Abstract
The gradual disappearance of host antidonor isohemagglutinins after major ABO-mismatched hematopoietic stem cell (HSC) allografts has been attributed to the gradual destruction of host plasma cells by graft-versus-host effects. To corroborate this hypothesis, we retrospectively analyzed results from 383 major or major/minor ABO-mismatched unrelated and related HSC allografts performed between 1983 and 1998. All patients were conditioned by high-dose pretransplant therapy and given methotrexate/cyclosporine for graft-versus-host disease (GvHD) prophylaxis. Of the 383 patients, 155 had HLA-matched related and 228 had unrelated grafts. We asked whether unrelated recipients experienced a more rapid disappearance of isohemagglutinins than related recipients, and whether, within the groups of related and unrelated recipients, the titer disappeared faster in patients with GvHD than in those without GvHD. The median time to reach undetectable antidonor IgG and IgM titers was significantly shorter in unrelated recipients (46 versus 61 days; P = .016). In addition, related recipients with GvHD had a 2.2-fold increased likelihood (1.12-4.39,95% CI; P = .02) of reaching undetectable titers within 100 days than patients without GvHD. The persistence of antidonor isohemagglutinins led to significantly increased red blood cell (RBC) transfusion requirements in the ABO-mismatched related patients compared with ABO-matched counterparts. However, time to neutrophil and platelet engraftment, incidence of GvHD, and survival were not influenced by ABO incompatibility. In conclusion, our results corroborate the hypothesis that the rate of disappearance of antidonor isohemagglutinins after ABO-mismatched allogeneic HSC grafts is influenced by the degree of genetic disparity between donor and recipient, suggesting a graft-versus-plasma cell effect.
ABO incompatibility between donor and recipient is not a barrier for successful allogeneic hematopoietic stem cell (HSC) transplantation even though it is well established that major ABO incompatibility may lead to prolonged destruction of donor-derived erythrocytes and prolonged transfusion requirements.1-8Plasma exchange in the recipient and red blood cell (RBC) depletion of the donor marrow are widely adopted strategies to minimize the possibility of acute hemolytic complications associated with infusion of major ABO-mismatched grafts.9-12 Antibody-producing plasma cells are terminally differentiated and nonproliferative and therefore relatively resistant to chemotherapy and radiotherapy.13-15 This, in turn, explains persistence of isohemagglutinins for extended periods in some recipients of major ABO-mismatched allografts. If prolonged survival of isohemagglutinin-producing plasma cells in the patient was the key mechanism accounting for elevated posttransplant antidonor isohemagglutinin titers, it is reasonable to speculate that the degree of genetic disparity between donor and recipient with a resulting graft-versus-host (GvH) effect might influence the rate of titer disappearance after ABO-mismatched allogeneic HSC transplantation.
In this study we addressed this question and asked, (1) whether unrelated recipients of HSC transplants experienced a more rapid disappearance of isohemagglutinins than related recipients because of the greater degree of genetic disparity between donors and hosts; and (2) whether within the groups of related and unrelated recipients, the rates of titer disappearance were faster in patients with graft-versus-host disease (GvHD) than in those without this complication. We retrospectively analyzed results from 383 major or major/minor ABO-mismatched unrelated and related HSC allografts performed between 1983 and 1998 for treatment of leukemia, lymphoma, and myelodysplastic syndromes. We found that the median time to reach clinically irrelevant isohemagglutinin titers was significantly shorter in unrelated recipients than in related recipients. In addition, related recipients with GvHD had a significantly increased likelihood of reaching undetectable isohemagglutinin titers within 100 days after transplant than patients without GvHD. Persistence of antidonor hemagglutinins was also associated with increased RBC transfusion requirements; however, time to neutrophil and platelet engraftment, incidence of GvHD, and survival were not influenced by ABO incompatibility. These findings suggest that the degree of genetic disparity between donor and host influences the rate of isohemagglutinin titer disappearance in the host, which is evidence for a graft-versus-plasma cell effect.
Patients and methods
Patients
A total of 1676 patients transplanted between 1983 and 1998 with marrow from HLA-matched related donors (MRD) (n = 921) or unrelated donors (MUD) (n = 755) were included in this study. These patients were selected because complete data on ABO-blood groups in donors and recipients were available, and a standard regimen of methotrexate plus cyclosporine was used for GvHD prophylaxis.16 Transplants were performed for acute leukemia, chronic myeloid leukemia, lymphoma, and myelodysplastic syndromes. Two hundred ninety-six patients in the MRD group (32.1%), and 420 patients in the MUD group (55.6%) received marrow from ABO-incompatible donors. Details regarding the degree of ABO-mismatching, conditioning regimens, and other patient characteristics are summarized in Table 1.
Patient characteristics, preparative regimens and degree of ABO-incompatibility
| Parameter . | Donor type . | |
|---|---|---|
| Matched related . | Matched unrelated . | |
| Total no of patients | 921 | 755 |
| Gender (% male) | 57% | 56% |
| Age at transplant (y) | ||
| Median (range) | 35.3 (0.6-67.8) | 32.3 (0.5-56.3) |
| Diagnoses (no of patients [%]) | ||
| ALL | 97 (11) | 129 (17) |
| ANL | 211 (23) | 152 (20) |
| CML | 444 (48) | 381 (50) |
| HD | 22 (2) | 3 (1) |
| NHL | 65 (7) | 15 (2) |
| MDS | 82 (9) | 75 (10) |
| Acute GvHD* (no of patients [%]) | ||
| Grades 0-I | 491 (54) | 124 (17) |
| Grades II-IV | 427 (47) | 624 (83) |
| Preparative regimen† (no of patients [%]) | ||
| Cy/TBI | 506 (56) | 615 (81) |
| Bu/Cy | 256 (28) | 37 (5) |
| Other TBI-containing regimens | 114 (13) | 88 (12) |
| Other non-TBI-containing regimens | 35 (4) | 15 (2) |
| Antibody depletion (no of patients [%]) | ||
| Plasma exchange | 14 (2) | 146 (19) |
| Plasma absorption | 3 (0.3) | 1 (0.1) |
| ABO-compatible (no of patients [%]) | 625 (68) | 335 (44) |
| ABO-incompatible (no of patients [%]) | ||
| Major mismatch | 150 (16) | 164 (22) |
| Minor mismatch | 116 (13) | 183 (24) |
| Major/minor mismatch | 30 (3) | 73 (10) |
| Parameter . | Donor type . | |
|---|---|---|
| Matched related . | Matched unrelated . | |
| Total no of patients | 921 | 755 |
| Gender (% male) | 57% | 56% |
| Age at transplant (y) | ||
| Median (range) | 35.3 (0.6-67.8) | 32.3 (0.5-56.3) |
| Diagnoses (no of patients [%]) | ||
| ALL | 97 (11) | 129 (17) |
| ANL | 211 (23) | 152 (20) |
| CML | 444 (48) | 381 (50) |
| HD | 22 (2) | 3 (1) |
| NHL | 65 (7) | 15 (2) |
| MDS | 82 (9) | 75 (10) |
| Acute GvHD* (no of patients [%]) | ||
| Grades 0-I | 491 (54) | 124 (17) |
| Grades II-IV | 427 (47) | 624 (83) |
| Preparative regimen† (no of patients [%]) | ||
| Cy/TBI | 506 (56) | 615 (81) |
| Bu/Cy | 256 (28) | 37 (5) |
| Other TBI-containing regimens | 114 (13) | 88 (12) |
| Other non-TBI-containing regimens | 35 (4) | 15 (2) |
| Antibody depletion (no of patients [%]) | ||
| Plasma exchange | 14 (2) | 146 (19) |
| Plasma absorption | 3 (0.3) | 1 (0.1) |
| ABO-compatible (no of patients [%]) | 625 (68) | 335 (44) |
| ABO-incompatible (no of patients [%]) | ||
| Major mismatch | 150 (16) | 164 (22) |
| Minor mismatch | 116 (13) | 183 (24) |
| Major/minor mismatch | 30 (3) | 73 (10) |
In patients transplanted between 1983 and 1998 with marrow from HLA-matched related or HLA-matched unrelated donors.
GvHD, graft-versus-host disease, ALL, acute lymphoblastic leukemia; ANL, acute nonlymphocytic leukemia; CML, chronic myelogenous leukemia; HD, Hodgkin disease; NHL, non-Hodgkin disease; MDS, myelodysplastic syndrome; Cy/TBI, cytoxan/total body irradiation; TBI, total body irradiation.
Ten patients did not have an acute GvHD grade available at the time of analysis.
Ten patients were missing data pertaining to their preparative regimen.
Standard procedures for ABO-incompatible transplants
The following pretransplant and posttransplant standard practice guidelines were in place at the Fred Hutchinson Cancer Research Center for ABO-mismatched transplants:
Major ABO-mismatch.
(Presence of hemagglutinins in the recipient against erythrocyte antigens of the donor.) For MRD transplants, donor marrow was RBC-depleted; for MUD transplants, plasma exchange with pooled AB-plasma was performed in the recipient when antidonor erythrocyte IgG or IgM titers were greater less 1:16. Posttransplant isohemagglutinin IgG and IgM titers were followed on a weekly basis, and RBC units with blood group O were used for transfusions until isohemagglutinin titers were undetectable for 2 consecutive weeks. At that point, RBC transfusions were switched over to stem cell donor blood type.
Minor ABO-mismatch.
(Presence of hemagglutinins in the donor against erythrocyte antigens of the recipient.) Unmanipulated donor marrow was infused when antirecipient hemagglutinin titers were less than 1:128. Plasma depletion of donor marrow was performed if antirecipient-ABO titers were greater than 1:128. RBCs of stem cell donor blood group were transfused after transplant.
Major plus minor ABO-mismatch.
(Bi-directional presence of hemagglutinins.) In addition to the antibody-removal guidelines used for major ABO-mismatched MRD and MUD transplants, donor marrow was plasma depleted if antirecipient-ABO titers were greater than 1:128. Posttransplant RBC transfusion guidelines were according to major ABO-mismatched transplants.
Monitoring of hemagglutinin titers after transplant
After transplant, IgG and IgM anti-A and B titers were measured weekly in recipients as previously described.17 To quantitate posttransplant donor-directed hemagglutinin titers in different subgroups of patients, charts of all major and major/minor ABO-mismatched recipients were reviewed. Among the 414 patients in this group, 383 patients had evaluable data for the following 3 titer endpoints: (1) days after transplant when IgG-isohemagglutinins were only detectable in undiluted serum (“IgG-undiluted”), (2) days after transplant when IgM-isohemagglutinins were only detectable in undiluted serum (“IgM-undiluted”), and (3) days after transplant when IgG plus IgM-isohemagglutinins were undetectable (“IgG/IgM-nil”). Because plasma exchange of the recipient led to transiently low or even undetectable hemagglutinin titers, we did not consider isohemagglutinin titers within the first 7 days after transplant for this analysis.
Statistical methods
Summary statistics such as medians and ranges are presented. Wilcoxon rank sum tests were used to compare time to events in which all subjects reached the titer endpoints. Kaplan-Meier estimates summarize survival within ABO compatibility groups for MRD and MUD patients.18 For survival analysis, follow-up time was censored at the date of last contact for surviving patients. Incidences for acute GvHD and attainment of hemagglutinin titer endpoints were calculated using cumulative incidence estimates.19 The time each patient contributed to these analyses was the minimum of time to the endpoint of interest, death, or last contact. Death before reaching the endpoint was treated as a competing risk, and time was censored at the date of last contact for surviving patients who did not reach the endpoint of interest. Log rank statistics were used for comparisons between all time-to-event endpoints involving censored data.19 Cox proportional hazard regression models were used to assess the impacts of MRD versus MUD, acute GvHD and plasma exchange on time to hemagglutinin titer endpoints.20 Acute GvHD was treated as a time-dependent covariate in this analysis. For acute GvHD and hemagglutinin titer endpoints, when using the log rank statistic and Cox proportional hazards regression, the censoring time was either the date of last contact or death for patients not reaching the endpoint of interest.
Results
Donor-directed hemagglutinin titers after MRD versus MUD marrow transplantation
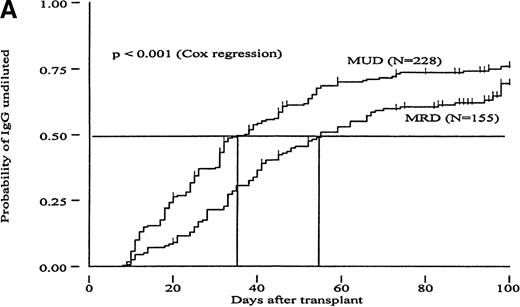
To investigate whether the time course of isohemagglutinin titer disappearance was influenced by genetic disparity between donor and recipient, we determined the number of posttransplant days required to reach different isohemagglutinin titer endpoints in major or major/minor ABO-mismatched MRD versus MUD recipients. The results are summarized in Table 2 and demonstrate that all 3 chosen titer endpoints were reached significantly earlier in recipients of MUD compared with recipients of MRD transplants. This analysis, however, only considered patients who reached the titer endpoints during the follow-up period. Figure1 shows the cumulative incidence curves and Table 3 the relative risks (RR) from Cox regression analysis for attaining different titer endpoints for MRD versus MUD patients. This approach also included patients who did not reach the titer endpoints during follow-up and showed that MUD patients had a significantly higher probability of clearing their IgG isohemagglutinins (“IgG-undiluted”) than MRD patients (P < .001; Cox regression analysis). The P-value for using “IgM-undiluted” as an endpoint, however, was only borderline significant (P = .057). When “IgG/IgM-nil” was used as an endpoint, isohemagglutinin titer disappearance was not significantly different between the MUD and MRD groups (P = .61). In addition, a Cox proportional hazard regression analysis was performed to evaluate the effect of donor type on titer disappearance while adjusting for the effects of TBI. The results of this analysis showed no effect of TBI use or dose on titer disappearance, nor did they change the effect donor type had on titer disappearance. In summary, accounting for the difference in TBI use and dose between MRD and MUD recipients did not ameliorate the difference in hemagglutinin titer disappearance seen between MRD and MUD transplants.
Median time after transplant needed to reach different hemagglutinin titer endpoints
| Titer endpoint . | Median (range) days among those reaching endpoint (number of patients) . | ||
|---|---|---|---|
| MRD . | MUD . | P value* . | |
| IgG-undiluted | 38 (9-101) (n = 99) | 30 (8-151) (n = 169) | <.001 |
| IgM-undiluted | 47 (9-143) (n = 82) | 32 (9-205) (n = 133) | .004 |
| IgG/IgM-nil | 61 (10-182) (n = 44) | 46 (10-172) (n = 76) | .016 |
| Titer endpoint . | Median (range) days among those reaching endpoint (number of patients) . | ||
|---|---|---|---|
| MRD . | MUD . | P value* . | |
| IgG-undiluted | 38 (9-101) (n = 99) | 30 (8-151) (n = 169) | <.001 |
| IgM-undiluted | 47 (9-143) (n = 82) | 32 (9-205) (n = 133) | .004 |
| IgG/IgM-nil | 61 (10-182) (n = 44) | 46 (10-172) (n = 76) | .016 |
For HLA-matched related (MRD) and HLA-matched unrelated donors (MUD) among patients reaching the endpoint. (“IgG-undiluted”), days after transplant when IgG-hemagglutinins were only detectable in undiluted serum; (“IgM-undiluted”), days after transplant when IgM-hemagglutinins were only detectable in undiluted serum; (“IgG/IgM-nil”), days after transplant when IgG plus IgM-hemagglutinins were undetectable.
From the Wilcoxon rank sum test.
Cumulative incidence curves showing probability of reaching different hemagglutinin titer endpoints during 100 days after transplant in recipients of marrow from HLA-matched related (MRD) or HLA-matched unrelated donors (MUD).
(A, “IgG-undiluted”), days after transplant when IgG-hemagglutinins were only detectable in undiluted serum; (B, “IgM-undiluted”), days after transplant when IgM-hemagglutinins were only detectable in undiluted serum; (C, “IgG/IgM-nil”), days after transplant when IgG plus IgM-hemagglutinins were undetectable.
Cumulative incidence curves showing probability of reaching different hemagglutinin titer endpoints during 100 days after transplant in recipients of marrow from HLA-matched related (MRD) or HLA-matched unrelated donors (MUD).
(A, “IgG-undiluted”), days after transplant when IgG-hemagglutinins were only detectable in undiluted serum; (B, “IgM-undiluted”), days after transplant when IgM-hemagglutinins were only detectable in undiluted serum; (C, “IgG/IgM-nil”), days after transplant when IgG plus IgM-hemagglutinins were undetectable.
Associations between donor status (HLA-matched related, MRD vs HLA-matched unrelated, MUD) and the likelihood of reaching different hemagglutinin titer endpoints
| Titer endpoint . | Patient group . | Univariate RR . | P value . | 95% CI . |
|---|---|---|---|---|
| IgG-undiluted | MRD | 1.0 | — | — |
| MUD | 1.60 | <.001 | (1.24, 2.05) | |
| IgM-undiluted | MRD | 1.0 | — | — |
| MUD | 1.31 | .057 | (0.99, 1.72) | |
| IgG/IgM-nil | MRD | 1.0 | — | — |
| MUD | 1.10 | .61 | (0.76, 1.60) |
| Titer endpoint . | Patient group . | Univariate RR . | P value . | 95% CI . |
|---|---|---|---|---|
| IgG-undiluted | MRD | 1.0 | — | — |
| MUD | 1.60 | <.001 | (1.24, 2.05) | |
| IgM-undiluted | MRD | 1.0 | — | — |
| MUD | 1.31 | .057 | (0.99, 1.72) | |
| IgG/IgM-nil | MRD | 1.0 | — | — |
| MUD | 1.10 | .61 | (0.76, 1.60) |
See Table 2 for abbreviations.
Influence of acute GvHD on time to disappearance of isohemagglutinin titers
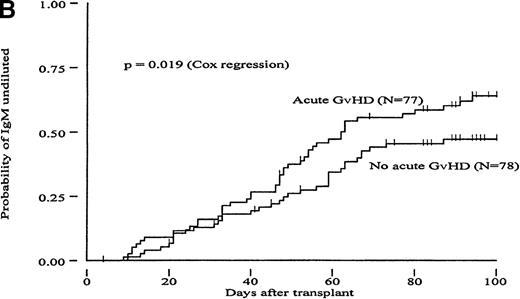
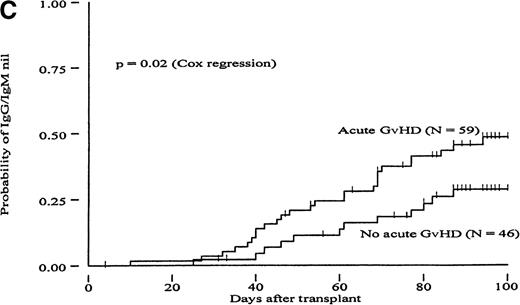
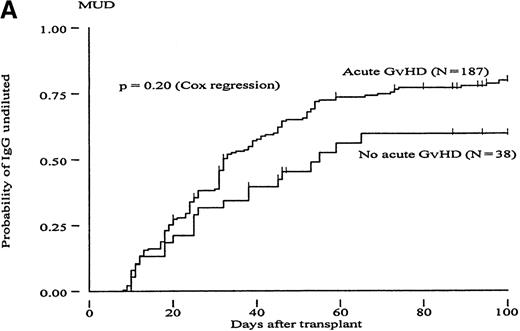
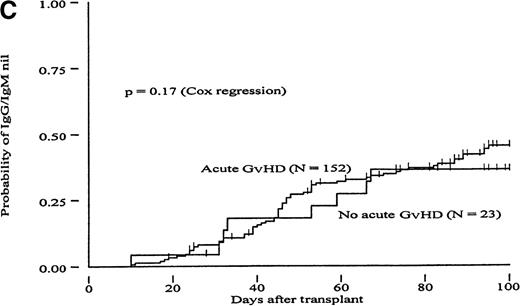
To determine whether GvH reactions could constitute a mechanism to explain the more rapid clearance of isohemagglutinin titers in the MUD group, we carried out a Cox proportional hazard regression analysis to evaluate the RR of reaching different titer endpoints using acute GvHD as a time-dependent covariate (Table 4). In the group of MRD transplants, patients with acute GvHD grades II to IV had a 1.71- to 2.22-fold increased likelihood of reaching all 3 titer endpoints compared with patients with acute GvHD grades 0 to I; these differences were statistically significant (P < .02). A trend in the same direction was observed in the group of MUD patients (increase in RR between 1.26 and 1.59). However, these differences did not reach statistical significance. Figures2 and 3 show the probabilities of reaching different isohemagglutinin titer endpoints until day 100 in the groups of MRD and MUD transplants according to the presence or absence of clinically significant acute GvHD. To create these figures, however, groups were formed based on GvHD, which is a time-dependent covariate, and differences may have become exaggerated as a result of a selection bias. Therefore, the data presented in Figures 2 and 3 should be interpreted in conjunction with the regression analysis shown in Table 4.
Association between acute graft-versus-host disease and the likelihood of reaching different hemagglutinin titer endpoints
| Titer endpoint . | Patient group . | Acute GvHD . | Univariate RR . | P value . | 95% CI . |
|---|---|---|---|---|---|
| IgG-undiluted | MRD | Grades 0-I | 1.0 | — | — |
| Grades II-IV | 1.89 | .002 | (1.26, 2.83) | ||
| MUD | Grades 0-I | 1.0 | — | — | |
| Grades II-IV | 1.26 | .20 | (0.88, 1.81) | ||
| IgM-undiluted | MRD | Grades 0-I | 1.0 | — | — |
| Grades II-IV | 1.71 | .019 | (1.09, 2.68) | ||
| MUD | Grades 0-I | 1.0 | — | — | |
| Grades II-IV | 1.39 | .14 | (0.90, 2.16) | ||
| IgG/IgM-nil | MRD | Grades 0-I | 1.0 | — | — |
| Grades II-IV | 2.22 | .02 | (1.12, 4.39) | ||
| MUD | Grades 0-I | 1.0 | — | — | |
| Grades II-IV | 1.59 | .17 | (0.81, 3.11) |
| Titer endpoint . | Patient group . | Acute GvHD . | Univariate RR . | P value . | 95% CI . |
|---|---|---|---|---|---|
| IgG-undiluted | MRD | Grades 0-I | 1.0 | — | — |
| Grades II-IV | 1.89 | .002 | (1.26, 2.83) | ||
| MUD | Grades 0-I | 1.0 | — | — | |
| Grades II-IV | 1.26 | .20 | (0.88, 1.81) | ||
| IgM-undiluted | MRD | Grades 0-I | 1.0 | — | — |
| Grades II-IV | 1.71 | .019 | (1.09, 2.68) | ||
| MUD | Grades 0-I | 1.0 | — | — | |
| Grades II-IV | 1.39 | .14 | (0.90, 2.16) | ||
| IgG/IgM-nil | MRD | Grades 0-I | 1.0 | — | — |
| Grades II-IV | 2.22 | .02 | (1.12, 4.39) | ||
| MUD | Grades 0-I | 1.0 | — | — | |
| Grades II-IV | 1.59 | .17 | (0.81, 3.11) |
GvHD = graft-versus-host disease; RR = relative risks.
See Table 2 for abbreviations.
Cumulative incidence curves showing probability of reaching different hemagglutinin titer endpoints during 100 days after transplant in recipients of marrow from HLA-matched related donors (MRD) in patients with or without acute GvHD.
(A, “IgG-undiluted”), days after transplant when IgG-hemagglutinins were only detectable in undiluted serum; (B, “IgM-undiluted”), days after transplant when IgM-hemagglutinins were only detectable in undiluted serum; (C, “IgG/IgM-nil”), days after transplant when IgG plus IgM-hemagglutinins were undetectable.
Cumulative incidence curves showing probability of reaching different hemagglutinin titer endpoints during 100 days after transplant in recipients of marrow from HLA-matched related donors (MRD) in patients with or without acute GvHD.
(A, “IgG-undiluted”), days after transplant when IgG-hemagglutinins were only detectable in undiluted serum; (B, “IgM-undiluted”), days after transplant when IgM-hemagglutinins were only detectable in undiluted serum; (C, “IgG/IgM-nil”), days after transplant when IgG plus IgM-hemagglutinins were undetectable.
Cumulative incidence curves showing probability of reaching different hemagglutinin titer endpoints during 100 days after transplant in recipients of marrow from HLA-matched unrelated donors (MUD) in patients with or without acute GvHD.
(A, “IgG-undiluted”), days after transplant when IgG-hemagglutinins were only detectable in undiluted serum; (B, “IgM-undiluted”), days after transplant when IgM-hemagglutinins were only detectable in undiluted serum; (C, “IgG/IgM-nil”), days after transplant when IgG plus IgM-hemagglutinins were undetectable.
Cumulative incidence curves showing probability of reaching different hemagglutinin titer endpoints during 100 days after transplant in recipients of marrow from HLA-matched unrelated donors (MUD) in patients with or without acute GvHD.
(A, “IgG-undiluted”), days after transplant when IgG-hemagglutinins were only detectable in undiluted serum; (B, “IgM-undiluted”), days after transplant when IgM-hemagglutinins were only detectable in undiluted serum; (C, “IgG/IgM-nil”), days after transplant when IgG plus IgM-hemagglutinins were undetectable.
Influence of plasma exchange on time to disappearance of isohemagglutinin titers
Plasma exchange significantly decreased the likelihood of reaching the titer endpoints in both MRD and MUD patients. In the group of MRD transplants, plasma exchange significantly decreased the likelihood of reaching the titer endpoints to 0.07 to 0.09 (P < .015). In the MUD group, plasma exchange also decreased the likelihood of a relatively rapid disappearance of isohemagglutinin titers (RR 0.33-0.45; P < .001). Therefore, plasma exchange could only transiently lower antibody titers without long-lasting effects presumably because of production of new antibodies as well as redistribution from peripheral tissue.
Red blood cell transfusion requirements
The median numbers of RBC units transfused within the first 60 days after transplant were compared between ABO-matched and major or major/minor-mismatched transplants (Table5). ABO-matched MRD patients received a median of 6 RBC units (range 0-73), whereas ABO-mismatched MRD patients required 10 RBC units (range 0-49) within the same period. This difference was statistically significant (P < .0001). In contrast, there was no difference in RBC transfusion requirements between ABO-mismatched and matched recipients of MUD marrow (10 RBC units vs 10 units; range 0-65 and 0-74, respectively;P = .11), although ABO-matched MUD recipients required more transfusions than their MRD counterparts (P < .0001).
Median red blood cell transfusion requirements during 60 days after transplant in patients transplanted with ABO-matched versus major or major/minor ABO-mismatched marrow
| Donor type . | Number evaluable patients . | Median number of units transfused (range) . | P value . |
|---|---|---|---|
| HLA-matched related: | |||
| ABO-incompatible | 150 | 10.0 (0-49)5-150 | |
| ABO-compatible | 502 | 6.0 (0-73)5-151 | <.0001 |
| HLA-matched unrelated: | |||
| ABO-incompatible | 236 | 10.0 (0-65) | |
| ABO-compatible | 335 | 10.0 (0-74) | .11 |
| Donor type . | Number evaluable patients . | Median number of units transfused (range) . | P value . |
|---|---|---|---|
| HLA-matched related: | |||
| ABO-incompatible | 150 | 10.0 (0-49)5-150 | |
| ABO-compatible | 502 | 6.0 (0-73)5-151 | <.0001 |
| HLA-matched unrelated: | |||
| ABO-incompatible | 236 | 10.0 (0-65) | |
| ABO-compatible | 335 | 10.0 (0-74) | .11 |
P = .19 compared with HLA-matched unrelated, ABO-incompatible group.
P < .0001 compared with HLA-matched unrelated, ABO-compatible group.
ABO-incompatibility and hematopoietic reconstitution
Neutrophil and platelet recoveries were compared between ABO-matched and ABO-major or major/minor mismatched transplants. Separate analysis of MRD (Figure 4A) and MUD (Figure 4B) transplants did not show significant differences in median neutrophil and platelet counts during 60 days after transplant between major or major/minor ABO-matched and mismatched transplants. The median time (range) to reach absolute neutrophil counts (ANC) greater than 1 000/μL and platelet counts greater than 50 000/μL were 24 (10-50) days and 25 (10-94) days in the ABO-matched and mismatched MRD groups (P = .18 and .50), and 24 (9-44) days and 24 (11-94) days in the MUD groups (P = .18 and .50), respectively.
ABO incompatibility and engraftment.
Median absolute neutrophil counts (ANC) and platelet counts (per μL) during 60 days after transplant in recipients of ABO-compatible (circle) and major or major/minor ABO-incompatible (triangle) marrow in recipients from (A) HLA-matched related donors and (B) HLA-matched unrelated donors.
ABO incompatibility and engraftment.
Median absolute neutrophil counts (ANC) and platelet counts (per μL) during 60 days after transplant in recipients of ABO-compatible (circle) and major or major/minor ABO-incompatible (triangle) marrow in recipients from (A) HLA-matched related donors and (B) HLA-matched unrelated donors.
ABO-incompatibility and probability of acute GvHD
We compared the probability of developing acute GvHD between subgroups with different degrees of ABO-incompatibilities. The overall incidence of acute GvHD grades II to IV was 47% in MRD transplants (n = 918), and 83% in MUD transplants (n = 748). Within the group of MRD transplants, the incidence of acute GvHD in recipients of ABO matched, major mismatched, minor mismatched, and major/minor mismatched marrow was 47%, 45%, 43%, and 60% (P > .22 [ABO matched vs mismatched]), respectively. Among recipients of MUD allografts, the corresponding GvHD incidence was 83%, 83%, 85%, and 82% (P = .81 [ABO matched vs mismatched]), respectively. Therefore, mismatching for ABO antigens did not influence incidence of GvHD in MRD and MUD transplants.
Survival
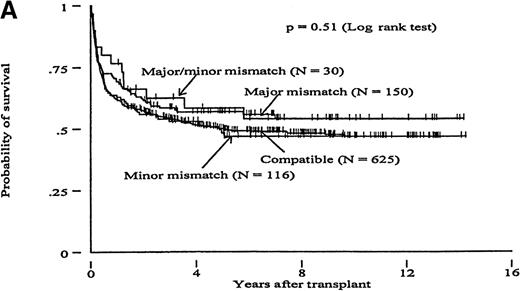
Overall survival was analyzed by comparing ABO-matched transplants with subgroups of major, minor, and major/minor ABO-mismatched transplants (Figure 5). Surviving patients were followed for a median of 6.5 (range .2-14.3) and 4.4 (range .3-6.2) years for patients with MRD and MUD, respectively. The overall survival at 5 years after transplant was 51% in MRD and 36% in MUD transplants. In both groups, ABO incompatibility did not appear to influence survival (P = .51 and .89 for MRD and MUD transplants, respectively; log rank test).
ABO incompatibility and survival.
Kaplan-Meier curves showing overall survival in patients transplanted with ABO-compatible versus ABO-incompatible marrow from (A) HLA-matched related (MRD) or (B) HLA-matched unrelated donors (MUD).
ABO incompatibility and survival.
Kaplan-Meier curves showing overall survival in patients transplanted with ABO-compatible versus ABO-incompatible marrow from (A) HLA-matched related (MRD) or (B) HLA-matched unrelated donors (MUD).
Discussion
The comparison of posttransplant isohemagglutinins titers between MRD and MUD patients in our study showed that the median times required to reach the 3 different titer endpoints was between 8 and 15 days shorter in MUD compared with MRD recipients (P < .016) (Table 2). Because acute GvHD is a much more frequent complication after MUD than after MRD transplantation,21 22 we analyzed whether there was a correlation between development of acute GvHD and relatively rapid disappearance of isohemagglutinins. We clearly demonstrated that a more rapid clearance of donor-directed anti-A and B was positively correlated with the development of acute GvHD in MRD recipients (Table 4; Figure 2). However, this correlation did not reach statistical significance in MUD recipients, although a trend in the same direction was consistently apparent in this group (Table 4; Figure3). The less striking correlation between acute GvHD and disappearance of isohemagglutinins in the MUD group may be due to the small sample size of MUD patients with minimal or no acute GvHD (n = 38) that limited the statistical power of the analysis. Furthermore, MUD recipients without clinically significant acute GvHD may still experience subclinical GvH effects that exceed those seen in their MRD counterparts.
The accelerated disappearance of donor-directed hemagglutinins in patients with acute GvHD can be interpreted to suggest that host-directed donor T cells lead to more rapid elimination of residual mature antibody-producing host lymphocytes and plasma cells. This is supported by the observation that GvHD-related differences in hemagglutinin titers in our study were detectable for both IgG and IgM. Host-derived IgG can diffuse from tissue pools into the plasma, whereas reappearance of IgM-hemagglutinins implies ongoing production by residual host-immune cells because there is essentially no tissue pool for IgM.1
The findings made here with nonmalignant host plasma cells may have additional relevance in light of recent reports on findings with malignant myeloma cells because GvH reactions may play a role in achieving and maintaining remissions in patient with multiple myelomas who were transplanted with allogeneic marrow.23-29 However, it is unclear whether our findings made with nonmalignant plasma cells are applicable to their malignant counterparts. VanTol et al30 showed that acute GvHD seemed to suppress IgG production by residual host plasma cells in a study that included 19 patients transplanted with allogeneic marrow. Their conclusion was based on informative IgG allotype differences that allowed the distinction between host-derived and donor-derived IgG.
Plasma exchange in the recipient is the preferred approach to prevent acute hemolytic transfusion reactions in major ABO-mismatched MUD transplants because it avoids manipulation of the donor marrow. Therefore, one could argue that the more rapid decrease of hemagglutinin titers in MUD patients could be due to the higher frequency of plasma exchanges performed in this subgroup. Nevertheless, our data clearly demonstrated that the RR of reaching all titer endpoints was significantly lower in patients treated with plasma exchange compared with patients who did not receive this treatment. This paradox is likely to be attributable to the fact that plasma exchange was selectively performed in those patients who had the highest hemagglutinin titers before transplant.
Immunosuppressive therapy with glucocorticoids, which constitutes the mainstay of acute GvHD therapy, could also have contributed to suppression of isohemagglutinin production. Our study did not allow us to differentiate between GvHD and GvHD therapy as potential causes for a rapid titer disappearance. Graft-versus-plasma cell effects would only result in suppression of host-derived antibodies such as isohemagglutinins without affecting immunoglobulins of donor cell origin. Immunoglobulin-allotype analysis incorporated in the design of future studies would be a useful tool to discern between these 2 mechanisms.
Our analysis of RBC transfusion requirements (Table 5) showed that within the group of ABO-matched transplants, MUD recipients required significantly more transfusions during the first 60 days after transplant than MRD recipients (P < .001). This was expected given the fact that more frequent and more severe acute GvHD that is known to occur in the MUD group, leads to an increased transplant-related morbidity, resulting in more infection and bleeding. In contrast, ABO-mismatched MUD recipients did not require more RBC transfusions than their MRD counterparts (P = .19). Ongoing hemolysis due to a major ABO-mismatch and acute GvHD with resulting infections and bleeding are potential causes for requiring prolonged transfusion support. In the ABO-mismatched MRD group, hemolysis might have been a more relevant mechanism than GvHD as a result of the longer persistence of isohemagglutinins. These competing mechanisms, however, would be reversed in the MUD group, resulting in comparable RBC transfusion requirements.
This study, which is based on 1676 HLA-identical related and unrelated donor marrow transplants, confirmed that ABO-mismatching between donor and recipient does not influence posttransplant survival. Benjamin et al31 found in retrospective analysis, that patients with AML or MDS who received major or minor ABO-mismatched marrow (n = 66) had an 85% greater risk of death within 100 days after transplant compared with recipients of ABO-matched allografts. This correlation was only consistent within the MRD group. Our analysis of the same subgroup of patients, however, could not confirm this observation (MRD recipients with AML or MDS, n = 293, P = .34, log rank test; data not shown). The differences seen between the Benjamin study and our study may be attributable to their transplant protocol that does not require reduction of antidonor ABO titers in the recipient before transplant. Alternatively, extensive subgroup analysis may have led to positive findings by chance which was not reproducible in our much larger study.
There was also no delay in neutrophil or platelet reconstitution and no increase in incidence of acute GvHD in ABO-incompatible compared with compatible transplants, which was in keeping with most previous reports.17,32-36 However, Hows et al37 reported delayed neutrophil, platelet, lymphocyte, and reticulocyte recovery after ABO-incompatible HLA-identical allogeneic marrow transplantation for aplastic anemia in a relatively small study, including only 24 patients. An increased incidence of acute GvHD associated with minor ABO-mismatched marrow transplantation, as reported by Bacigalupo et al,38 was also not observed in our study. This could be due to differences in GvHD prophylaxis; Bacigalupo et al16 used either methotrexate or cyclosporine alone, whereas a combination of both agents was used in our patient cohort.
In conclusion, our results corroborate the hypothesis that the rate of disappearance of antidonor isohemagglutinins after ABO-mismatched allogeneic HSC transplantation is influenced by the degree of genetic disparity between donor and recipient. On the basis of a relatively large study group, we could also validate that neutrophil and platelet engraftment, incidence of acute GvHD, and survival were not influenced by ABO-mismatching. These findings provide evidence for a graft-versus-plasma cell effect, and, at the same time raise the question as to whether GvH reactions may suppress residual host-derived humoral immune responses during the early phase after allogeneic HSC transplantation which, in turn, might contribute to the higher incidence of infectious complications seen in patients with GvHD.
Acknowledgments
We thank Helen Crawford and Bonnie Larson for typing the manuscript.
Supported in part by grants HL36444, CA15704, CA18221, DK51417, and CA18029 from the National Institutes of Health, DHHS, Bethesda, MD.
Reprints:Marco Mielcarek, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; email: mielcar@u.washington.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal