Abstract
The spleen plays a major role in immune surveillance, but the impact that splenectomy exerts on the immune competence of an individual is not fully resolved. Here we show that neonatal splenectomy in sheep does not abrogate the development of a large, nonrecirculating pool of lymphocytes and that it has no effect on the acquisition of a normal blood lymphocyte profile. Splenectomy did, however, result in a significant decrease in blood residency time of recirculating lymphocytes and in an enhanced accumulation of recirculating lymphocytes in lymph nodes. Furthermore, nonrecirculating peripheral blood lymphocytes were less likely to migrate to the lung, possibly because of saturation of the marginal pool by recirculating lymphocytes. Although splenectomy has little effect on the development or distribution of lymphocyte subsets in blood and lymph, it has marked effects on the rate of recirculation of lymphocytes, which may have significant implications for peripheral immune surveillance in patients who undergo splenectomy.
As the single largest lymphoid organ in the body, more lymphocytes migrate through the spleen each day than through any other tissue.1 Data have clearly demonstrated alterations in the distribution of lymphocyte subsets and the activation status of peripheral blood lymphocytes (PBL) after splenectomy.2,3 In the clinical setting, splenectomy often leads to increased susceptibility to specific infections and abnormal immune reponses.4 Although splenectomy has been reported to cause acute decreases in the concentration of circulating IgM antibody, the most serious consequence is overwhelming postsplenectomy infection (OPSI).5,6 Although the risk for postsplenectomy sepsis is significant in adults, this danger is markedly increased in very young children and has been reported in clinical studies to be as high as 13.8% in children from birth to 5 years of age.7 The precise mechanism of this immunosuppression is unclear, but it seems that the spleen contributes significantly to the acquisition of reactivity to the polysaccharide antigens of pneumococcus and other encapsulated bacteria, which are the principal agents of OPSI.6
To understand more fully the role of the spleen in the mammalian immune system, investigators have examined acute and chronic effects of splenectomy on the distribution and numbers of peripheral blood leukocyte subsets. In rats, splenectomy appears to result in a selective increase in peripheral blood B cells and in CD8+T cells,8 which may relate to the recently described nonrecirculating lymphocyte pool (NRLP) found in the blood and spleen of sheep but not in other lymphoid organs.9-11 Despite the fact that this blood-borne pool differs functionally and phenotypically from the recirculating lymphocyte pool (RLP) found in lymph, its function remains poorly defined. We have used the well-established sheep model to examine the effects of neonatal splenectomy on the development of the RLP and the NRLP and the resultant effects on lymphocyte recirculation.
Study design
Experimental animals
Randomly bred sheep (Versuchsbetrieb Sennweid, Olsberg, Switzerland) of either sex were splenectomized 19 to 21 days after birth. All animals were of average size and weight relative to nonsplenectomized animals throughout the experimental period. At roughly 2 years of age, efferent prescapular lymphatics and the jugular vein were cannulated, as described, for use in the recirculation studies delineated below.12
Lymph collection and labeling
Fluorescent cell tracking.
Efferent lymph cells and 350 mL blood were collected and labeled with CFSE6 and DiI-DS, respectively (Molecular Probes, Eugene, OR). For DiI-DS labeling, cells were harvested by centrifugation at 450g for 7 minutes, washed 3 times with phosphate-buffered saline (PBS), and resuspended at 2 × 108 cells/mL RPMI prewarmed to 37°C. Twelve micrograms DiI-DS/108 cells was dissolved in 300 μL dimethyl sulfoxide and diluted with RPMI to an equivalent volume to the cells. Then cells and DiI-DS were mixed gently and incubated for 30 minutes at 37°C. Cells were washed 3 times in PBS and resuspended in saline for reinjection into the jugular vein. To determine the proportions of labeled cells, blood samples were drawn at 15-minute intervals for 4 hours after injection, and blood and lymph were analyzed at 24, 48, 72, and 96 hours after injection as described.9
Radioactive tracking.
PBL and efferent lymph lymphocytes (ELL) were collected, labeled with 111-Indium Oxine (In111) and Sodium 51-Chromate (CR51)12 and reinjected intravenously. Animals were killed 8 hours later using T-61 euthanasia solution (Cobra II Autogamma Gamma Spectrometer; Canberra–Packard, Downer's Grove, IL), and samples of tissues were collected for weighing and radioactive counting as described.12
Immunophenotyping
Lymph and blood samples prepared as described above were collected and incubated with antibodies specific for CD4 (mAb 17D),13CD8 (mAb 7C2),9 γδ-TcR(mAb 86D),14 CD72 (mAb 2-104),9 and CD21 (mAb 2-87),9 as previously described,9 followed by phycoerythrin, fluorescein isothiocyanate (Southern Biotechnology Associates, Birmingham, AL), or allophycocyanin (Molecular Probes)-conjugated secondary antibodies.
Statistics
Phenotypic results are presented as the mean percentage of total lymphocytes reactive with antibodies directed against the lymphocyte subsets described above. To assess differences in the migration of labeled lymphocyte subsets in normal and splenectomized sheep, the percentage of each subset within the labeled population in the blood was determined by dual-color flow cytometry. The mean concentration of each subset as measured in 4 animals was then compared to the overall concentration within the blood and between the labeled ELL and PBL recovered from the blood (repeated measures analysis of variance with Tukey post-test; GraphPad InStat version 3.0 for Windows; GraphPad Software, San Diego, CA). To compare the ability of radiolabeled PBL and ELL to home to various tissues in splenectomized and intact animals, the percentage of total injected radioactivity recovered from each gram of tissue was calculated, and the means of 4 animals were calculated. For each tissue, the value obtained for labeled PBL was compared between splenectomized and intact animals, and the value of ELL was similarly compared (Student t test; GraphPad Software).
Results and discussion
Development and recirculation of PBL
The distribution of T- and B-cell subsets in splenectomized and normal animals is shown in Table 1. Although there did appear to be a slight increase in the percentage of B cells in the peripheral blood of the splenectomized animals, this varied widely between individuals and was not significant. In contrast to previous studies in rodents,1,8 we were unable to identify significant changes in the blood profile after splenectomy. This could be explained as species differences, or it could be that the extended period of time that elapsed between splenectomy and observation in our model (which examined chronic rather than acute immunologic consequences of splenectomy) allowed physiological compensatory mechanisms to function. In rats, the most severe hematologic alterations are observed immediately after splenectomy, with progressive decreases in peripheral blood cell numbers during the following months.8 Nonetheless, the fact that the numbers of B- and T-cell subsets in the blood were normal in the absence of the spleen, which would normally contain a large number of non-recirculating CD21−ve B cells, indicated that the size of the nonrecirculating lymphocyte pool develops and is regulated independently of the spleen. This agreed with previous data obtained in the mouse indicating that the number and distribution of the peripheral T- and B-cell pools are regulated at the pool level rather than through thymic input.15 16
Proportions of B- and T-lymphocyte subsets in blood and efferent subcutaneous lymph of 2-year-old splenectomized and normal sheep
| Cell type . | Blood . | Efferent lymph . | ||
|---|---|---|---|---|
| Intact . | Splenectomized . | Intact . | Splenectomized . | |
| B cells | 43.8 ± 7.5* | 56.7 ± 4.1 | 24.4 ± 3.5 | 23.4 ± 3.2 |
| CD4 | 18.0 ± 4.1 | 14.6 ± 1.7 | 35.0 ± 3.4 | 42.8 ± 1.5 |
| CD8 | 14.2 ± 3.3 | 12.2 ± 1.3 | 15.1 ± 3.8 | 14.0 ± 1.3 |
| γδ | 12.3 ± 7.5 | 9.9 ± 2.1 | 13.5 ± 2.1 | 11.3 ± 1.0 |
| Cell type . | Blood . | Efferent lymph . | ||
|---|---|---|---|---|
| Intact . | Splenectomized . | Intact . | Splenectomized . | |
| B cells | 43.8 ± 7.5* | 56.7 ± 4.1 | 24.4 ± 3.5 | 23.4 ± 3.2 |
| CD4 | 18.0 ± 4.1 | 14.6 ± 1.7 | 35.0 ± 3.4 | 42.8 ± 1.5 |
| CD8 | 14.2 ± 3.3 | 12.2 ± 1.3 | 15.1 ± 3.8 | 14.0 ± 1.3 |
| γδ | 12.3 ± 7.5 | 9.9 ± 2.1 | 13.5 ± 2.1 | 11.3 ± 1.0 |
Average percentage ± SEM.
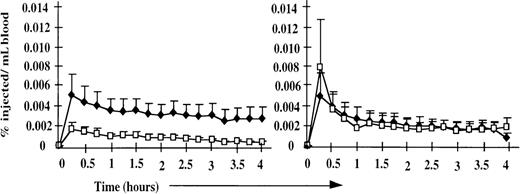
Because the peripheral blood and efferent lymph phenotypic profiles were similar between normal and splenectomized sheep, the starting populations for the cell-labeling experiments described below were equivalent, and the results were directly comparable between the 2 groups. Despite the normal composition of blood and lymph, marked alterations in the physiology of lymphocyte recirculation were observed in the splenectomized animals (Figure 1). In normal animals, labeled PBL (representing the NRLP) and ELL (representing the RLP) disappeared from the bloodstream at similar rates, whereas in splenectomized animals, there was a marked enhancement in the ability of labeled ELL to leave the blood. These differences resulted from the different rates of disappearance of the 2 populations; by 24 hours after injection, the distributions of labeled PBL and ELL were similar in splenectomized and normal animals. The selective retention of labeled peripheral blood CD21−ve B cells previously associated with the NRLP9 was observed to an even greater degree in splenectomized animals (Table 2). This enhanced retention of the NRLP can be explained as solely owing to the absence of the spleen, to which many cells would normally localize after intravenous injection. In contrast, the rapid mobility of the recirculating lymphocyte pool suggests that the spleen normally slows lymphocyte recirculation out of the blood and that other compensatory mechanisms replace the spleen to regulate the rate of lymphocyte recirculation and the distribution of the RLP.
Disappearance of labeled ELL and PBL from the peripheral blood of splenectomized and normal animals (n = 4).
In normal animals (right), labeled PBL (♦) and ELL (□) disappeared from the bloodstream at similar rates. In splenectomized animals (left), the disappearance of labeled ELL (□) was markedly enhanced, but the disappearance of labeled PBL (♦) was unchanged. Data are presented as a percentage of injected cells recovered per milliliter of blood for each population.
Disappearance of labeled ELL and PBL from the peripheral blood of splenectomized and normal animals (n = 4).
In normal animals (right), labeled PBL (♦) and ELL (□) disappeared from the bloodstream at similar rates. In splenectomized animals (left), the disappearance of labeled ELL (□) was markedly enhanced, but the disappearance of labeled PBL (♦) was unchanged. Data are presented as a percentage of injected cells recovered per milliliter of blood for each population.
Proportion of labeled B- and T-lymphocyte subsets in blood 24 hours after the intravenous injection of labeled PBL and ELL (n = 4)
| Subset . | Lymphocyte population in blood . | ||
|---|---|---|---|
| Total . | Labeled PBLs . | Labeled ELLs . | |
| CD4 | 15 ± 1.7 | 4 ± 0.8* | 26 ± 3.3*,† |
| CD8 | 12 ± 1.3 | 2 ± 0.2* | 7 ± 1.7*,† |
| γδ | 10 ± 2.1 | 7 ± 1.3 | 29 ± 3.2*,† |
| B | 57 ± 4.1 | 75 ± 6.5 | 23 ± 8.6*,† |
| CD21 | 24 ± 6.3 | 25 ± 8.9 | 25 ± 1.7 |
| Subset . | Lymphocyte population in blood . | ||
|---|---|---|---|
| Total . | Labeled PBLs . | Labeled ELLs . | |
| CD4 | 15 ± 1.7 | 4 ± 0.8* | 26 ± 3.3*,† |
| CD8 | 12 ± 1.3 | 2 ± 0.2* | 7 ± 1.7*,† |
| γδ | 10 ± 2.1 | 7 ± 1.3 | 29 ± 3.2*,† |
| B | 57 ± 4.1 | 75 ± 6.5 | 23 ± 8.6*,† |
| CD21 | 24 ± 6.3 | 25 ± 8.9 | 25 ± 1.7 |
Significantly different (P < .05) than total (unlabeled) PBLs.
Significantly different (P < .05) than labeled PBLs.
These results are particularly interesting, given previous data obtained in splenectomized rats, pigs, and humans that demonstrated a significantly slower exit of labeled cells from the peripheral blood.17-19 Although it is possible that our results could be explained by species differences, a more likely explanation is that the previous studies used labeled PBL as the test population. Although we observed no significant difference in the equilibration time of labeled PBL in normal and splenectomized sheep, there was a tendency for the labeled PBL to be recovered at higher levels in the splenectomized animals (not shown). It should be stressed that this effect was less marked than the significant differences observed with labeled recirculating lymphocytes harvested from lymph. Because ELL were not used in previous studies, our results do not conflict but, in fact, suggest that though the NRLP has not been phenotypically defined in other species, it can be functionally identified by its reduced ability to migrate out of the blood after splenectomy. It would, therefore, be expected that labeled human, rat, and pig ELL recirculate more effectively after splenectomy.
Enhanced migration of the RLP after splenectomy
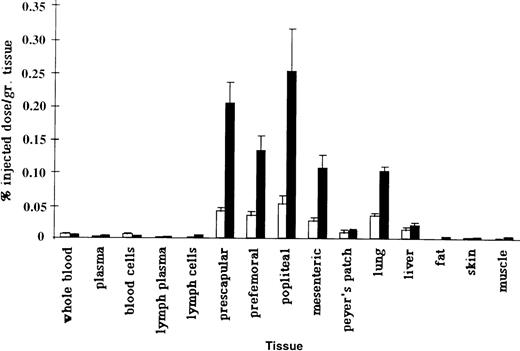
To assess whether splenectomy significantly altered the tissue distribution of the NRLP or the RLP, we measured the ability of radiolabeled PBL and ELL to home to various tissues in normal and splenectomized animals (Figure 2). The innate ability of the RLP to migrate more effectively than the NRLP to lymphoid tissues was significantly enhanced in splenectomized animals (Table 3). Labeled ELL were recovered in significantly higher numbers in subcutaneous lymph nodes and in the liver after splenectomy, whereas the ability of labeled PBL to migrate to the lung was significantly inhibited. Assuming that the tissue weights in splenectomized animals were in the normal range, it can be estimated that roughly 3% of labeled PBL and 15% of labeled ELL localized to 100 g lymph nodes in the splenectomized animals versus 2.5% of PBL and 5% of ELL in the intact animals. There was also a significant increase in the ability of the RLP to migrate to the liver after splenectomy, so that roughly 14% of ELL localized to the liver in splenectomized animals versus 8% in the intact sheep.20Although no tissue appeared to replace the spleen as a preferential homing site of the NRLP, there was a significant inhibition in the migration of labeled PBL to the lung. Based on the measured radioactivity per gram of tissue and normal organ weights for adult sheep, 9% of labeled PBL and 30% of labeled ELL would be expected to localize to the lung in splenectomized animals compared with roughly 20% of labeled PBL and 25% of labeled ELL in intact animals.20
Recovery of PBL and ELL.
Recovery of labeled PBL and ELL in various tissues of splenectomized animals. Labeled ELL (solid bars) migrated more efficiently to lymphoid tissues than PBL. In normal animals, significant numbers of labeled PBL (open bars) migrate to the spleen.9 In splenectomized animals, labeled PBL do not home to any tissue in greater numbers than labeled ELL. Data are presented as a percentage of injected radioactivity recovered per gram of tissue (n = 4).
Recovery of PBL and ELL.
Recovery of labeled PBL and ELL in various tissues of splenectomized animals. Labeled ELL (solid bars) migrated more efficiently to lymphoid tissues than PBL. In normal animals, significant numbers of labeled PBL (open bars) migrate to the spleen.9 In splenectomized animals, labeled PBL do not home to any tissue in greater numbers than labeled ELL. Data are presented as a percentage of injected radioactivity recovered per gram of tissue (n = 4).
Comparison of the distribution of labeled PBL and ELL in normal and splenectomized animals 8 hours after intravenous injection
| Tissue . | Labeled PBLs . | Labeled ELLs . | ||
|---|---|---|---|---|
| Splenectomized . | Intact . | Splenectomized . | Intact . | |
| Prescapular LN | 0.40 ± 0.053-150 | 0.24 ± 0.08 | 2.04 ± 0.303-151 | 0.43 ± 0.11 |
| Mesenteric LN | 0.20 ± 0.05 | 0.21 ± 0.08 | 1.07 ± 0.19 | 0.65 ± 0.20 |
| Lung | 0.30 ± 0.053-151 | 0.65 ± 0.12 | 1.06 ± 0.07 | 0.75 ± 0.16 |
| Peyer's patch | 0.08 ± 0.04 | 0.05 ± 0.02 | 0.12 ± 0.02 | 0.20 ± 0.05 |
| Liver | 0.12 ± 0.04 | 0.14 ± 0.03 | 0.20 ± 0.043-151 | 0.11 ± 0.01 |
| Blood cells | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.003 | 0.02 ± 0.01 |
| Tissue . | Labeled PBLs . | Labeled ELLs . | ||
|---|---|---|---|---|
| Splenectomized . | Intact . | Splenectomized . | Intact . | |
| Prescapular LN | 0.40 ± 0.053-150 | 0.24 ± 0.08 | 2.04 ± 0.303-151 | 0.43 ± 0.11 |
| Mesenteric LN | 0.20 ± 0.05 | 0.21 ± 0.08 | 1.07 ± 0.19 | 0.65 ± 0.20 |
| Lung | 0.30 ± 0.053-151 | 0.65 ± 0.12 | 1.06 ± 0.07 | 0.75 ± 0.16 |
| Peyer's patch | 0.08 ± 0.04 | 0.05 ± 0.02 | 0.12 ± 0.02 | 0.20 ± 0.05 |
| Liver | 0.12 ± 0.04 | 0.14 ± 0.03 | 0.20 ± 0.043-151 | 0.11 ± 0.01 |
| Blood cells | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.003 | 0.02 ± 0.01 |
Percentage of injected cells per 10 grams of tissue.
Significantly different (P < .05, Student t test) from the corresponding cell migration in the intact animal.
Pabst and colleagues1,21 have described the existence of a “marginal pool” in the lung, which represent a large reservoir of recirculating lymphocytes. One potential explanation for the rapid disappearance of labeled ELL from the blood in splenectomized animals would be an increased rate of systemic dissemination and migration of the RLP, as evidenced by the significantly enhanced ability of ELL to migrate to lymph nodes. The reduced propensity for labeled PBL to be recovered from the lung could be caused by a physical “blockage” of binding sites on lung endothelium by recirculating ELL within this marginal pool. It is particularly interesting that there was also an increased tendency for the RLP to localize to the liver in the splenectomized sheep. It has been hypothesized that one of the principal immune deficiencies in splenectomized individuals may be the loss of the marginal zone macrophages.22,23 One therapeutic possibility is to enhance the presence of specific antibodies against T-independent antigens by immunization, which may enhance the effect of other phagocytic cells in the body, such as the Kupffer cells in the liver.22 24 It may be that the enhanced ability of the RLP to localize in the liver after intravenous injection reflects an enhanced role for the liver in the immune system of our long-term splenectomized animals. Together, these data would indicate that splenectomy results in a more mobile recirculating lymphocyte pool that rapidly disseminates to the lymphoid organs, liver, and the marginal pool of the lung.
Conclusion
Clearly, the numbers of recirculating lymphocytes in the blood were regulated independently of the spleen. Previous experiments measuring factors regulating peripheral T- and B-cell hemostasis have suggested complex mechanisms that specifically regulate the number of peripheral T cells.15,16 Furthermore, the composition of the peripheral T-cell pool appears to be independently regulated so that a normal CD4:CD8 T-cell ratio is attained after cell transfer experiments, regardless of the number of injected T cells.15 Although newly formed T cells from the thymus appear to be exempt from this control, numbers of mature T cells are regulated by an undefined external mechanism capable of independently measuring the composition of the circulating T-cell pool.25Similarly, it appears that the number and composition of the peripheral blood lymphocyte pool are equally regulated, so that normal proportions of the recirculating and nonrecirculating lymphocyte pools are found in the blood of long-term splenectomized sheep. Despite the clear role of the spleen as a site of deposition for the NRLP, the blood lymphocyte profile and relative distribution of the NRLP and the RLP in the peripheral blood developed normally. However, a consequence of this normal profile was a clear alteration in the recirculation kinetics of the RLP, which may be a mechanism to maintain the normal balance of the NRLP and RLP within the blood. After injection, the recirculating lymphocyte pool rapidly equilibrated to baseline values, whereas the NRLP appeared to equilibrate more slowly.
It is tempting to speculate that the systemic immune deficiencies previously reported in splenectomized individuals may have more to do with the lack of normal splenic architecture and resultant specialized microenvironments than with a deficiency in appropriate cells. The cells may very well be present but unable to respond without the normal framework of appropriate accessory cells and molecules. It is known that a principal cause of OPSI is an inability to respond to T-independent antigens, such as the surface antigens of pneumococcus, a response that normally occurs within the marginal zone of the spleen.6,23 The fact that human development of the marginal zone coincides with a reduction in susceptibility to OPSI supports the concept that an adequate immune response requires not only antigen-specific lymphocytes but a specialized network of accessory cells in an appropriately structured microenvironment.26,27Although autotransplanted spleen fragments and immunization with pneumococcal vaccines have shown some benefit in overcoming this deficiency, complete immune reconstitution is still lacking.24 27-29 Given the importance of the RLP to immunologic surveillance and the dissemination of memory, it is possible that the enhanced rate of recirculation observed in splenectomized individuals could be exploited in vaccination strategies to increase peripheral immunosurveillance and to compensate for a lack of systemic immunity. Future experiments should be directed toward investigating the potential for peripheral immune mechanisms to compensate for this lack of systemic immunity.
Acknowledgments
We thank Drs Alexandre Potocnik and Marco Colonna for critical reading of the manuscript and Dr Jack Hay for helpful discussion.
The Basel Institute for Immunology was founded and is supported by F. Hoffmann LaRoche Ltd.
Reprints:Alan J. Young, c/o Steven Mentzer, Brigham and Women's Hospital, Department of Thoracic Surgery, 75 Francis Street, Boston, MA 02115; e-mail: shepherd666@hotmail.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal