Abstract
The AML1-MTG8 fusion transcription factor generated by t(8;21) translocation is thought to dysregulate genes that are crucial for normal differentiation and proliferation of hematopoietic progenitors to cause acute myelogenous leukemia (AML). Although AML1-MTG8 has been shown to repress the transcription of AML1 targets, none of the known targets of AML1 are probably responsible for AML1-MTG8-mediated leukemogenesis. In this study, 24 genes under the downstream control of AML1-MTG8 were isolated by using a differential display technique. Analysis with deletion mutants of AML1-MTG8 demonstrated that the regulation of the majority of these genes requires the region of 51 residues (488-538) containing the Nervy homology region 2 (NHR2), through which AML1-MTG8 interacts with MTGR1. Among the 24 genes identified, 10 were considered to be genes under the control of AML1, because their expression was altered by AML1b or AML1a or both. However, the other 14 genes were not affected by either AML1b or AML1a, suggesting the possibility that AML1-MTG8 regulates a number of specific target genes that are not normally regulated by AML1. Furthermore, an up-regulated gene, TIS11b (ERF-1,cMG1), was highly expressed in t(8;21) leukemic cells, and the overexpression of TIS11b induced myeloid cell proliferation in response to granulocyte colony-stimulating factor. These results suggest that the high-level expression of TIS11b contributes to AML1-MTG8-mediated leukemogenesis.
Transcription factor genes are the frequent targets of chromosomal translocations associated with human leukemia. Translocation leads to the dysregulation of downstream target genes under the control of the transcription factor and ultimately results in differentiation arrest and aberrant proliferation of hematopoietic progenitor cells.1 The t(8;21)(q22;q22) translocation is observed in approximately 40% of acute myeloid leukemia subtype M2 (AML-M2)2 and juxtaposes the AML1 gene3on chromosome 21, which encodes a transcription factor, with theMTG8 (ETO) gene on chromosome 8, generating the AML1-MTG8 (ETO) fusion transcription factor.4-7 This fusion protein consists of the N-terminal portion of AML1 fused in frame to the nearly full-length MTG8.
AML1 forms heterodimeric complexes with CBFβ (PEBP2β) and regulates the transcription of target genes by binding to the DNA sequence TGT/cGGT.8-10 A 128-amino acid region has been shown to be required for heterodimerization with CBFβ and for DNA binding and is referred to as the Runt homology domain (RHD),11-13 because it shares extensive homology with the product of the Drosophilasegmentation gene runt.14 Alternative splicing produces at least 3 forms of AML1 protein. Major isoforms AML1b and AML1c (453 and 480 amino acids, respectively) contain RHD and the C-terminal transcriptional activation domain, whereas a minor isoform, AML1a (250 amino acids), contains RHD but lacks the transcriptional activation domain.15 We recently found that AML1 interacts through its transactivation domain with the multifunctional transcriptional coactivators p300 and CBP,16 which activate transcription by acetylating chromatin17,18 and recruiting basal transcription factors.19 AML1 is an important regulator of a number of hematopoietic-specific genes including genes encoding interleukin-3 (IL-3),20 granulocyte-macrophage colony-stimulating factor (GM-CSF),21,22 colony-stimulating factor 1 (CSF1/M-CSF) receptor,23 myeloperoxidase, neutrophil elastase,24 granzyme B,25 and T-cell antigen receptors (TCRs).10,26,27 Targeted disruption has demonstrated that both AML1 and CBFβ are essential for definitive hematopoiesis of all lineages in mouse fetal liver.28-32MTG8 contains 2 putative zinc fingers and several proline-rich regions.6,33 We have isolated 2 family members, MTGR134 and MTG16,35 which are closely related to MTG8. Comparison of the amino acid sequences of MTG8, MTGR1, and MTG16 with their Drosophila homologue, Nervy,36reveals 4 evolutionarily conserved regions termed NHR (Nervy homology region) 1, NHR2, NHR3, and NHR4 (corresponding to a zinc finger domain). MTG8 is normally expressed in brain tissue6 and in CD34+ hematopoietic cells mobilized to peripheral blood by growth factor stimulation,37 whereas MTGR1 and MTG16 are ubiquitously expressed. Recently, MTG8 was found to interact with the nuclear receptor corepressor (N-CoR)/mSin3/histone deacetylase (HDAC) complex,38-40 which mediates transcriptional repression by deacetylating histones and creating repressive chromatic structures.41 42
The AML1-MTG8 fusion protein contains the DNA-binding domain (RHD) of AML1 and the MTG8 portion that interacts with the N-CoR/mSin3/HDAC corepressor complex,38-40 but lacks the C-terminal region of AML1 that interacts with the p300 and CBP histone acetyltransferase coactivators.16 Therefore, AML1-MTG8 could recruit HDAC activity instead of histone acetylase to the promoter of AML1-responsive target genes, resulting in histone deacetylation and transcriptional repression. Reporter assays have shown that AML1-MTG8 dominantly inhibits AML1-dependent transactivation of the TCRβ enhancer43 and the GM-CSF promoter.21 InAML1-MTG8 knock-in mice, definitive hematopoiesis from the normal fetal liver is completely absent44,45 as inAML1 knock-out mice, indicating that AML1-MTG8 also blocks normal AML1 functions in vivo through a dominant-negative mechanism. In contrast to AML1 knock-out mice, AML1-MTG8 knock-in mice produced apparently normal macrophage colonies in yolk sac cultures.44 They also contain dysplastic multilineage hematopoietic progenitors within their fetal livers, which have an abnormally high self-renewal capacity in vitro.45 These observations suggested that AML1-MTG8 has novel gain-of-function activities that alter the expression of genes not normally regulated by AML1. Most of the targets of AML1-MTG8, however, have been taken to be targets of AML1 whose transactivation is inhibited by AML1-MTG8. Besides, already known AML1 target genes including GM-CSF,TCRs, and IL-3 are probably not responsible for AML1-MTG8–mediated leukemogenesis.
We previously found that ectopic expression of AML1-MTG8 in L-G cells induces G-CSF–dependent cell proliferation and blocks differentiation to mature neutrophils.34 These findings opened the possibility that L-G cells might be suitable for differential screening and that some of the genes whose expression is altered in AML1-MTG8–expressing cells might affect myeloid cell proliferation and differentiation. In this study, we identified 24 downstream genes of AML1-MTG8 by a differential display technique. We show here that the region containing NHR2, through which AML1-MTG8 interacts with MTGR1,34 plays a major role in AML1-MTG8–mediated regulation in L-G cells, and that AML1-MTG8 may not only repress the transcription of AML1 target genes but also regulate specific downstream genes. In addition, we demonstrate that the TIS11b(ERF-1, cMG1) gene46-48 may contribute to AML1-MTG8–mediated leukemogenesis.
Materials and methods
Cells and retroviruses
The L-G cells49 were cultured in RPMI1640 medium supplemented with 10% fetal calf serum (FCS), 0.1 ng/mL recombinant mouse IL-3 (a generous gift from Kirin Brewery, Tokyo, Japan), and 50 μmol/L β-mercaptoethanol. For production of retroviruses, we used BOSC23 cells50 and pLNSX and pLNCX vectors.51BOSC23 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS and transfected with pLNSX- or pLNCX-derived plasmid DNAs by a calcium phosphate precipitation method; then the culture supernatants were saved as retrovirus solutions 48 hours after transfection. L-G cells (1 × 105) were incubated with 1 mL of the retrovirus solutions for 24 hours, and the infected cells were selected with 1 mg/mL G418 for 7 days. When L-G cells were exposed to G-CSF, the cells maintained in the presence of IL-3 were washed twice with phosphate-buffered saline (PBS) and incubated in medium containing 10 ng/mL recombinant human G-CSF (a generous gift from Chugai Pharmaceutical, Tokyo, Japan) in place of IL-3. Viable cells were counted with a Coulter counter (Beckman Coulter, Fullerton, CA). Nuclear morphologies were observed after staining with May-Grünwald solution and Giemsa solution (Merck, Darmstadt, Germany).
Patient samples
Leukemic cells and control cells were obtained from the bone marrow of patients with AML and from those with idiopathic thrombocytopenic purpura, respectively. Diagnosis was based on morphologic and cytochemical examinations of peripheral blood smears and bone marrow aspirates on admission. Cytogenetic analysis with G-banding techniques was performed on bone marrow samples from each patient with AML. The AML patients with t(8;21) were also diagnosed by reverse transcription-polymerase chain reaction (RT-PCR) amplification of the AML1-MTG8 fusion transcript.
Plasmid construction
The pLNSX-derived plasmids for HA-tagged AML1-MTG8, AML1b, AML1a, AML1-MTG8Δ538, and AML1-MTG8Δ487 were described previously.34 A pLNCX-derived plasmid for the expression of HA-tagged AML1a was constructed by inserting AML1a complementary DNA(cDNA) digested with StuI and ClaI and treated with Klenow polymerase into a HpaI site of pLNCX. The cDNA for HA-tagged mouse TIS11b was produced by performing RT-PCR on the total RNA of L-G cells. The PCR primers were designed to introduce an HA-tag at the N-terminus and HindIII sites at both ends, and had the following sequences: 5′-AAGCTTAGGCCTCTAGACCATGGCATACCCATACGACGTGCCT- GACTACGCCTCCACCACCACCCTCGTGTCCGC-3′ and 5′-AAGCTTAGTCATCTGAGATGGAGAG-3′. This TIS11b cDNA was digested with HindIII, and cloned into a HindIII site of pLNCX. The integrity of the insert was confirmed by nucleotide sequencing.
Differential display analysis
Total RNAs were isolated by the acid guanidinium thiocyanate/phenol/chloroform method52 from the retrovirus-infected L-G cells that had been cultivated in the presence of IL-3 and harvested within the 5-day period after G418 selection. Differential display analysis was performed essentially as described by Ito et al.53 Briefly, cDNAs were synthesized by using 4 oligo-dT primers (GT15MN; M = A+C+G, n = A, C, G, or T) and SuperScript II reverse transcriptase (Gibco-BRL, Rockville, MD). PCR amplification was carried out between the same oligo-dT primers and arbitrary 10-mers (Operon Technologies, Alameda, CA) using Taq DNA polymerase (Perkin-Elmer, Norwalk, CT or Boehringer Mannheim, Mannheim, Germany). A thermal cycling profile composed of 1 cycle of 94°C for 3 minutes, 40°C for 5 minutes, and 72°C for 5 minutes, and 24 cycles of 95°C for 15 seconds, 40°C for 2 minutes, and 72°C for 1 minute followed by an extension step at 72°C for 5 minutes was used. The reaction products were separated by 6% native polyacrylamide gel electrophoresis and detected by FluorImager SI (Amersham Pharmacia Biotech, Uppsala, Sweden) after staining of the gel with SYBR Green I (Amresco, Solon, OH). Bands that displayed more than 3-fold visible differences in intensity were excised from the gel and reamplified by PCR using the same set of primers. The reamplified products were cloned into a plasmid vector, pGEM-T Easy (Promega, Madison, WI), and sequenced. The cloned cDNA fragments were confirmed to correspond to the bands that we identified, by Southern blotting of the differential display reaction products using the cDNA fragments as probes. The identities of the sequences and their homologies to already known sequences were determined by performing BLAST searches in the Genbank/EMBL/DDBJ database. The nucleotide sequences of cDNA fragments were registered with the DDBJ database. Their data appear in the Genbank/EMBL/DDBJ database with the accession numbers AB030390 toAB030433.
Northern blotting
Total RNAs were isolated by the acid guanidinium thiocyanate/phenol/chloroform method. Five micrograms of each RNA was electrophoresed on a 1.0% agarose gel containing 2.2 mol/L formaldehyde in 20 mmol/L MOPS pH 7.0/8 mmol/L sodium acetate/1 mmol/L EDTA, and transferred to Hybond-N membranes (Amersham Pharmacia Biotech). To examine the tissue specificity of gene expression, a human multiple tissue Northern (MTN) blot (Clontech, Palo Alto, CA) was used. Hybridization was performed at 42°C for 20 hours in 6 × standard saline citrate (SSC)/10% dextran sulfate/1% sodium dodecyl sulfate (SDS)/1 × Denhardt's solution/50% formamide/100 μg/mL denatured salmon sperm DNA. The membranes were washed several times in 0.1 × SSC/0.1% SDS at 65°C, and the hybridized transcripts were detected with a BAS2000 image analyzer (Fuji Film, Tokyo, Japan). The cloned cDNA fragments in pGEM-T Easy were used as hybridization probes after EcoRI digestion and agarose gel electrophoresis purification. The probe for the humanTIS11b gene was produced by RT-PCR on total RNA from the bone marrow cells of a t(8;21) AML patient. The PCR primers were designed according to the known cDNA sequence as follows: 5′-CCACCTAACATAAGGACAAGT-3′ and 5′-CTTCCTGCAGACCTACACAA-3′. To confirm the amount of RNA loaded onto each lane, all membranes were successively hybridized with human glyceraldehide-3-phosphate-dehydrogenase (G3PDH) or β-actin cDNA probe (Clontech).
Western blotting
Cells were harvested and suspended in 62.5 mmol/L Tris-HCl pH 6.8/2% SDS/5% β-mercaptoethanol/10% glycerol at 2 × 107 cells/mL. After boiling and centrifugation, cell lysates were electrophoresed with 10% SDS-polyacrylamide gels and transferred to Hybond ECL membranes (Amersham Pharmacia Biotech) by electroblotting. The membranes were blocked in 5% low-fat milk dissolved in PBS containing 0.1% Tween-20 (PBS-T) at room temperature for 2 hours or at 4°C overnight, and incubated at room temperature for 1 hour with the anti-HA monoclonal antibody (3F10, Boehringer Mannheim) dissolved in PBS-T. After washing with PBS-T, the membranes were incubated at room temperature for 1 hour with horseradish peroxidase-conjugated second antibody dissolved in PBS-T. After extensive washing with PBS-T, the immunocomplexes were visualized by an ECL detection system (Amersham Pharmacia Biotech).
Results
Identification of genes whose expression is altered by AML1-MTG8
The L-G cell is an IL-3–dependent murine myeloid precursor cell line that can be induced to differentiate into mature neutrophils in response to G-CSF.49 Ectopic expression of AML1-MTG8 in L-G cells induces G-CSF–dependent cell proliferation and blocks differentiation into mature neutrophils.34 To identify genes involved in AML1-MTG8–mediated leukemogenesis, we applied the differential display technique53,54 using RNA from control L-G cells and L-G cells ectopically expressing AML1-MTG8. From 1600 differential display reactions obtained by using 400 arbitrary 10-mers and 4 oligo-dT primers in different combinations, we obtained 49 cDNA bands that displayed more than 3-fold visible differences in intensity. The reproducibility of the differential expression pattern was confirmed by using more than 3 pairs of L-G infectants infected with control and AML1-MTG8–carrying retroviruses at different times. Each band was excised from the gel, reamplified, and cloned, resulting in the isolation of 47 differentially expressed cDNA clones except for 2 bands that resisted reamplification. Sequencing analysis and database searching of these 47 clones led to the identification of 24 nonredundant genes (Table 1). These 24 genes consisted of 19 genes up-regulated by AML1-MTG8, which we termed AMUGs, and 5 genes down-regulated by AML1-MTG8, which we termed AMDGs. Eight of the 19 AMUGs and 1 of the 5 AMDGs did not have any significant homology with known genes in the database. None of these genes has been previously identified as being regulated by AML1-MTG8 or AML1, except for the granzyme B gene previously described as a putative target of AML1.25
Gene identities and expression patterns of cDNA fragments cloned by differential display
| cDNA fragment* . | Identity/homology† . | Differential expression in‡ . | Transcript size (kb)1-153 . | ||||
|---|---|---|---|---|---|---|---|
| AML1-MTG8 . | Δ538 . | Δ487 . | AML1b . | AML1a . | |||
| A6C390, I9C440 | MRP14 (M83219) | ↑ | ↓ | → | ↓ | ↑ | 0.6 |
| A6C520 | Unknown | ↑ | ↑ | → | ↓ | ↑ | ND |
| B6G440, D7T390 | Stefin 3 (M92419) | ↑ | → | → | ↓ | ↑ | 0.4 |
| B15T180 | Human Grb10 (D86962) | ↑ | → | → | → | → | 5.0 |
| B16G120 | Unknown | ↑ | ↑ | → | ↓ | ↑ | 3.0 |
| B16G290, L1C1300, L5C1200, P15C280 | Mast cell carboxypeptidase A (J05118) | ↑ | ↑ | → | → | ↑ | 1.4 |
| B16G340, G8T730, H9G390, I6G700, I11G470, I18T1000/950, I20G800/700/440, J3A700, J7G400/290, K13T290, K20A340, K20T220, L16G550, R10G1000, S17T1000 | Granzyme B (X04072) | ↓ | ↓ | → | ↑ | ↓ | 1.6 |
| C3G950 | Unknown | ↑ | ↑ | → | → | → | 2.1 |
| C18A360 | Unknown | ↑ | ↑ | → | → | → | ND |
| G9T200 | Rat β-galactoside-α2,6-syalyltransferase (M83143) | ↓ | ↓ | → | → | → | ND |
| G9T360 | Unknown | ↑ | ↑ | → | → | → | 2.0 |
| G10C360 | Uridine phosphorylase (D44464) | ↑ | ↑ | → | ↓ | ↑ | 1.4 |
| G19T460 | Unknown | ↑ | ↑ | → | → | → | ND |
| I5G520, L1C480, M18G800/200 | Human monocyte/neutrophil elastase inhibitor (M93056) | ↑ | ↑ | → | → | → | 1.5 |
| I19C700/600 | CTLA-2-β (X15592) | ↑ | ↑ | → | → | → | 0.9 |
| J6G550, J11T470 | Rat interferon-induced mRNA (X61381) | ↓ | ↓ | → | → | → | 0.9 |
| J13T600 | Bovine aspartyl-β-hydroxylase (M91213) | ↑ | ↑ | → | → | → | 3.1 |
| J15G700 | Unknown | ↑ | ↑ | → | → | → | >10 |
| K6A220, O4T230 | TIS11b (M58566) | ↑ | ↑ | → | → | → | 2.7 |
| M3A230 | Pim-2 (L41495) | ↓ | ↓ | → | → | ↓ | 2.0 |
| N9T310 | Unknown | ↑ | ↑ | → | → | → | ND |
| P10A370 | Unknown | ↓ | ↓ | → | → | ↓ | ND |
| P13C350 | MIP1α receptor like 1 gene (U28405) | ↑ | ↑ | → | → | → | ND |
| S14T480 | Rat CD53 (M57276) | ↑ | ↑ | → | → | ↑ | 1.7 |
| cDNA fragment* . | Identity/homology† . | Differential expression in‡ . | Transcript size (kb)1-153 . | ||||
|---|---|---|---|---|---|---|---|
| AML1-MTG8 . | Δ538 . | Δ487 . | AML1b . | AML1a . | |||
| A6C390, I9C440 | MRP14 (M83219) | ↑ | ↓ | → | ↓ | ↑ | 0.6 |
| A6C520 | Unknown | ↑ | ↑ | → | ↓ | ↑ | ND |
| B6G440, D7T390 | Stefin 3 (M92419) | ↑ | → | → | ↓ | ↑ | 0.4 |
| B15T180 | Human Grb10 (D86962) | ↑ | → | → | → | → | 5.0 |
| B16G120 | Unknown | ↑ | ↑ | → | ↓ | ↑ | 3.0 |
| B16G290, L1C1300, L5C1200, P15C280 | Mast cell carboxypeptidase A (J05118) | ↑ | ↑ | → | → | ↑ | 1.4 |
| B16G340, G8T730, H9G390, I6G700, I11G470, I18T1000/950, I20G800/700/440, J3A700, J7G400/290, K13T290, K20A340, K20T220, L16G550, R10G1000, S17T1000 | Granzyme B (X04072) | ↓ | ↓ | → | ↑ | ↓ | 1.6 |
| C3G950 | Unknown | ↑ | ↑ | → | → | → | 2.1 |
| C18A360 | Unknown | ↑ | ↑ | → | → | → | ND |
| G9T200 | Rat β-galactoside-α2,6-syalyltransferase (M83143) | ↓ | ↓ | → | → | → | ND |
| G9T360 | Unknown | ↑ | ↑ | → | → | → | 2.0 |
| G10C360 | Uridine phosphorylase (D44464) | ↑ | ↑ | → | ↓ | ↑ | 1.4 |
| G19T460 | Unknown | ↑ | ↑ | → | → | → | ND |
| I5G520, L1C480, M18G800/200 | Human monocyte/neutrophil elastase inhibitor (M93056) | ↑ | ↑ | → | → | → | 1.5 |
| I19C700/600 | CTLA-2-β (X15592) | ↑ | ↑ | → | → | → | 0.9 |
| J6G550, J11T470 | Rat interferon-induced mRNA (X61381) | ↓ | ↓ | → | → | → | 0.9 |
| J13T600 | Bovine aspartyl-β-hydroxylase (M91213) | ↑ | ↑ | → | → | → | 3.1 |
| J15G700 | Unknown | ↑ | ↑ | → | → | → | >10 |
| K6A220, O4T230 | TIS11b (M58566) | ↑ | ↑ | → | → | → | 2.7 |
| M3A230 | Pim-2 (L41495) | ↓ | ↓ | → | → | ↓ | 2.0 |
| N9T310 | Unknown | ↑ | ↑ | → | → | → | ND |
| P10A370 | Unknown | ↓ | ↓ | → | → | ↓ | ND |
| P13C350 | MIP1α receptor like 1 gene (U28405) | ↑ | ↑ | → | → | → | ND |
| S14T480 | Rat CD53 (M57276) | ↑ | ↑ | → | → | ↑ | 1.7 |
cDNA fragment designation was coded from its arbitrary 10-mer, anchor primer, and band size (eg, S14T480 is a 480-bp fragment amplified with S14 10-mer and GT15MT oligo-dT primer).
The origin of each fragment was identified or presumed from the highest scoring sequence in BLAST search of GenBank/EMBL/DDBJ database. The accession number of the highest scoring sequence is also shown in parenthesis.
Arrows pointing up indicate that the differential display band was more intense in the AML1-MTG8, Δ538, Δ487, AML1b, or AML1a lanes than in the control lane, and arrows pointing down indicate the reverse. Arrows pointing right indicate the invariable change by visual inspection.
The transcript size of the major species was determined by Northern blotting analysis. ND indicates not detectable.
The regulation of the majority of AMUGs and AMDGs requires the region containing NHR2
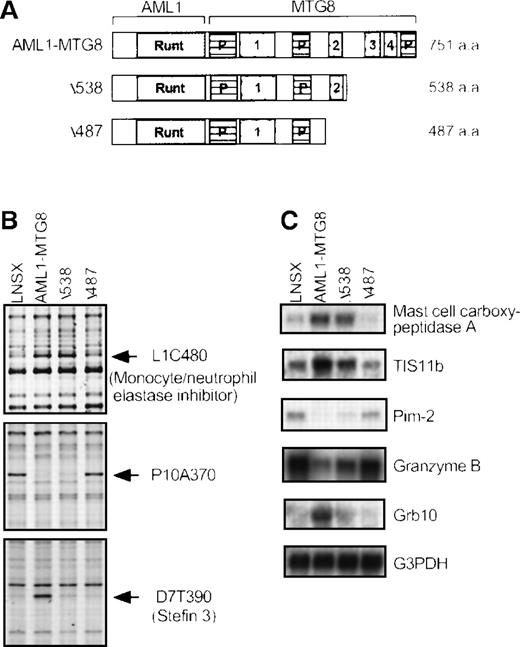
To examine the importance of the NHR2 portion of AML1-MTG8 in the alteration in expression of AMUGs and AMDGs, we investigated their differential expression patterns in L-G cells expressing C-terminal deletion mutants of AML1-MTG8 (Δ538 and Δ487) using the differential display technique and Northern blotting (Figure1B and C). The region of 51 residues (488-538) containing NHR2 is essential for complex formation between AML1-MTG8 and MTGR1 as well as for induction of G-CSF–dependent proliferation and differentiation arrest of L-G cells.34The Δ538 mutant lacks NHR3 and NHR4 but retains NHR2, whereas the Δ487 mutant is devoid of NHR2 as well as NHR3 and NHR4 (Figure 1A). As summarized in Table 1, most of the genes were differentially expressed by Δ538 like the wild-type AML1-MTG8, whereas no genes were differentially expressed by Δ487. This result indicates that the regulation of the majority of these genes requires the region containing NHR2. Exceptionally, the expression of Grb10 andStefin 3 was not affected by Δ538, indicating that the regulation of these genes requires the region containing NHR3 and NHR4. In addition, it was puzzling that the expression of MRP14 was repressed by Δ538 despite being induced by AML1-MTG8.
Regulation of AMUGs and AMDGs by AML1-MTG8 deletion mutants.
(A) Schematic representation of the structures of AML1-MTG8 and AML1-MTG8 deletion mutants Δ538 and Δ487. Runt homology domain (Runt), proline-rich regions (P), and Nervy homology regions (numbered 1-4) are indicated. (B) Differential display patterns of 3 cDNA fragments in control (LNSX) and AML1-MTG8-, Δ538-, and Δ487-expressing L-G cells. AML1-MTG8, Δ538, and Δ487 were introduced on an LNSX vector by retrovirus infection, and control cells were infected with a mock vector. The expression of L1C480 and P10A370 was altered, but the expression of D7T390 was not altered by the NHR2-containing mutant Δ538. Each fragment is indicated by an arrow. (C) Northern blotting analysis of the expression of 3 AMUGs and 2 AMDGs in control and AML1-MTG8-, Δ538-, and Δ487-expressing L-G cells. The membranes were hybridized with the corresponding cDNA fragments isolated by the differential display analysis. Human G3PDH cDNA was used as a control probe. The expression of mast cell carboxypeptidase A, TIS11b, Pim-2, and granzyme B was altered, but the expression of Grb10 was not altered by Δ538.
Regulation of AMUGs and AMDGs by AML1-MTG8 deletion mutants.
(A) Schematic representation of the structures of AML1-MTG8 and AML1-MTG8 deletion mutants Δ538 and Δ487. Runt homology domain (Runt), proline-rich regions (P), and Nervy homology regions (numbered 1-4) are indicated. (B) Differential display patterns of 3 cDNA fragments in control (LNSX) and AML1-MTG8-, Δ538-, and Δ487-expressing L-G cells. AML1-MTG8, Δ538, and Δ487 were introduced on an LNSX vector by retrovirus infection, and control cells were infected with a mock vector. The expression of L1C480 and P10A370 was altered, but the expression of D7T390 was not altered by the NHR2-containing mutant Δ538. Each fragment is indicated by an arrow. (C) Northern blotting analysis of the expression of 3 AMUGs and 2 AMDGs in control and AML1-MTG8-, Δ538-, and Δ487-expressing L-G cells. The membranes were hybridized with the corresponding cDNA fragments isolated by the differential display analysis. Human G3PDH cDNA was used as a control probe. The expression of mast cell carboxypeptidase A, TIS11b, Pim-2, and granzyme B was altered, but the expression of Grb10 was not altered by Δ538.
Regulation of AMUGs and AMDGs by AML1b and AML1a
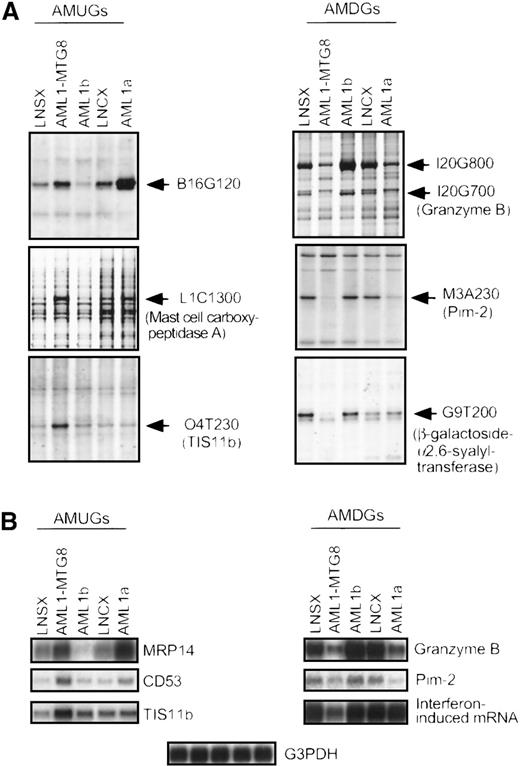
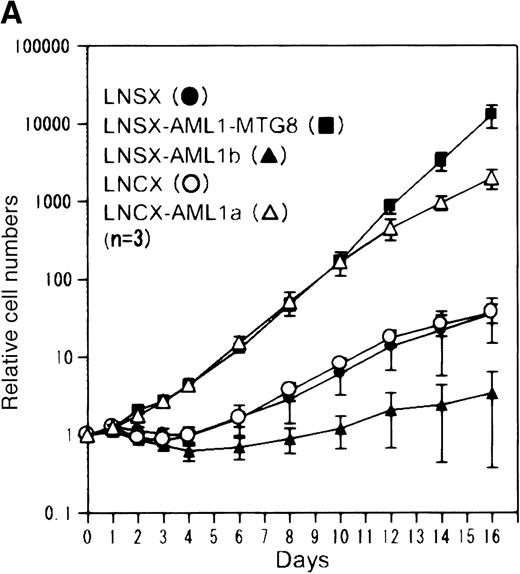
AML1-MTG8 blocks AML1-dependent transcriptional activation. To examine whether AML1b alters the expression of AMUGs and AMDGs, we investigated their differential expression patterns in L-G cells overexpressing AML1b using the differential display technique and Northern blotting (Figure 2). Surprisingly, AML1b altered the expression of only 6 of the 24 genes as summarized in Table 1. The exogenously expressed AML1b protein, however, may not alter the expression of some AML1b-regulated genes, because L-G cells express AML1b endogenously. Thus, we investigated the differential expression patterns of AMUGs and AMDGs in L-G cells overexpressing AML1a (Figure 2), which was thought to function as a competitive inhibitor of AML1b. AML1a lacks the transactivation domain and has been shown to interfere with AML1b-activated transcription of the TCRβ enhancer.43 Besides, overexpression of AML1a inhibits myeloid cell differentiation and stimulates cell proliferation of 32Dcl3 in response to G-CSF, and this effect is canceled by the concomitant overexpression of AML1b.55 We previously reported that overexpression of AML1a in L-G cells by the SV40 promoter of LNSX vector does not stimulate cell proliferation in response to G-CSF.34 However, we show here that further overexpression of AML1a by the cytomegalovirus promoter of the LNCX vector can induce cell proliferation in response to G-CSF (Figure3), although it does not inhibit differentiation as fully as AML1-MTG8 (data not shown). The cells that overexpressed AML1a showed slight growth retardation in the presence of IL-3–like cells overexpressing AML1-MTG8 (data not shown). In this system, AML1a is considered to competitively inhibit the function of AML1b. Thus the CMV promoter-directed overexpression of AML1a is expected to alter the expression of the masked AML1b-regulated genes. The expression of all the genes affected by the exogenously expressed AML1b protein, such as MRP14, A6C520, stefin 3,B16G120, granzyme B, and uridine phosphorylase, was altered by the overexpression of AML1a with the same pattern as seen with AML1-MTG8. In addition, among 18 genes unaffected by the exogenously expressed AML1b protein, the overexpression of AML1a altered the expression of 4 genes with the same pattern as seen with AML1-MTG8. This result indicates that these 4 genes are under the downstream control of AML1b and have been fully regulated by endogenous AML1b. The other 14 genes may be regulated in an AML1b-independent manner.
Regulation of AMUGs and AMDGs by AML1b and AML1a.
(A) Differential display patterns of 6 cDNA fragments in control (LNSX and LNCX) and AML1-MTG8-, AML1b-, and AML1a-expressing L-G cells. AML1-MTG8 and AML1b were introduced on an LNSX vector by retrovirus infection, and AML1a was introduced on an LNCX vector. Control cells were infected with the respective mock vectors. Each fragment is indicated by an arrow. (B) Northern blotting analysis of the expression of 3 AMUGs and 3 AMDGs in control and AML1-MTG8-, AML1b-, and AML1a-expressing L-G cells. The membranes were hybridized with the corresponding cDNA fragments isolated by the differential display analysis. Human G3PDH cDNA was used as a control probe. The expression of B16G120, MRP14, and granzyme B was counterregulated by AML1b and AML1-MTG8. These genes were up- or down-regulated by AML1a as by AML1-MTG8. The expression of mast cell carboxypeptidase A, CD53, and Pim-2 was not altered by AML1b, but altered by AML1a as by AML1-MTG8. The AML1a-mediated alteration, even though faint as seen in the case ofmast cell carboxypeptidase A, was considered to reflect the competitive inhibition of endogenous AML1b-dependent transcription. The expression of TIS11b,β-galactoside-α2,6-syalyltransferase,and interferon-induced mRNA was specifically altered by AML1-MTG8 but not altered by either AML1b or AML1a.
Regulation of AMUGs and AMDGs by AML1b and AML1a.
(A) Differential display patterns of 6 cDNA fragments in control (LNSX and LNCX) and AML1-MTG8-, AML1b-, and AML1a-expressing L-G cells. AML1-MTG8 and AML1b were introduced on an LNSX vector by retrovirus infection, and AML1a was introduced on an LNCX vector. Control cells were infected with the respective mock vectors. Each fragment is indicated by an arrow. (B) Northern blotting analysis of the expression of 3 AMUGs and 3 AMDGs in control and AML1-MTG8-, AML1b-, and AML1a-expressing L-G cells. The membranes were hybridized with the corresponding cDNA fragments isolated by the differential display analysis. Human G3PDH cDNA was used as a control probe. The expression of B16G120, MRP14, and granzyme B was counterregulated by AML1b and AML1-MTG8. These genes were up- or down-regulated by AML1a as by AML1-MTG8. The expression of mast cell carboxypeptidase A, CD53, and Pim-2 was not altered by AML1b, but altered by AML1a as by AML1-MTG8. The AML1a-mediated alteration, even though faint as seen in the case ofmast cell carboxypeptidase A, was considered to reflect the competitive inhibition of endogenous AML1b-dependent transcription. The expression of TIS11b,β-galactoside-α2,6-syalyltransferase,and interferon-induced mRNA was specifically altered by AML1-MTG8 but not altered by either AML1b or AML1a.
Overexpression of AML1a.
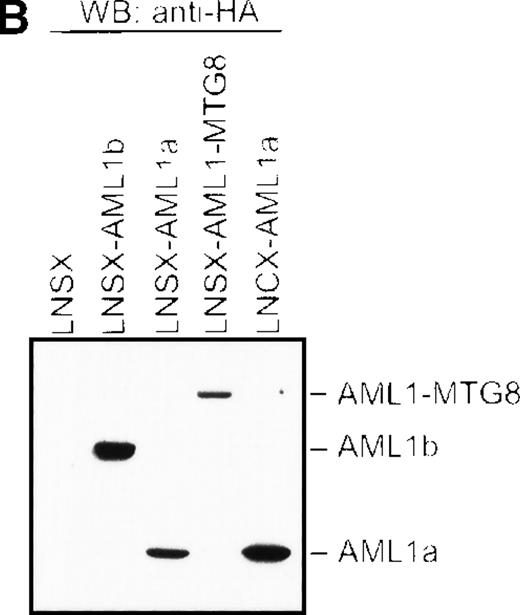
Overexpression of AML1a induced G-CSF–dependent proliferation of L-G cells. (A) G-CSF–dependent growth of control (LNSX and LNCX) and AML1-MTG8-, AML1b-, and AML1a-expressing L-G cells. Three infections were independently performed for each construct. These infected cells were separately cultured in the presence of 10 ng/mL G-CSF instead of IL-3. The averages of these 3 experiments are shown. Error bars indicate standard deviations. (B) Western blotting analysis of the expression of AML1-MTG8, AML1b, and AML1a in the infected L-G cells. Each protein was detected with anti-HA antibody. The positions of bands of AML1-MTG8, AML1b, and AML1a are indicated on the right.
Overexpression of AML1a.
Overexpression of AML1a induced G-CSF–dependent proliferation of L-G cells. (A) G-CSF–dependent growth of control (LNSX and LNCX) and AML1-MTG8-, AML1b-, and AML1a-expressing L-G cells. Three infections were independently performed for each construct. These infected cells were separately cultured in the presence of 10 ng/mL G-CSF instead of IL-3. The averages of these 3 experiments are shown. Error bars indicate standard deviations. (B) Western blotting analysis of the expression of AML1-MTG8, AML1b, and AML1a in the infected L-G cells. Each protein was detected with anti-HA antibody. The positions of bands of AML1-MTG8, AML1b, and AML1a are indicated on the right.
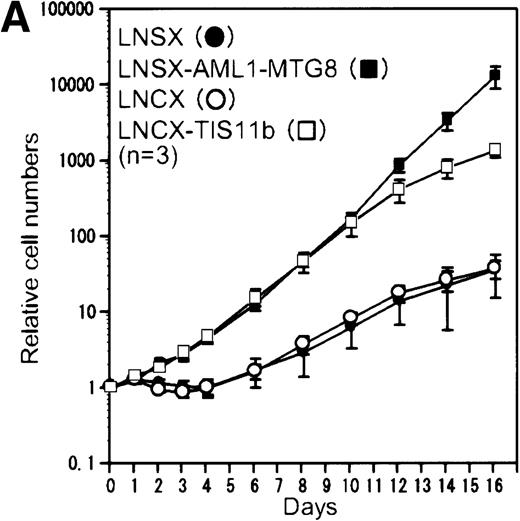
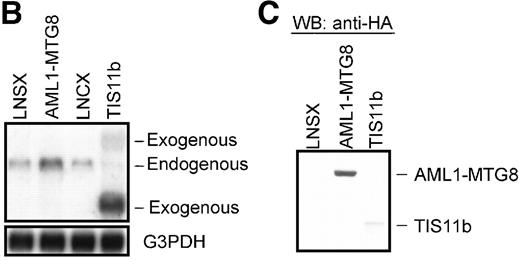
Overexpression of the TIS11b gene induced myeloid cell proliferation in response to G-CSF and delayed granulocytic differentiation
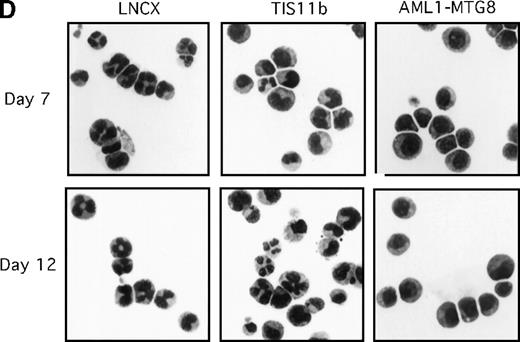
To examine whether the genes identified in this study actually have an effect on cell proliferation or differentiation, we introduced AMUGs and AMDGs into L-G cells and AML1-MTG8–expressing L-G cells, respectively, and then examined G-CSF–dependent growth and differentiation. We evaluated the effect of Grb10,Pim-2, and TIS11b overexpression as candidates. Although overexpression of Grb10 and Pim-2 did not affect cell proliferation and differentiation (data not shown), overexpression of TIS11b clearly induced G-CSF–dependent cell proliferation as shown in Figure 4A, with the growth rate decreasing after days 10 to 12. We confirmed the exogenous expression of TIS11b in L-G cells by Northern blotting analysis (Figure 4B) and by Western blotting analysis with the anti-HA antibody (Figure 4C). The TIS11b expression was maintained up to day 14 (data not shown). To examine whether TIS11b inhibits granulocytic differentiation, we chronologically followed the cell morphology of the L-G infectants in the presence of G-CSF. Before G-CSF treatment, we could not find any significant difference in cell morphology between AML1-MTG8- and TIS11b-expressing cells and control cells (data not shown). After 7 days of cultivation in the presence of G-CSF, the majority of the control cells differentiated into mature granulocytes containing segmented nuclei, whereas AML1-MTG8–expressing cells maintained an immature morphology even after day 12 as shown in Figure4D. On the other hand, TIS11b-expressing cells had not fully differentiated at day 7, and the majority of the cells comprised promyelocyte-, myelocyte-, and metamyelocyte-like immature cells. At day 12, the cell population consisted of myeloid elements in all stages of maturation, containing 20% to 30% mature granulocytes. Thus, TIS11b delayed the maturation of the L-G cells but did not block their terminal differentiation as completely as AML1-MTG8.
Overexpression of TIS11b.
Overexpression of TIS11b induced G-CSF–dependent proliferation of L-G cells but did not block their terminal differentiation as completely as AML1-MTG8. (A) G-CSF–dependent growth of control (LNSX and LNCX) and AML1-MTG8- and TIS11b-expressing L-G cells. Three infections were independently performed for each construct. These infected cells were separately cultured in the presence of 10 ng/mL G-CSF instead of IL-3. The averages of these 3 experiments are shown. Error bars indicate standard deviations. (B) Comparison of the expression of TIS11bby Northern blotting analysis in control (LNSX and LNCX) and AML1-MTG8- and TIS11b-expressing L-G cells. The membrane was successively hybridized with mouse TIS11b cDNA and human G3PDH cDNA. The positions of bands representing the endogenous TIS11b and the exogenousTIS11b genes, whose expression was directed by the LTR (upper) and the CMV promoter (lower) of the LNCX vector, are indicated on the right. The signal of the lower exogenous TIS11b gene transcript was about 10-fold higher than that of the endogenous TIS11bgene transcript in control cells. (C) Western blotting analysis of the expression of AML1-MTG8 and TIS11b in the infected L-G cells. Each protein was detected with anti-HA antibody. The positions of bands of AML1-MTG8 and TIS11b are indicated on the right. (D) Nuclear morphology of the L-G infectants when cultured in the presence of G-CSF. The cells cultured in the presence of G-CSF were stained with May-Grünwald and Giemsa solutions at the indicated days.
Overexpression of TIS11b.
Overexpression of TIS11b induced G-CSF–dependent proliferation of L-G cells but did not block their terminal differentiation as completely as AML1-MTG8. (A) G-CSF–dependent growth of control (LNSX and LNCX) and AML1-MTG8- and TIS11b-expressing L-G cells. Three infections were independently performed for each construct. These infected cells were separately cultured in the presence of 10 ng/mL G-CSF instead of IL-3. The averages of these 3 experiments are shown. Error bars indicate standard deviations. (B) Comparison of the expression of TIS11bby Northern blotting analysis in control (LNSX and LNCX) and AML1-MTG8- and TIS11b-expressing L-G cells. The membrane was successively hybridized with mouse TIS11b cDNA and human G3PDH cDNA. The positions of bands representing the endogenous TIS11b and the exogenousTIS11b genes, whose expression was directed by the LTR (upper) and the CMV promoter (lower) of the LNCX vector, are indicated on the right. The signal of the lower exogenous TIS11b gene transcript was about 10-fold higher than that of the endogenous TIS11bgene transcript in control cells. (C) Western blotting analysis of the expression of AML1-MTG8 and TIS11b in the infected L-G cells. Each protein was detected with anti-HA antibody. The positions of bands of AML1-MTG8 and TIS11b are indicated on the right. (D) Nuclear morphology of the L-G infectants when cultured in the presence of G-CSF. The cells cultured in the presence of G-CSF were stained with May-Grünwald and Giemsa solutions at the indicated days.
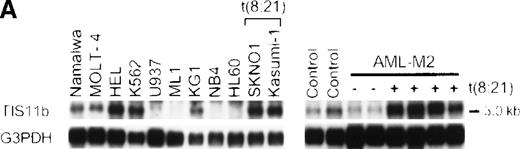
Expression of the TIS11b gene is increased in leukemic cells carrying t(8;21)
To examine whether TIS11b gene expression is associated with leukemia phenotypes, we compared the TIS11b gene expression in leukemia cell lines carrying t(8;21) (Kasumi-1 and SKNO1) to the expression in myeloid cell lines without t(8;21) (ML1, KG1, NB4, and HL60), a monocyte cell line (U937), erythroid cell lines (HEL, K562), and lymphoid cell lines (Namalwa and MOLT-4) by Northern blotting. As shown in Figure 5A, Kasumi-1 and SKNO1 cells displayed significantly higher expression of TIS11bcompared to the monocyte and other myeloid cell lines. Erythroid and lymphoid leukemia cell lines also showed high expression ofTIS11b. As well, TIS11b gene expression was higher in bone marrow cells from patients with t(8;21) AML-M2 compared to non-t(8;21) AML-M2 cells and the controls. These findings are compatible with the results showing that TIS11b gene expression is elevated in L-G cells expressing AML1-MTG8.
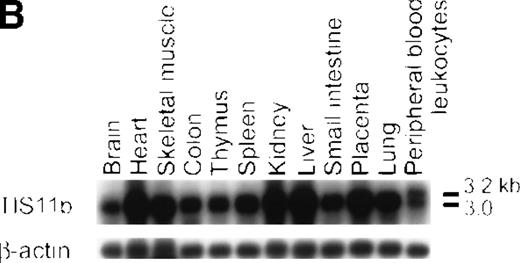
Northern blotting analysis.
The TIS11b expression was examined in leukemia cell lines and in bone marrow cells from patients with t(8;21) AML-M2 and non-t(8;21) AML-M2 (A) and in normal human tissues (B). The membranes were hybridized with human TIS11b cDNA as a probe and rehybridized with human G3PDH or β-actin cDNA. The expression of TIS11b gene is increased in leukemic cells carrying the t(8;21) translocation.
Northern blotting analysis.
The TIS11b expression was examined in leukemia cell lines and in bone marrow cells from patients with t(8;21) AML-M2 and non-t(8;21) AML-M2 (A) and in normal human tissues (B). The membranes were hybridized with human TIS11b cDNA as a probe and rehybridized with human G3PDH or β-actin cDNA. The expression of TIS11b gene is increased in leukemic cells carrying the t(8;21) translocation.
We also examined the tissue specificity of TIS11b gene expression. A 3.0-kb TIS11b gene transcript was ubiquitously expressed in all the tissues examined, with higher expression in heart, skeletal muscle, kidney, liver, and placenta. An additional 3.2-kb transcript was also observed in peripheral blood leukocytes (Figure5B).
Discussion
In this report, we identified 24 genes whose expression was altered by AML1-MTG8 in myeloid precursor cells. Nineteen were up-regulated by AML1-MTG8 (AMUGs) and 5 were down-regulated by AML1-MTG8 (AMDGs). None of these genes has ever been identified as being regulated by AML1-MTG8, although the granzyme B gene was expected to be regulated by AML1 on the basis of promoter analysis.25 In addition to granzyme B, theTIS11b, Pim-2 and mast cell carboxypeptidase Agenes have putative AML1-binding sites within their promoters. On the other hand, the MRP14, monocyte/neutrophil elastase inhibitor, and uridine phosphorylasegenes have no AML1-binding sites within their known promoter sequences. At present, however, it is unknown whether the genes identified in this study are regulated directly or indirectly. Some of them may be secondary targets whose expression is regulated by transcription factors that are induced or repressed by AML1-MTG8 or regulated in a more complex manner. However, even though these genes are regulated indirectly, our results provide important information on AML1-MTG8–mediated transcriptional regulation and leukemogenesis.
We previously found that AML1-MTG8 interacts with MTGR1 through the region of 51 residues (488-538) containing NHR2, and that this region is essential for the induction of G-CSF–dependent proliferation of L-G cells.34 These findings suggest the importance of this region in AML1-MTG8–mediated leukemogenesis. We have shown here that 21 of the 24 genes identified in this study were differentially expressed by Δ538 but not Δ487, indicating that the alteration in expression of these genes requires the region containing NHR2. Previously it was shown that this region is essential for AML1-MTG8 to interfere with AML1b-dependent transcription of the TCRβ enhancer56 and C/EBPα and AML1b-synergistic activation of the neutrophil protein 3 (NP-3) promoter.57 As well, the region containing NHR2 is essential for AML1-MTG8 to synergize with AML1b to activate the M-CSF receptor promoter.58 On the other hand, AML1-MTG8–mediated transcriptional activation of the BCL-2 promoter59 requires the region containing NHR4 (2 zinc finger motifs) but not NHR2. AML1-MTG8–mediated transcriptional repression of the multidrug resistance 1 (MDR-1) promoter60requires the region containing both NHR2 and NHR4. Thus, the regions that are required for AML1-MTG8–mediated transcriptional regulation depend on the target gene, although only a few genes have been examined on the basis of reporter assays to date. Our results, however, indicate that the region containing NHR2 is required for the regulation of the majority of the endogenous downstream genes of AML1-MTG8 in myeloid precursor cells. Because this region is essential for interaction of AML1-MTG8 with MTGR1,34 the AML1-MTG8/MTGR1 complex may play a major role in AML1-MTG8–mediated regulation at least in L-G cells.
AML1-MTG8 represses the expression of AML1 target genes. In this study, however, the exogenously expressed AML1b protein altered the expression of only 6 of the 24 AML1-MTG8–regulated genes. Taking into consideration the possibility that endogenous AML1b in L-G cells may fully regulate some of the other 18 genes, we examined their differential expression pattern in L-G cells overexpressing AML1a, a competitive inhibitor of AML1b. Among the 18 genes unaffected by AML1b, 4 genes were differentially expressed by AML1a, indicating that these genes are actually under the downstream control of AML1b. On the other hand, the other 14 genes were not affected by AML1a, indicating that the majority of the 24 genes identified in this study are not affected by either AML1b or AML1a. Although it is not obvious whether all of these 14 genes are regulated in an AML1-MTG8–specific manner, this result suggests the possibility that AML1-MTG8 not only represses the transcription of AML1 target genes but also regulates a number of specific downstream genes, such as BCL-2,59 that are not normally regulated by AML1. Furthermore, this finding supports the idea that AML1-MTG8 may have additional functions besides blocking normal AML1 functions, as suggested by the AML1-MTG8 knock-in analyses.44 45 Thus leukemic transformation may require aberrant expression of specific downstream genes regulated by AML1-MTG8 in addition to dysregulation of genes under the control of AML1.
The list shown in Table 1 includes a cell surface antigen gene and its related gene (CD53, β-galactoside-α2,6-syalyltransferase) and inflammatory-associated genes (MRP14, stefin 3,mast cell carboxypeptidase, monocyte/neutrophil elastase inhibitor, MIP1α receptor-like 1). We did not perform further analyses of these genes because they did not appear to be responsible for cell proliferation and differentiation. Among the others, we evaluated the effect of Grb10, Pim-2, andTIS11b on L-G cell proliferation and differentiation. Grb10and TIS11b are AMUGs whose expression is not altered by either AML1b or AML1a. Pim-2 is an AMDG whose expression is not altered by AML1b but down-regulated by AML1a. The first candidate,Grb10, encodes an adapter protein containing a proline-rich region, a central Pleckstrin homology domain, and a C-terminal Src homology 2 domain.61 Recently, the Grb10/BCR-ABL interaction has been shown to contribute to the BCR-ABL–induced IL-3 independence of Ba/F3 cells.62 Overexpression ofGrb10, however, did not affect the proliferation and differentiation of L-G cells. This finding is not surprising, because the AML1-MTG8Δ538 mutant that stimulates cell proliferation does not alter Grb10 expression, as described in this report. The second candidate, Pim-2, encodes a serine/threonine protein kinase63 and is a member of the Pim proto-oncogene family. Although our data that Pim-2 is down-regulated by AML1-MTG8 are in contradiction with the oncogenic potential ofPim-2, another family oncoprotein Pim-1 has been shown to stimulate c-Myc–mediated apoptosis upstream of caspase-3–like protease activation.64 We expected that Pim-2 might affect the growth of AML1-MTG8–expressing L-G cells by stimulating cell apoptosis. However, overexpression of Pim-2 did not affect G-CSF–dependent cell proliferation. The last candidate,TIS11b46-48 is 1 of 3 members of the TIS11early response gene family, which encode a zinc finger protein of the unusual CCCH class. TIS11b-overexpressing L-G cells showed slight growth retardation in the presence of IL-3 (data not shown). In response to G-CSF, however, overexpression of TIS11b clearly induced cell proliferation and delayed differentiation. This effect is different from that of AML1-MTG8, which completely blocks cell differentiation, suggesting that the transforming activity of AML1-MTG8 depends on the dysregulation of multiple downstream targets of AML1-MTG8.
Members of the TIS11 gene family of early response genes are rapidly and transiently induced in a wide variety of tissues by phorbol esters and polypeptide mitogens.65 Little is known about the functions of TIS11b compared to the well-characterized member of the family, TIS11 (TTP, Nup475).66-68 TIS11 destabilizes tumor necrosis factor-α messenger RNA (mRNA) by binding to its AU-rich element through the CCCH zinc fingers.69,70 Like TIS11, TIS11b might be an RNA-binding protein that functions as a posttranscriptional regulator. Structurally, TIS11b, TIS11, and the third member TIS11d are highly homologous over a 67-amino acid region including tandem YKTELC X8CX5CX3H sequences completely conserved among the 3 members.48TIS11bgene expression was 3-fold induced by AML1-MTG8, but the expression of TIS11 and TIS11d was not (data not shown), indicating that only TIS11b among the members of theTIS11 gene family is implicated in AML1-MTG8–mediated induction of L-G cell proliferation in response to G-CSF.
Northern blotting analysis in leukemia cell lines and bone marrow cells from patients indicates that the expression of TIS11b is increased in both of the t(8;21) cell lines, Kasumi-1 and SKNO1, and in patient samples with t(8;21) AML-M2. On the other hand, TIS11bis very weakly expressed in the other myeloid cell lines, ML1, NB4, and HL60, and in 2 non-t(8;21) AML-M2 patient samples. Given that the progenitors of t(8;21) leukemic cells also make little TIS11b mRNA, we can be fairly certain that the production of the AML1-MTG8 fusion protein elevates TIS11b gene expression in t(8;21) leukemic cells on the grounds that ectopic expression of AML1-MTG8 in L-G cells induces TIS11b gene expression. In conclusion, the enhanced expression of TIS11b in t(8;21) leukemic cells as well as its biologic function in L-G cells suggests that TIS11b is important to AML1-MTG8–mediated leukemogenesis.
Acknowledgments
We thank Dr A. D. Miller for providing retrovirus vectors, Dr D. Baltimore for providing BOSC23 cells, Dr T. Honjyo for providing L-G cells, and Dr T. Ito for suggestions about the mRNA differential display technique. We also thank M. Mori and C. Hatanaka for technical assistance. We are grateful to Drs M. Sasaki, K. Sugita, S. Tanitsu, T. Ishii, and H. Takeuchi for providing patient samples.
Supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science and Culture; by a grant from the Special Coordination Funds for the Promotion of Science and Technology from the Science and Technology Agency; by a Grant-in-Aid for the 2nd Term Comprehensive 10-year Strategy for Cancer Control and a Research Grant on Human Genome and Gene Therapy from the Ministry of Health and Welfare; and by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion, and Product Review of Japan. H. S. and S. N. are Awardees of Research Resident Fellowships from the Foundation for Promotion of Cancer Research in Japan.
Reprints:Hitoshi Ichikawa, Cancer Genomics Division, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; e-mail: hichikaw@ncc.go.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal