Abstract
Familial Mediterranean fever (FMF) is an inherited disease whose manifestations are acute but reversible attacks of sterile inflammation affecting synovial and serosal spaces. The FMF gene (MEFV) was recently cloned, and it codes for a protein (pyrin/marenostrin) homologous to known nuclear factors. We previously reported the deficient activity of a C5a/interleukin (IL)–8 inhibitor, a physiologic regulator of inflammatory processes, in FMF serosal and synovial fluids. We now describe the concomitant expression ofMEFV and C5a/IL-8–inhibitor activity in primary cultures of human fibroblasts. Fibroblasts grown from synovial and peritoneal tissues displayed C5a/IL-8–inhibitor activity that could be further induced with phorbol myristate acetate (PMA) and IL-1β. Very low levels of chemotactic inhibitor were evident in skin fibroblast cultures or in peritoneal and skin fibroblasts obtained from FMF patients. MEFV was expressed in peritoneal and skin fibroblasts at a lower level than in neutrophils and could be further induced by PMA and IL-1β. In the FMF cultures, the MEFV transcript carried the M694V mutation, consistent with the genetic defect found in patients with this disease. MEFV was also expressed in other cell lines that do not produce C5a/IL-8 inhibitor. These findings suggest that human primary fibroblast cultures express MEFV and produce C5a/IL-8–inhibitor activity. The interrelationship between pyrin, the MEFV product, and the C5a/IL-8 inhibitor requires further investigation.
The etiology of familial Mediterranean fever (FMF), an inherited disease characterized by recurrent episodes of unprovoked inflammation involving serosal and synovial spaces, was long a puzzle.1-3 Several years ago, we postulated a defect in an inhibitor of inflammation as the cause of this disease. Because the release of chemotactic factor is one of the first events occurring in an inflammatory response, we postulated that the most effective site of action for such an inhibitor would be at the level of the chemotactic response. Accordingly, we described a serine protease that inactivates C5a and interleukin (IL)–8.4-7 The activity of this enzyme, designated C5a/IL-8 inhibitor, was documented in peritoneal and synovial fluids but was absent from nonconcentrated human serum4,5; it was produced by synovial and peritoneal fibroblasts but not by skin fibroblasts.8 In addition, it displayed reduced activity in serosal fluids from FMF patients.7 9-12 These findings suggested that the C5a/IL-8 inhibitor acts to prevent inappropriate inflammation in serosal tissues and that its reduced activity in FMF could account for the attacks of sterile inflammation characteristic of this disease.
Recently, the FMF gene designated MEFV was identified by positional cloning.13 14 Several point mutations were recognized, and homozygous patients for these mutations were found to be suffering from FMF. The gene codes for a protein termed pyrin/marenostrin that by computer alignment resembles previously described nuclear factors. As MEFV expression was described mainly in mature neutrophils, the authors suggested that it plays a role in the inflammatory process.
In an attempt to shed light on the pathogenesis of the inflammatory attacks in FMF, we examined the expression pattern of MEFV in human primary fibroblast cultures from normal and FMF patients, and compared it with C5a-inhibitor activity in these cultures.
Materials and methods
Materials
Recombinant C5a (rC5a) was purchased from Sigma (St Louis, MO) and was dissolved in distilled-H2O containing 2.5 mg/mL of bovine serum albumin (BSA). Media and buffers (Dulbecco's phosphate-buffered saline, Hank's balanced salt solution (HBSS), Dulbecco's modified Eagle's medium, F-10 medium, and supplements) were obtained from Biological Industries (Beit Ha'emek, Israel), and fetal calf serum (FCS) from Gibco (Grand Island, NY). IL-1β was purchased from Pepro Tech (Rocky Hill, NJ). All other chemicals were of reagent grade and were purchased from Sigma.
Cell cultures
Human promyelocytic leukemia (HL-60) cells, generously provided by Eithan Fibach, PhD, Hadassah University Hospital, were cultured and induced with retinoic acid as previously described by us.15 M9K and JMN (mesothelioma cell lines) were generously provided by Brenda Gerwin, PhD, National Institutes of Health (Bethesda, MD). Synovial cultures were prepared from surgical specimens obtained from patients with osteoarthritis who were undergoing orthopedic surgical procedures. Peritoneal biopsies were obtained from FMF patients and otherwise healthy patients undergoing surgical procedures because of hernia or suspected appendicitis. Skin biopsies were obtained from FMF patients and healthy volunteers. The patients and volunteers signed an informed consent approved by the Human Studies Committee of Hadassah Hospital.
Primary cultures were established and maintained by a modification of the method of Matzner et al.8 Briefly, the biopsy material was incubated in 0.025% EDTA/0.125% trypsin solution at 4°C overnight and then transferred to new, identical solution, incubated at 37°C for 20 minutes, and teased apart. The monolayers thus obtained were identified as fibroblasts by their morphology and by positive immunofluorescent staining for fibronectin and negative staining for cytokeratin. They were grown in F-10 medium containing 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 20% FCS and were passaged with 0.05% EDTA/0.25% trypsin when confluent (every 2 to 4 weeks).
Conditioned media were obtained within 3 to 5 days from confluent monolayer cultures (105 cells per 10 mm/well in a 24-well dish) and tested for C5a-inhibitor activity. One day before a scheduled assay, the medium was replaced with serum-free medium. Inducers were added for the time indicated (phorbol myristate acetate [PMA] for 1 hour and IL-1β for 24 hours), after which the cultures were washed with HBSS and re-fed with serum-free medium for 24 hours. Supernatants and cells were then harvested for C5a-induced myeloperoxidase (MPO) release or reverse transcription–polymerase chain reaction (RT-PCR) assays, respectively.
MPO release
C5a-induced MPO release from neutrophils was used to measure C5a-inhibitor activity in conditioned media of human primary fibroblast cultures, as described.12 Briefly, 50 μL of 5 nmol/L rC5a and 50 μL of the conditioned media derived from 3 wells of a 24-well dish were each loaded into 3 wells in a 96-well microtiter plate and incubated for 20 minutes at 37°C. To each well, we added 25 μL freshly prepared human neutrophils (4 × 106cells/mL in HBSS/25 mmol/L Hepes/0.25% BSA) that had been incubated with 5 μg/mL cytochalasin B for 10 minutes at 37°C. Degranulation was allowed to proceed for 10 minutes at 37°C, and MPO release was then measured. The results were corrected for MPO release in the absence of rC5a and compared with those obtained in wells containing rC5a in the absence of a putative source of C5a inhibitor.
Reverse transcription PCR
Total RNA from the various cultured fibroblasts was prepared with the use of Tri Reagent (Molecular Research Center, Cincinnati, OH). Complementary DNA (cDNA) was synthesized with the use of oligo (dT)12-18 primer and SuperScript II reverse transcriptase (Gibco BRL, Gaithersburg, MD). The cDNA was amplified with Red Hot DNA polymerase (Advanced Biotechnologies, Surrey, UK) and primers designed from exon 10 of MEFV: forward common, 5′ TGACAGCTGTATCATTGTTCTGGGCT C TCCG 3′; reverse normal, 5′ TCGGGGGAACGCTGGACGCCTGGTACTCATTTTCC TCCT 3′; reverse M694V mutant, 5′ CGGGGGAACGCTGGACGCCTGGTACTCATTTT CCTTCCC 3′.16 Mixtures were incubated in a thermocycler (MJ Research, Watertown, MA) under the following conditions: 1 cycle of 10 minutes at 94°C followed by 34 cycles each consisting of 10 seconds at 94°C, 10 seconds at 60°C, and 30 seconds at 72°C and, at the end, followed by 1 cycle of 10 minutes at 72°C. The amplified products were separated by electrophoresis on a 2% agarose gel. Ethidium bromide staining of the agarose gel was used to detect the amplified fragments. Amplification of a fragment of the housekeeping gene β actin (220–base pair [bp] fragment) was always used as a positive control for successful amplification of the cDNA.17 Negative controls included replacement of the cDNA mixture with H2O in the PCR reaction and performance of the RT reaction in the absence of reverse transcriptase. Additional primer pairs used were primers from exons 8 and 10 (forward, 5′ TTCAATGTTCCAGAGCTG 3′; reverse, 5′ TGTAGTCCACGAAGATGC 3′, respectively) and 9 and 10 (forward, 5′ GATTGGCGCTCAGGCACATGCTGTTA 3′; reverse, 5′ GTCGGGGGAACGC TGGACGCCTGGTA 3′, respectively). The experiments with FMF cultures were carried out with the primer pair designed from exon 10 in order to locate the M694V mutation previously described.16
Semiquantitative RT-PCR was performed as described with the use of the primers from exons 8 and 10. Mixtures were incubated in a thermocycler under the following conditions: 1 cycle of 2 minutes at 95°C followed by 25 cycles for β actin and 33 cycles for MEFV, each consisting of 30 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C and, at the end, followed by 1 cycle of 10 minutes at 72°C.
Calculations
MPO release was corrected for enzyme release in the absence of rC5a. Inhibition of MPO release was calculated in comparison with that in wells containing rC5a in the absence of a putative source of C5a inhibitor (“culture”) as follows:
Conditioned medium from FMF culture was assayed in comparison with that of non-FMF culture at the same passage. Induced culture medium was always assayed in comparison with culture medium in the absence of inducer. Significance was determined by the paired t test.
Results
C5a-inhibitor activity in primary fibroblast cultures
We previously reported that supernatants of cultured human peritoneal and synovial fibroblasts, but not skin fibroblasts, inhibit C5a-induced neutrophil chemotaxis.8 We have now extended those studies, using the more accurate and less laborious C5a-induced MPO release assay, and compared the results with those obtained from cultures established from FMF patients (Table1). C5a-inhibitor activity was always present between the third and fifth passage of the normal synovial and peritoneal cultures where more than 95% of the cells were fibroblasts. After the fifth passage, most of these primary cultures lost their ability to express C5a-inhibitor activity. As previously documented, skin fibroblast cultures display very low levels of this activity. During the last decade, we established 3 peritoneal and 2 skin fibroblast cultures from FMF patients. Assay of these cultures and of normal cultures at the same passage indicated that the FMF cultures fail to secrete significant C5a-inhibitor activity (Table 1).
C5a-inhibitor activity in various fibroblast cultures
| Culture . | Number of cultures . | Number of experiments . | C5a inhibition (%) . |
|---|---|---|---|
| Synovial, normal | 4 | 15 | 30.4 ± 3.1 |
| Peritoneal, normal | 10 | 30 | 55.2 ± 4.5 |
| Peritoneal, FMF | 3 | 8 | −9.0 ± 0.9 |
| Skin, normal | 6 | 22 | 3.8 ± 3.2 |
| Skin, FMF | 2 | 6 | 8.4 ± 1.5 |
| Culture . | Number of cultures . | Number of experiments . | C5a inhibition (%) . |
|---|---|---|---|
| Synovial, normal | 4 | 15 | 30.4 ± 3.1 |
| Peritoneal, normal | 10 | 30 | 55.2 ± 4.5 |
| Peritoneal, FMF | 3 | 8 | −9.0 ± 0.9 |
| Skin, normal | 6 | 22 | 3.8 ± 3.2 |
| Skin, FMF | 2 | 6 | 8.4 ± 1.5 |
FMF indicates familial Mediterranean fever. C5a-inhibitor activity was measured as the percentage of inhibition of recombinant C5a (rC5a)–induced release of myeloperoxidase (MPO) from neutrophils as described. A 50-μL volume of serum-free culture medium (3rd through 5th passage) was incubated with 50 μL of 5 nmol/L rC5a for 20 minutes at 37°C before the MPO release assay. For each assay carried out with a putative inhibitor source, a control assay was carried out under conditions identical in every respect except that the inhibitor source was replaced by an equal volume of serum-free medium. For each FMF culture, a culture from the same normal tissue, at the same passage, was assayed. A490 readings for control MPO release were 0.35 to 0.67. The results are expressed as the mean ± SE. The percentage of C5a inhibition produced by all the synovial and peritoneal normal cultures was highly significant (P < .001), whereas no significant inhibition was found in the normal skin or in FMF skin and peritoneal fibroblast cultures.
The induction of the C5a inhibitor by PMA is shown in Table2. Peritoneal fibroblast cultures expressing maximal C5a-inhibitor activity at the third to fifth passage could not be further induced by PMA (100 nmol/L for 1 hour). On the other hand, cultures that had lost this ability, either partially or completely, could be induced, at least in part, by the same concentrations of PMA. Induction of those cultures with IL-1β (10 ng/mL for 24 hours) revealed similar effects (Table3). In addition, skin fibroblast cultures, lacking C5a-inhibitor activity, could be partially induced by PMA (Table 2) and IL-1β (Table 3). C5a-inhibitor activity could be induced by 50 to 500 nmol/L PMA after incubation for 30 to 60 minutes. There was no induction of activity when PMA at 500 nm remained in the culture for 24 hours or when PMA was added following preincubation of the culture with the protein kinase C inhibitor staurosporine (results not shown). FMF cultures, both peritoneal and skin, could not be induced by PMA (Table 2) or IL-1β (Table 3).
Induction of C5a-inhibitor activity in various fibroblast cultures by phorbol myristate acetate (PMA)
| Culture . | Passages . | Number of cultures . | Number of experiments . | C5a inhibition (%) . | |
|---|---|---|---|---|---|
| −PMA . | +PMA . | ||||
| Peritoneal, normal | 3-5 | 4 | 4 | 46.5 ± 6.6 | 40.3 ± 5.1 |
| Peritoneal, normal | 5-6 | 4 | 1 | 14.1 ± 4.2 | 27.0 ± 7.1* |
| Peritoneal, normal | 6-12 | 9 | 15 | 1.5 ± 2.8 | 21.5 ± 2.7† |
| Peritoneal, FMF | 3 | 3 | 8 | −9.0 ± 0.9 | 3.0 ± 2.9 |
| Skin, normal | 3-5 | 3 | 3 | −3.3 ± 6.2 | 18.1 ± 6.6* |
| Skin, FMF | 3-5 | 2 | 6 | 8.4 ± 1.5 | 7.2 ± 0.7 |
| Culture . | Passages . | Number of cultures . | Number of experiments . | C5a inhibition (%) . | |
|---|---|---|---|---|---|
| −PMA . | +PMA . | ||||
| Peritoneal, normal | 3-5 | 4 | 4 | 46.5 ± 6.6 | 40.3 ± 5.1 |
| Peritoneal, normal | 5-6 | 4 | 1 | 14.1 ± 4.2 | 27.0 ± 7.1* |
| Peritoneal, normal | 6-12 | 9 | 15 | 1.5 ± 2.8 | 21.5 ± 2.7† |
| Peritoneal, FMF | 3 | 3 | 8 | −9.0 ± 0.9 | 3.0 ± 2.9 |
| Skin, normal | 3-5 | 3 | 3 | −3.3 ± 6.2 | 18.1 ± 6.6* |
| Skin, FMF | 3-5 | 2 | 6 | 8.4 ± 1.5 | 7.2 ± 0.7 |
FMF indicates familial Mediterranean fever. C5a inhibition was measured as described (see footnote to Table 1) after culture induction with either PMA (100 nmol/L for 1 hour) or medium as described in “Materials and methods.” A490 readings for control myeloperoxidase release were 0.21 to 0.64. For each FMF culture with or without PMA, a normal control culture at the same passage was assayed.
P < .05 (versus noninduced cultures).
P < .001 (versus noninduced cultures).
Induction of C5a-inhibitor activity in late-passage fibroblast cultures by IL-1β
| Culture . | Passage . | Number of cultures . | Number of experiments . | C5a inhibition (%) . | |
|---|---|---|---|---|---|
| −IL-1β . | +IL-1β . | ||||
| Peritoneal, normal | 5-7 | 6 | 7 | 9.6 ± 3.4 | 23.2 ± 2.63-150 |
| Skin, normal | 7-12 | 2 | 4 | 3.6 ± 1.8 | 8.8 ± 3.9 |
| Peritoneal, FMF | 6 | 1 | 2 | 0.2 ± 0.2 | −3.7 ± 0.9 |
| Culture . | Passage . | Number of cultures . | Number of experiments . | C5a inhibition (%) . | |
|---|---|---|---|---|---|
| −IL-1β . | +IL-1β . | ||||
| Peritoneal, normal | 5-7 | 6 | 7 | 9.6 ± 3.4 | 23.2 ± 2.63-150 |
| Skin, normal | 7-12 | 2 | 4 | 3.6 ± 1.8 | 8.8 ± 3.9 |
| Peritoneal, FMF | 6 | 1 | 2 | 0.2 ± 0.2 | −3.7 ± 0.9 |
FMF indicates familial Mediterranean fever. C5a inhibition was measured as described (see footnote to Table 1) after culture induction with either IL-1β (10 ng/mL for 24 hours) or medium, as described in “Materials and methods.” A490 readings for control myeloperoxidase release were 0.29 to 0.75.
P < .01 (versus noninduced cultures).
MEFV expression in primary fibroblast cultures
The recently identified FMF gene, MEFV, was found to be expressed mainly in mature neutrophils.13,14 We have now extended this finding and compared MEFV expression with C5a-inhibitor activity in various cell cultures. MEFVexpression was detected by RT-PCR analysis of total RNA extracted from the studied cells with the use of several primer pairs designed from different exons as described in “Materials and methods.”
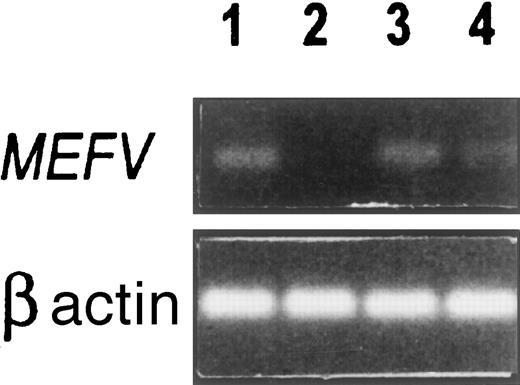
As shown in Figure 1, MEFV was expressed in human neutrophils (lane 1), absent from native HL-60 cells (lane 2), but expressed in retinoic-acid–induced HL-60 cells (lane 3). This suggests that MEFV is expressed during early stages of myeloid differentiation, coincidental with the appearance of the primary granules.18 In addition,MEFV was also expressed in primary fibroblast cultures obtained from normal peritoneal tissue (lane 4). Semiquantitative RT-PCR assays suggested 2- to 3-log lower expression of MEFV in the fibroblast cultures as compared with neutrophils (Figure2). The low expression of MEFV in the fibroblast cultures could be further induced with the use of certain agents (Abedat et al, unpublished data, February 2000). One example of the use of IL-1β as an inducer is shown in Figure 2 and suggests a 1- to 2-log increase in MEFVexpression in peritoneal fibroblasts after the induction. These results suggest that MEFV may be up-regulated during certain inflammatory conditions.
MEFV expression in various cells.
RT-PCR analysis was performed on total RNA with the use of common and normal primers designed from exon 10 as described in “Materials and methods.” The size of the amplified MEFV fragment was 200 bp. Lane 1: Normal human neutrophils. Lane 2: HL-60 cells, uninduced. Lane 3: Retinoic acid–induced HL-60 cells after 5 days incubation. Lane 4: Peritoneal fibroblasts, third passage.
MEFV expression in various cells.
RT-PCR analysis was performed on total RNA with the use of common and normal primers designed from exon 10 as described in “Materials and methods.” The size of the amplified MEFV fragment was 200 bp. Lane 1: Normal human neutrophils. Lane 2: HL-60 cells, uninduced. Lane 3: Retinoic acid–induced HL-60 cells after 5 days incubation. Lane 4: Peritoneal fibroblasts, third passage.
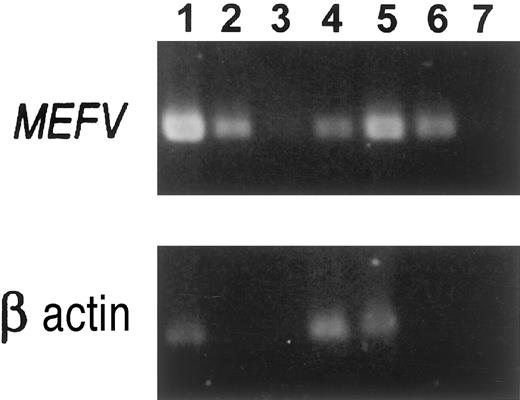
Semiquantitative RT-PCR assay of MEFV expression.
RT-PCR analysis was performed with the use of primers from exons 8 and 10 as described in “Materials and methods.” The size of the amplified MEFV fragments was 420 bp. Lane 1: Normal human neutrophils; undiluted cDNA sample. Lane 2: Neutrophil cDNA diluted 1:100. Lane 3: Neutrophil cDNA diluted 1:1000. Lane 4: Normal peritoneal fibroblasts, fourth passage; undiluted sample. Lane 5: Normal peritoneal fibroblasts induced for 24 hours with 10 ng/mL IL-1β, undiluted sample. Lane 6: IL-1β–induced peritoneal fibroblasts; cDNA diluted 1:10. Lane 7: IL-1β–induced peritoneal fibroblasts; cDNA diluted 1:100. All undiluted samples included the same amount of RNA in the RT reaction.
Semiquantitative RT-PCR assay of MEFV expression.
RT-PCR analysis was performed with the use of primers from exons 8 and 10 as described in “Materials and methods.” The size of the amplified MEFV fragments was 420 bp. Lane 1: Normal human neutrophils; undiluted cDNA sample. Lane 2: Neutrophil cDNA diluted 1:100. Lane 3: Neutrophil cDNA diluted 1:1000. Lane 4: Normal peritoneal fibroblasts, fourth passage; undiluted sample. Lane 5: Normal peritoneal fibroblasts induced for 24 hours with 10 ng/mL IL-1β, undiluted sample. Lane 6: IL-1β–induced peritoneal fibroblasts; cDNA diluted 1:10. Lane 7: IL-1β–induced peritoneal fibroblasts; cDNA diluted 1:100. All undiluted samples included the same amount of RNA in the RT reaction.
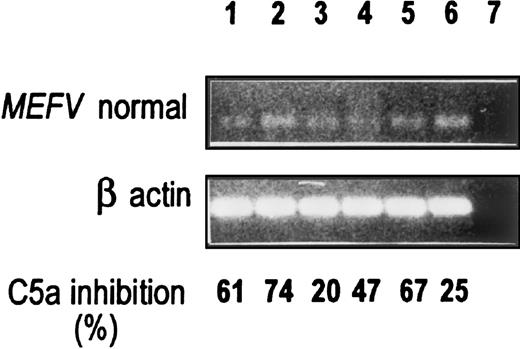
We then compared MEFV expression with C5a-inhibitor activity. The expression of MEFV in all 6 normal primary fibroblast cultures (5 peritoneal and 1 synovial) is shown in Figure3. All of the culture media displayed C5a-inhibitor activity in the absence of stimulus. Other cell lines that express MEFV (ie, the mesothelioma cell lines M9K and JMN) did not secrete C5a-inhibitor activity (results not shown).
MEFV expression and C5a-inhibitor activity in normal peritoneal and synovial fibroblast cell cultures.
RT-PCR was performed with the use of the normal primers as described in Figure 1. C5a-inhibitor activity is shown at the bottom and was assayed by the MPO release assay as described in “Materials and methods.” A490 readings for control C5a-induced MPO release were 0.39 to 0.62. Lanes 1 to 5: Five different normal peritoneal fibroblasts (lanes 1, 3, 4, and 5, third and fourth passage; lane 2, second passage). Lane 6: Normal synovial fibroblasts, third passage. Lane 7: H2O.
MEFV expression and C5a-inhibitor activity in normal peritoneal and synovial fibroblast cell cultures.
RT-PCR was performed with the use of the normal primers as described in Figure 1. C5a-inhibitor activity is shown at the bottom and was assayed by the MPO release assay as described in “Materials and methods.” A490 readings for control C5a-induced MPO release were 0.39 to 0.62. Lanes 1 to 5: Five different normal peritoneal fibroblasts (lanes 1, 3, 4, and 5, third and fourth passage; lane 2, second passage). Lane 6: Normal synovial fibroblasts, third passage. Lane 7: H2O.
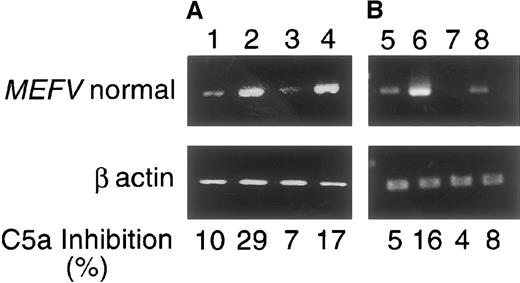
The expression of MEFV and the activity of C5a inhibitor in primary fibroblast cultures could be further induced by PMA or IL-1β. Figure 4 shows the induction of both peritoneal and skin fibroblast cultures at late passages (the skin cultures lack C5a-inhibitor activity while the peritoneal ones lost the activity, either partially or completely) (Tables 2, 3). Following induction with both inducers, concomitant increase inMEFV expression and C5a-inhibitor activity was observed in the peritoneal and, to a lesser extent, in skin-fibroblast cultures.
MEFV expression and C5a-inhibitor activity in normal skin and peritoneal fibroblast cell cultures induced with (A) IL-1β or (B) PMA.
RT-PCR was performed with the use of primers from exons 9 and 10 as described in “Materials and methods.” The size of the amplifiedMEFV fragment was 350 bp. C5a-inhibitor activity is shown at the bottom (see legend to Figure 3). Lanes 1-4: Cultures induced with 10 ng/mL IL-1β for 24 hours. Lanes 5-8: Cultures induced with PMA 100 nmol/L for 1 hour. Lane 1: Normal peritoneal fibroblasts. Lane 2: IL-1β–induced peritoneal fibroblasts. Lane 3: Normal skin fibroblasts. Lane 4: IL-1β–induced skin fibroblasts. Lane 5: Normal peritoneal fibroblasts. Lane 6: PMA-induced peritoneal fibroblasts. Lane 7: Normal skin fibroblasts. Lane 8: PMA-induced skin fibroblasts. The experiments were performed with cells from passage 6 and above. A representative experiment is shown in the figure.
MEFV expression and C5a-inhibitor activity in normal skin and peritoneal fibroblast cell cultures induced with (A) IL-1β or (B) PMA.
RT-PCR was performed with the use of primers from exons 9 and 10 as described in “Materials and methods.” The size of the amplifiedMEFV fragment was 350 bp. C5a-inhibitor activity is shown at the bottom (see legend to Figure 3). Lanes 1-4: Cultures induced with 10 ng/mL IL-1β for 24 hours. Lanes 5-8: Cultures induced with PMA 100 nmol/L for 1 hour. Lane 1: Normal peritoneal fibroblasts. Lane 2: IL-1β–induced peritoneal fibroblasts. Lane 3: Normal skin fibroblasts. Lane 4: IL-1β–induced skin fibroblasts. Lane 5: Normal peritoneal fibroblasts. Lane 6: PMA-induced peritoneal fibroblasts. Lane 7: Normal skin fibroblasts. Lane 8: PMA-induced skin fibroblasts. The experiments were performed with cells from passage 6 and above. A representative experiment is shown in the figure.
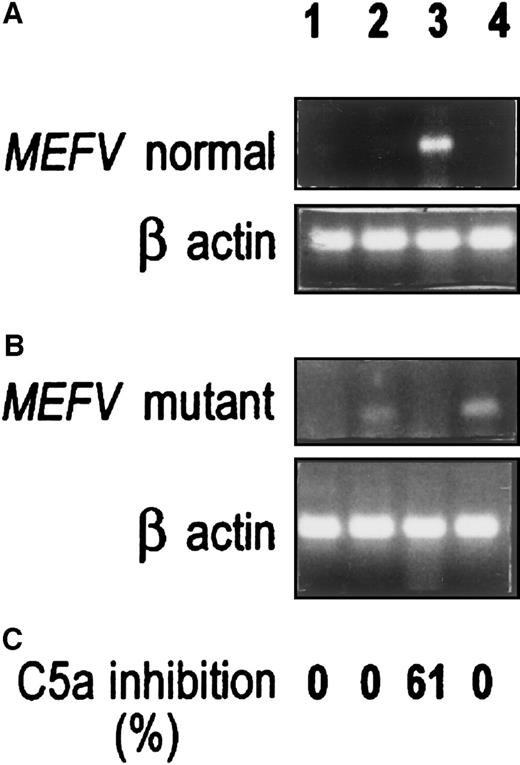
Figure 5 compares MEFV expression and C5a-inhibitor activity in normal and FMF skin and peritoneal fibroblast cultures. With the use of the normal primer (Figure 5A), strong MEFV expression was evident in normal peritoneal fibroblast culture (lane 3), which showed C5a-inhibitor activity. No PCR band or C5a-inhibitor activity could be detected in the FMF culture (lane 4). Normal skin fibroblasts, which lack spontaneous C5a-inhibitory activity, expressed weak MEFV expression (lane 1). The FMF skin culture lacked C5a-inhibitor activity as well as detectable PCR product when normal primer was used (lane 2).
MEFV expression in normal and FMF primary fibroblast cultures.
Lane 1: Normal skin fibroblasts. Lane 2: FMF skin fibroblasts. Lane 3: Normal peritoneal fibroblasts. Lane 4: FMF peritoneal fibroblasts. Three experiments were performed for each cell type, each with cells from a different passage (normal skin, passages 4, 6, 7; FMF skin, passages 1, 5, 9; normal peritoneum, passages 2, 3, 4; FMF peritoneum, passages 4, 5, 6). A representative experiment is shown in the figure. (A) RT-PCR performed with the use of the normal MEFV primer. (B) RT-PCR performed with the use of the mutant M694V MEFVprimer. Primers were designed from exon 10. (For details, see “Materials and methods” and the legend to Figure 1.) (C) Percentage of C5a inhibition. A490 readings for control C5a-induced MPO release were 0.43 to 0.58.
MEFV expression in normal and FMF primary fibroblast cultures.
Lane 1: Normal skin fibroblasts. Lane 2: FMF skin fibroblasts. Lane 3: Normal peritoneal fibroblasts. Lane 4: FMF peritoneal fibroblasts. Three experiments were performed for each cell type, each with cells from a different passage (normal skin, passages 4, 6, 7; FMF skin, passages 1, 5, 9; normal peritoneum, passages 2, 3, 4; FMF peritoneum, passages 4, 5, 6). A representative experiment is shown in the figure. (A) RT-PCR performed with the use of the normal MEFV primer. (B) RT-PCR performed with the use of the mutant M694V MEFVprimer. Primers were designed from exon 10. (For details, see “Materials and methods” and the legend to Figure 1.) (C) Percentage of C5a inhibition. A490 readings for control C5a-induced MPO release were 0.43 to 0.58.
PCR analysis of genomic DNA obtained from 3 FMF patients (2 from skin, 1 from peritoneum) revealed the M694V homozygous mutation.16 We therefore repeated the expression experiments, using the M694V mutant primer for the RT-PCR study. The experiment depicted in Figure 5B revealed a mirror image of the results shown in Figure 5A. When a normal primer was used, a PCR product in normal skin (weak) and peritoneal cells (strong) was observed, whereas no such product was observed in the corresponding FMF cells (Figure 5A). However, when an M694V mutant primer was used, a PCR product was amplified in the skin (weak) and peritoneal FMF cells (strong), but not in the normal cells (Figure 5B). As mentioned, C5a-inhibitor activity was detected only in the normal peritoneal fibroblast culture (Figure 5C).
Discussion
In the present study, we demonstrate expression of the FMF gene (MEFV) and C5a/IL-8–inhibitor activity in human primary fibroblast cultures.
Appreciating the inflammatory nature of FMF attacks, we previously suggested that they might result from an inborn deficiency in a physiologic regulator of the inflammatory response, just as hereditary angioedema is attributed to an inherited deficiency of C1-esterase inhibitor and certain hypercoagulable states may result from an inherited deficiency of endogenous anticoagulant(s). The putative inflammatory regulator was indeed detected by us in normal synovial and peritoneal fluids4-7 and was missing in serosal fluids obtained from FMF patients.7,9-12 It is a 50-kd serine protease, designated C5a inhibitor, that inactivates C5a and IL-8 by limited proteolysis.6 It was purified to homogeneity; neutralizing polyclonal and monoclonal antibodies were raised; and partial amino acid sequence was obtained.12 Concomitantly, we showed that the protein is produced by human primary fibroblast cultures from synovial and peritoneal tissues and is lacking in skin fibroblasts, suggesting high local anti-inflammatory activity in tissues that might be affected in FMF.8
We have now extended these studies to FMF patients and show the deficient production of C5a inhibitor by FMF cultures. By using PMA or IL-1β as stimulators, we demonstrate that skin fibroblasts, which express very low spontaneous C5a-inhibitor activity, could be partially induced. On the other hand, C5a-inhibitor activity could not be induced in any of the 5 FMF cultures. These results, which explain, at least in part, the serosal location of the inflammatory attacks of FMF, also suggest that the anti-inflammatory activity of the C5a inhibitor plays a role in other tissues as well. In fact, a transient erysipelas-like rash is an infrequent manifestation of FMF.1-3 19
MEFV was found to be expressed mainly in neutrophils.13,14 Centola et al18 did not detect MEFV expression in several synovial samples; neither was it detected in a single cDNA library established from peritoneal fibroblast primary culture.13 The French FMF Consortium did detect message by RT-PCR in a synovial sample taken from a patient with rheumatoid arthritis, but it was unknown whether this represented expression in synoviocytes or infiltrating inflammatory cells.14 Thus, it was of interest to investigateMEFV expression in serosal tissues that are affected in FMF. Such expression, if found, could give support for a possible relationship between the putative nuclear factor encoded byMEFV and the anti-inflammatory activity that was assumed to prevent undesired inflammatory attacks by means of C5a/IL-8 inactivation. Our results indicate the expression of MEFV and the production of C5a-inhibitor activity by serosal fibroblast cultures. Moreover, both could be further induced by the inflammatory mediators IL-1β and PMA. FMF cultures, in which MEFVtranscript carried the M694V mutation, lacked C5a-inhibitor activity.
On the basis of these results, one might hypothesize that the C5a inhibitor functions as a tissue-specific inflammatory mediator that may be dependent on pyrin/marenostrin for proper expression and secretion. Pyrin/marenostrin, which is encoded by MEFV, includes conserved domains characteristic of known transcription factors, such as the murine rpt-1 that down-regulates IL-2 expression.13 It could regulate the C5a inhibitor directly, or indirectly via one or more mediators.20 When pyrin/marenostrin expression is normal, it may result in up-regulation of the C5a inhibitor and, consequently, inhibit the development of an undesirable inflammatory response. On the other hand, dysfunction of pyrin/marenostrin would result in the observed decrease in C5a-inhibitor activity, resulting in the uncontrolled inflammatory attacks characteristic of FMF.21
This hypothesis requires further investigation since several cell lines other than serosal fibroblasts express MEFV at various levels without production of C5a-inhibitor activity. Once additional cultures from FMF patients are available, transfection experiments should lead to a better understanding of pyrin/marenostrin function and its interaction with the C5a inhibitor and other proteins involved in the regulation of inflammation.
Acknowledgments
We thank Dr B. M. Babior and Dr Alexandra Mahler for critical reading of the manuscript and the surgeons at the Hadassah Mount Scopus Hospital and Dr Vardiela Meiner from the Genetics Department for providing the synovial, peritoneal, and skin tissues.
Partially supported by the USA-Israel Binational grant BSF 95-00588.
Reprints:Yaacov Matzner, Hematology Unit, Hadassah University Hospital, Mount Scopus, Jerusalem, 91240 Israel; e-mail: matzner@cc.huji.ac.il.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal