by analyzing the expression of different cell surface activation markers (CD69, CD25, and CD71), and by PI staining. After 2 successive depletion steps by plastic adherence, the primary lymphocyte fraction (more than 99% purity) contained approximately 90.0% ± 1.7% CD3+ cells (which included 59.0% ± 4.9% CD4+ and 28.3% ± 4.9% CD8+ cells), 2.4% ± 0.5% CD56+ natural killer (NK) cells, and 10.0% ± 3.3% CD19+ B cells. Only very small numbers (less than 1%) of cells expressing the CD13 and/or CD14 monocyte/macrophage specific molecules could be detected. Although very few cells showed weak staining for some cell activation markers CD69 (1.8% ± 0.6%), CD25 (2.9% ± 0.9%), and CD71 (3.0% ± 1.0%), cell cycle analysis by PI staining showed that the majority of the cells (98.8% ± 0.4%) were in the G0/G1 phase. In all our experiments, the unstimulated cells could be maintained in a stable resting state (G0/G1, activation marker mostly negative) during cell culture for up to 10 days.
Unstimulated cells were transduced with VSV-G pseudotyped vector particles generated in the presence or the absence of APs. The results presented in Table 2 show that G0/G1lymphocytes were successfully transduced with pHR′PGK-EGFP vector stocks carrying all APs. The mean percentage of EGFP-expressing cells 3 days after transduction (90.2% ± 1.1%) was comparable to that observed in mitogen-activated lymphocytes transduced with similar vector preparation. Further, stable expression of EGFP was observed for up to 4 months in culture, in a considerable fraction of the cells that were stimulated with PHA and rhIL-2 after transduction (Table 2). In contrast, vector stocks assembled from APs negative packaging construct did not efficiently transduce resting lymphocytes as shown by the extremely low percentage of EGFP positive cells (4.8% ± 0.7%) at 3 days after transduction. In addition, no significant change in EGFP expression could be observed with time after stimulation with PHA and rhIL-2 (Table 2). These results indicate that effective transduction of unstimulated lymphocytes requires lentiviral vector preparations produced in the presence of APs. Similar results were obtained using the pHR′CMV-EGFP vector (data not shown). Cells kept in culture without mitogen stimulation for 10 days after HIV-1–vector transduction showed approximately 65% of EGFP positive cells if transduced with APs containing vectors, but only approximately 2% if APs negative vectors were used.
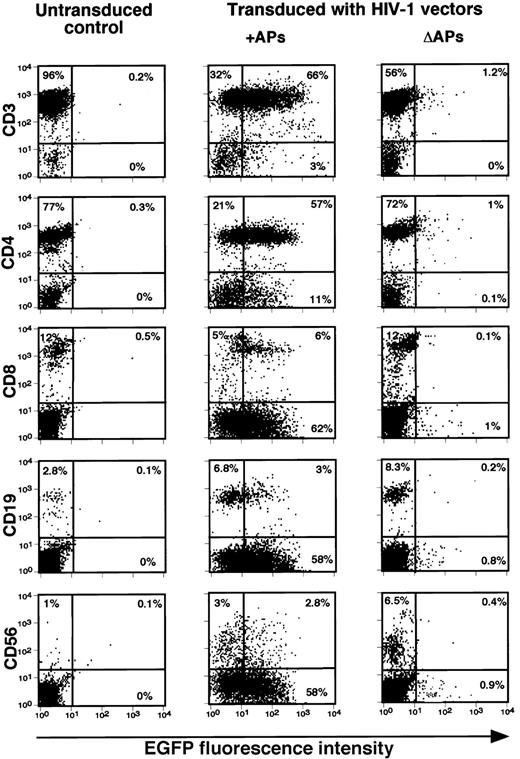
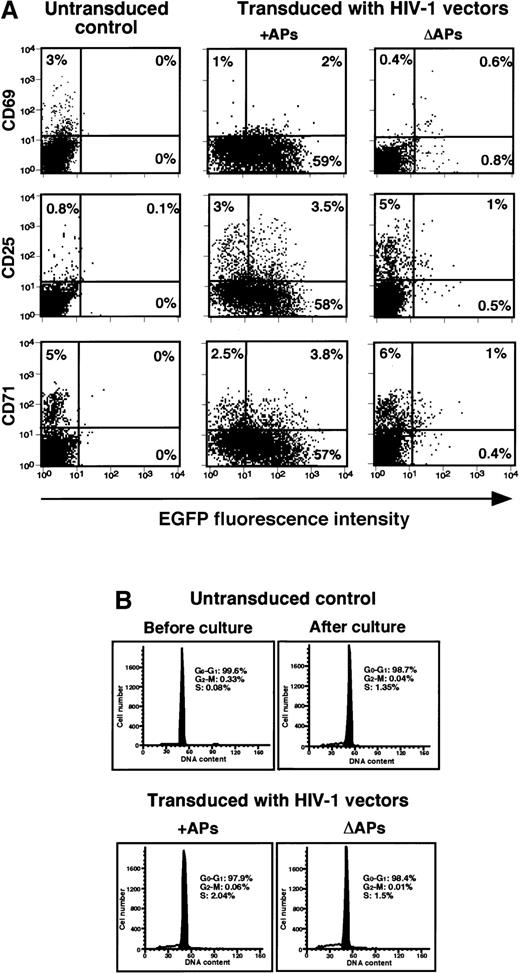
Finally, we examined the efficiency of these vectors in transducing and expressing EGFP in different subsets of unstimulated, resting lymphocytes. The results of a representative experiment with PBLs transduced with pHR′PGK-EGFP vectors and analyzed at 5 days after transduction are presented in Figure 3. CD4+ and CD8+ subsets of T cells, CD56+ NK cells, as well as CD19+ B cells, could be successfully transduced using vector pseudotypes containing APs, whereas the vector lacking APs failed to transduce these cells. To further define whether the transduction procedure-induced cellular activation was responsible for effective gene transfer, combined analysis of EGFP expression and cell surface activation markers by immunostaining or cell cycle analysis by PI staining was performed in transduced resting lymphocytes at 5 days after transduction. The results presented in Figure 4A indicate that a high-level expression of EGFP could be detected in majority of the cells, which were negative for the expression of activation markers, CD69, CD25, or CD71. Although a small increase (2%-4%) was observed in the number of cells expressing CD69, CD25, and CD71 among the transduced populations, irrespective of the vectors used (Figure4A), no significant differences in their cell cycle status could be detected (Figure 4B). These data suggest that the transduction procedure itself did not induce significant amount of cellular activation signals that could account for the successful transduction of resting lymphocytes by the lentiviral vector carrying APs.
HIV-1 vector-mediated gene transfer into various subsets of resting human lymphocytes.
Transduction of resting lymphocytes was carried out as described in “Materials and methods.” Relative gene transfer efficiencies of HIV-1 vectors generated in the presence (+) or the absence (Δ) of HIV-1 APs in lymphocyte subsets were determined by analyzing the number of EGFP positive cells 5 days after transduction and by staining with the indicated PE-conjugated mAbs. Data shown are from 1 of 2 independent experiments generating similar results.
HIV-1 vector-mediated gene transfer into various subsets of resting human lymphocytes.
Transduction of resting lymphocytes was carried out as described in “Materials and methods.” Relative gene transfer efficiencies of HIV-1 vectors generated in the presence (+) or the absence (Δ) of HIV-1 APs in lymphocyte subsets were determined by analyzing the number of EGFP positive cells 5 days after transduction and by staining with the indicated PE-conjugated mAbs. Data shown are from 1 of 2 independent experiments generating similar results.
HIV-1 vector-mediated transduction does not alter the activation status or cell cycle progression of resting human PBLs.
(A) Relative gene transfer efficiencies of HIV-1 vectors generated in the presence (+) or the absence (Δ) of HIV-1 APs as determined by dual analysis of EGFP and activation marker expression by FACS. Cells were analyzed 5 days after transduction. Data shown are representative of 2 independent experiments. (B) Cell cycle status of adherent cells- depleted primary human PBLs before and 5 days after transduction with HIV-1-based vectors in the presence (+) or the absence (Δ) of HIV-1 APs by PI staining and flow cytometry. Percentages of cells residing in each phase of the cell cycle are indicated.
HIV-1 vector-mediated transduction does not alter the activation status or cell cycle progression of resting human PBLs.
(A) Relative gene transfer efficiencies of HIV-1 vectors generated in the presence (+) or the absence (Δ) of HIV-1 APs as determined by dual analysis of EGFP and activation marker expression by FACS. Cells were analyzed 5 days after transduction. Data shown are representative of 2 independent experiments. (B) Cell cycle status of adherent cells- depleted primary human PBLs before and 5 days after transduction with HIV-1-based vectors in the presence (+) or the absence (Δ) of HIV-1 APs by PI staining and flow cytometry. Percentages of cells residing in each phase of the cell cycle are indicated.
Detection of integrated HIV-1 vector DNA in primary human lymphocytes transduced with HIV-1 vectors
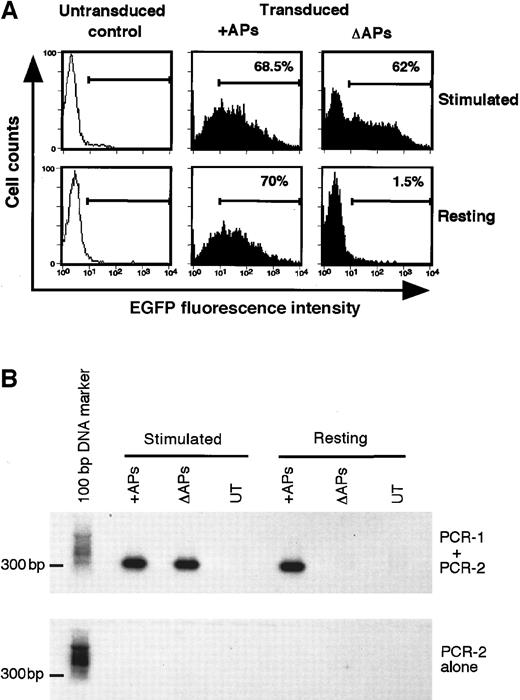
Although long-term EGFP expression is most likely attributable to vector integration, the transgene expression observed at 5 days after transduction could be due to pseudotransduction. To demonstrate vector integration at this early time points, genomic DNA extracted from transduced lymphocytes was analyzed using a previously described Alu-HIV-1 LTR PCR detection method.25 Alu regions are repetitive sequences scattered throughout the human genomic DNA and it is therefore likely that an integrated HIV-1 vector sequence would lie in the proximity of one such a region. Primers designed in the human Alu and HIV-1 LTR sequences allow amplification of the junctional fragments overlapping the integration of the HIV-1 vector in the host genomic DNA. As HIV-1 vectors can integrate in different sites within the same cell and among different cells, these PCR products are expected to have variable sizes and therefore to generate a “diffused” (smeared) signal after Southern blot and probing for HIV-1 LTR specific sequences. The second round of PCR using nested primers within the HIV-1 LTR results in the generation of a unique amplification product only from cells containing the Alu-HIV-1 LTR junctional fragment, which can be easily detected by Southern blotting. As shown in Figure 5, the expression of EGFP correlated with a detectable Alu-HIV-1 LTR PCR products, thus supporting the conclusion that EGFP expression detectable in transduced lymphocytes was the result of vector integration. In contrast, no PCR product could be detected in resting lymphocytes transduced with HIV-1 vectors devoid of APs, indicating that in the absence of APs no vector integration had taken place in these cells.
Analysis of integrated HIV-1 vector DNA in primary human lymphocytes.
(A) FACS analysis of EGFP expression in mitogen-stimulated and resting primary human PBLs transduced with pHR′PGK-EGFP vector in the presence (+) or the absence (Δ) of HIV-1 accessory proteins (APs) at 5 days after transduction. (B) Detection of integrated HIV-1 provirus by using Alu-HIV-1 LTR PCR. Genomic DNA isolated from mitogen-stimulated and resting primary human PBLs, untransduced (UT) or transduced with pHR′PGK-EGFP vector in the presence (+) or the absence (Δ) of HIV-1 accessory proteins (APs) at 5 days after transduction shown in Figure5A was subjected to Alu-HIV-1 LTR PCR procedure (PCR-1 + PCR-2) or only to the second round of amplification (PCR-2 alone). The amplification products were resolved in an agarose gel and bands were detected by Southern blot analysis by using an LTR-specific probe.
Analysis of integrated HIV-1 vector DNA in primary human lymphocytes.
(A) FACS analysis of EGFP expression in mitogen-stimulated and resting primary human PBLs transduced with pHR′PGK-EGFP vector in the presence (+) or the absence (Δ) of HIV-1 accessory proteins (APs) at 5 days after transduction. (B) Detection of integrated HIV-1 provirus by using Alu-HIV-1 LTR PCR. Genomic DNA isolated from mitogen-stimulated and resting primary human PBLs, untransduced (UT) or transduced with pHR′PGK-EGFP vector in the presence (+) or the absence (Δ) of HIV-1 accessory proteins (APs) at 5 days after transduction shown in Figure5A was subjected to Alu-HIV-1 LTR PCR procedure (PCR-1 + PCR-2) or only to the second round of amplification (PCR-2 alone). The amplification products were resolved in an agarose gel and bands were detected by Southern blot analysis by using an LTR-specific probe.
Discussion
Introduction of genes into primary resting lymphocytes may have applications in clinical gene therapy approaches where it is not desirable to induce cell proliferation. In contrast to onco-retroviruses that have provided the basis for most gene transfer vectors currently used for clinical applications, HIV-1 has the capacity to infect nondividing cells. However, differences exist in the relative ability of gene transfer vectors based on HIV-1 to integrate into the host DNA of different types of nondividing cells. In particular, some of the HIV-1 accessory genes appear to be required for efficient lentiviral vector-mediated gene transfer into specific tissues, as it is the case of hepatocytes where Vif and Vpr seem to be necessary.26 The molecular details of the HIV-1 infection of primary resting lymphocytes is now fairly well understood; however, little is known about the requirements for efficient transduction of these cells by HIV-1–based recombinant vector pseudotypes. Recently, Unutmaz et al27 have reported that an HIV-1 vector system pseudotyped with VSV-G similar to the APs defective pseudotypes described here, failed to transduce resting lymphocytes without cytokine prestimulation.27
Our results demonstrate that replication-defective, VSV-G pseudotyped HIV-1 vectors could efficiently transfer and stably express the EGFP transgene in lymphoid cell lines and various subsets of mitogen-stimulated and resting primary human lymphocytes. By comparing the ability of vectors produced in the presence or the absence of the 4 accessory genes, we found no significant differences in their ability to transduce T and B lymphoblastoid cells or actively proliferating primary lymphocytes. However, when the same vector preparations were used to transduce resting lymphocytes (residing in G0/G1 phase of cell cycle and negative for activation markers), striking differences were observed. In line with the results of Unutmaz et al,27 our data demonstrate that lentiviral vectors generated in the absence of APs were severely impaired in their ability to transduce these cells and that stimulation with PHA plus rhIL-2 after transduction did not increase the gene transfer efficiency (Table 2 and Figure 3). Interestingly, however, viral supernatants packaged in the presence of all APs could efficiently transduce resting T, NK, and B cells. EGFP expression was observed in significant proportions of cells up to 10 days after transduction in the absence of stimulation, and up to 4 months when the same cells were maintained in culture by periodical stimulation with PHA and rhIL-2. In addition to long-term expression, nested Alu-HIV-1 LTR PCR analysis of integrated HIV-1 proviral DNA indicated that the transgene expression observed in primary lympohocytes (both mitogen-stimulated and resting) was derived from the integrated vector sequences (Figure 5). We also observed a gradual decrease in the percentage of EGFP positive cells in the primary lymphocyte population over the course of several weeks of in vitro culture, from approximately 90% to 20%. This finding can be attributed to selective survival of a subset of lymphocytes or to extinction of viral gene expression. In vivo experiments in immunodeficient mice may help address this issue.
Experimental conditions were designed to ensure that our model was representative of the quiescent status of lymphocytes in vivo. To eliminate in vitro variables known to contribute to cell activation, a negative selection by stringent adherent cell depletion rather than a positive selection was used for lymphocyte isolation and low FBS concentration (1%) was used to supplement the culture medium. Under these conditions, more than 98% of the recovered cells were found in G0/G1 phase of the cell cycle. To monitor whether the transduction procedure itself induced metabolic activation, expression of cell activation antigens and changes in cycle status by PI staining were analyzed in 2 different experiments after exposure to lentiviral vector supernatants. No significant changes in the levels of expression were observed for CD69, CD25, and CD71 activation molecules in the target population, and only 1% to 2% of G0/G1 cells moved into the S phase during transduction. To the best of our analytical abilities therefore, the lymphocyte population used in this study is comparable to the quiescent lymphocytes found in the peripheral circulation in vivo.
In our hands, HIV-1 vector-mediated gene transfer and stable integration into resting lymphocytes, irrespective of their source, peripheral blood (adult) or umbilical cord blood (neonatal), did not require activation-induced cell proliferation, but was rather dependent on lentiviral vector preparations produced in the presence of HIV-1 APs. Taking into account our results and data from the literature, we envisage the following explanations for the apparent need for APs for efficient lentiviral-mediated transduction of resting lymphocytes; (a) the APs could induce early cell activation signals for example, phosphorylation of p62/NAK kinase,28NF-κB,29 or Vav,30 thus allowing the establishment of the infection of resting lymphocytes without driving cell proliferation or obvious cell activation status; or (b) the activity of some cellular cofactors produced from the virus producing 293T cells through their interaction with the HIV-1 accessory gene(s) or gene product(s) could exert a positive environment for effective transduction to occur in permissible populations (see below). However, the precise mechanisms by which APs seem to be required for efficient transduction of resting lymphocytes and the nature of APs-induced signals involved in this process remain to be elucidated.
Although HIV-1–based vectors are likely to behave differently from wild-type virus, some explanation for our findings may come from the knowledge of the roles played by different APs in the HIV-1 replicative cycle. It is now believed that several viral proteins, including p17 matrix, RT, Vpr, and Nef, are important to the establishment of HIV-1 infection in nondividing cells.18,31-34Considerable evidence is also available to suggest that Vif is likely to play an essential role during infection in vivo 34-37and in vitro in the case of primary PBLs and some lymphoid cell lines.38 Further, it has been proposed that Vif might be essential for the stability of proviral DNA complex.34,39,40 In addition, Vpr has been implicated in nuclear localization of viral nucleic acids in nondividing cells and its absence could affect nuclear import of the HIV-1 preintegration complex.41 Information is also beginning to emerge on the third early protein, Nef, which is expressed at a much higher level than other proteins in infected cells. In a variety of experimental studies, Nef was shown to down-regulate MHC class I molecule expression,39,42 enhance viral infectivity,43-46 and modulate cellular activation and signal transduction pathways, most probably through association with cellular signaling molecules.20,30,47 More importantly, Swingler et al48 have shown that resting T cells, which are normally refractory to productive infection, became activated and permissive to HIV-1 infection when exposed to supernatants from macrophages that were engineered to express Nef by adenoviral-mediated gene transfer. This effect was found to be mediated through chemokines (MIP1α and MIP1β) produced from the engineered macrophages and suggested that Nef might affect the early activation status of lymphocytes.48 On the basis of these findings, it is conceivable therefore that APs may similarly play a significant role in the context of HIV-1–based vectors.
In addition to these virus-derived gene products, other cellular molecules expressed by the virus-producing 293T cells in concomitance with or in response to the viral accessory gene product(s) may be important players. It has been reported that HIV-1 can incorporate a wide array of cell membrane molecules while budding from the infected cells.49-53 Of particular interest is the demonstration that increased incorporation of HLA-DR and ICAM molecules in HIV-1 virions resulted in enhanced viral infectivity on CD4 negative cell lines.50 In their recent work, Haase and coworkers17 suggested that interaction between resting T cells and major histocompatibility complex molecules may allow a low level of stimulation without the apparent commitment of the cells to full activation and progression through the cell cycle, but with alteration of the status of the cells to an extent sufficient to support HIV-1 infection and propagation. In this regard, it is possible to speculate that 293T cell-derived molecules may associate with pseudotyped lentiviral vectors and play a role in the transduction process.
In conclusion, our studies indicate that the multiply attenuated, VSV-G pseudotyped HIV-1–based vectors are highly efficient in transducing actively proliferating primary lymphocytes and established lymphoid cell lines. We have provided evidence, however, that the presence of HIV-1 APs is important for the establishment of stable transduction in quiescent lymphocytes. These findings may lead to additional insights into the mechanism whereby the HIV APs regulate T-cell activity and contribute to the pathogenesis of HIV infection.
HIV-1 vectors are currently being engineered to reduce the complement of genes needed for transduction of various cell types. As APs are associated with HIV-1 virulence, safe and efficient vector systems should exclude any nonessential viral proteins that may cause undesirable effects in the context of clinical applications. Dissection of the importance and role of the various APs by using different packaging constructs devoid of individual accessory genes22and elucidation of their potential cellular partners that allow transduction of resting lymphocytes therefore warrant further investigation that will lead to the development of safe and efficient lentivirus-based vectors for clinical gene transfer applications.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fabio Candotti, Clinical Gene Therapy Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda MD 20892-1851; e-mail: fabio@nhgri.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal