Abstract
In 21 patients with lymphoproliferative disease of granular lymphocytes (LDGL), we investigated the expression and the function of molecules belonging to TNF-receptor and TNF-ligand superfamilies (CD30/CD30L; CD40/CD40L; CD27/CD70; Fas [CD95]/FasL[CD95L]). Fourteen patients were characterized by a proliferation of granular lymphocytes (GLs) expressing the CD3+CD16+phenotype, whereas 7 cases showed the CD3−CD16+ CD56 ± phenotype. Our data show that both CD3+ and CD3-GLs are preferentially equipped with CD30, CD40, CD40L, CD70, and CD95 antigens; this pattern is usually associated with the lack of CD27 and CD30L antigens expression. CD95L was demonstrated in the cytoplasm in 14 of 21 cases by flow cytometry, but a definite signal was demonstrated in all cases studied using polymerase chain reaction analysis. On functional grounds, a stimulatory activity on rIL-2 mediated redirected-cytotoxicity against Fcγ+ P815 targets was demonstrated with anti-CD30, CD40, CD40L, CD70, CD95, and CD95L mAbs, although resting cells were unable to exhibit significant redirected-cell lysis. The addition of anti-CD30, CD30L, CD40, CD40L, CD95, and CD95L mAbs did not show any significant effect on cell proliferation at resting conditions or after rIL-2 stimulation, whereas anti-CD70 mAb mediated cell proliferation in 6 of 10 cases tested. This figure was not related to an increase in apoptotic cells, as investigated by Annexin-V expression. Our data indicate that both CD3+ and CD3− GLs are equipped with different costimulatory antigens, supporting the concept that these cells are in vivo activated and suggesting that these molecules might play a role in the cytotoxic mechanisms of GLs.
Lymphoproliferative disease of granular lymphocytes (LDGL) is characterized by a chronic proliferation of granular lymphocytes (GLs), which either belong to CD3+ T or CD3-NK cell lineage.1-4 TNF-α has been demonstrated to be involved in the mechanisms controlling GLs functional properties. Furthermore, we previously showed that both CD3+ and CD3− GLs of patients with LDGL in the majority of cases express a functional TNF receptor (TNF-R) p75 chain (CD120b), whereas they lack TNF-R p55 (CD120a).5 Since then, new molecules and techniques have been recognized that permit a better definition of functional properties of cells.
The TNF-R superfamily represents a growing family of receptors whose components bind surface membrane molecules, acting as cytokines, and which are referred to as the TNF ligand (TNF-L) superfamily.6-8 Members of the TNF-receptor and ligand superfamilies mediate interactions between different hematopoietic cells, including T cell/B cell, T cell/monocyte, and T cell/T cell. Signals can be transduced not only through the receptors but also through most of the ligands.6-8 As far as T and natural killer (NK) cells are concerned, a large number of reports emphasizes the functional role of TNF-R and TNF-L in mediating activation processes that ultimately lead to cell proliferation or death by apoptosis. CD30L has been reported to act both as a costimulator for the proliferation of T cells and a mediator of cytotoxicity through apoptosis.9,10 Enhancement of antitumor immunity has been shown for CD70 and CD40L.11-13 The generation of efficient cytotoxic T-lymphocytes (CTLs) has been demonstrated after CD40/CD40L interactions,14-16 and CD40L has a role in the establishment of CTL memory. It has been recently demonstrated that normal NK cells can be induced to express CD40L, which triggers cytotoxicity.17 Fas ligand (FasL) and Fas-mediated pathways are involved in the cytotoxic machinery of NK cells and CTLs,18 as well as in the transduction of activation signals in normal human T lymphocytes.19 However the role of these antigens in malignant disorders of T and NK cells is less clearly defined.
In this study we analyzed a large series of TNF-R and TNR-L molecules, which could putatively be involved in the balance of positive and negative signals ultimately leading to the maintenance of chronic GL lymphocytosis or which could be related to some properties of GLs that are relevant for the clinical manifestation of disease. To this aim, we investigated the pattern of distribution and the in vitro functional role of CD30-CD30L, CD40-CD40L, CD27-CD70, Fas (CD95)-FasL (CD95L) molecules by flow cytometry and reverse transcriptase-polymerase chain reaction (RT-PCR) in 21 patients with LDGL (11 CD3+TCR αβ+; 3 CD3+ TCR γδ+ and 7 CD3− cases).
Patients, materials, and methods
Patients
Twenty-one patients (9 males and 12 females, mean age 59 ± 16 years) were studied. In all cases, a chronic lymphocytosis (lasting more than 6 months) sustained by at least 2000 GL/μL was present in the peripheral blood.20 At the time of the study, none of the patients had received treatment, the only exception being patient 10 who is receiving methotrexate therapy for an associated disease (idiopathic pulmonary hypertension). With the use of Southern blot analysis, all patients with CD3+ LDGL were found to be monoclonally rearranged for TCR β and γ genes. Relevant immunologic data of cases under study are shown in Table1.
Surface phenotype of GLs from patients with LDGL reported in this study
| Case number . | CD3 (Leu4) . | CD4 (Leu3) . | CD8 (Leu2) . | CD16 (KD1) . | TCR αβ (WT31) . | TCRγδ (TCRδ1) . |
|---|---|---|---|---|---|---|
| 1 | + | − | + | + | + | − |
| 2 | + | − | + | + | + | − |
| 3 | + | − | + | + | + | − |
| 4 | + | − | + | + | + | − |
| 5 | + | − | + | + | + | − |
| 6 | + | − | + | + | + | − |
| 7 | + | − | + | + | + | − |
| 8 | + | − | + | + | + | − |
| 9 | + | − | + | + | + | − |
| 10 | + | − | + | + | + | − |
| 11 | + | + | + | + | + | − |
| 12 | + | − | − | + | − | + |
| 13 | + | − | − | + | − | + |
| 14 | + | − | − | + | − | + |
| 15 | − | − | − | + | − | − |
| 16 | − | − | − | + | − | − |
| 17 | − | − | − | + | − | − |
| 18 | − | − | − | + | − | − |
| 19 | − | − | − | + | − | − |
| 20 | − | − | − | + | − | − |
| 21 | − | − | − | + | − | − |
| Case number . | CD3 (Leu4) . | CD4 (Leu3) . | CD8 (Leu2) . | CD16 (KD1) . | TCR αβ (WT31) . | TCRγδ (TCRδ1) . |
|---|---|---|---|---|---|---|
| 1 | + | − | + | + | + | − |
| 2 | + | − | + | + | + | − |
| 3 | + | − | + | + | + | − |
| 4 | + | − | + | + | + | − |
| 5 | + | − | + | + | + | − |
| 6 | + | − | + | + | + | − |
| 7 | + | − | + | + | + | − |
| 8 | + | − | + | + | + | − |
| 9 | + | − | + | + | + | − |
| 10 | + | − | + | + | + | − |
| 11 | + | + | + | + | + | − |
| 12 | + | − | − | + | − | + |
| 13 | + | − | − | + | − | + |
| 14 | + | − | − | + | − | + |
| 15 | − | − | − | + | − | − |
| 16 | − | − | − | + | − | − |
| 17 | − | − | − | + | − | − |
| 18 | − | − | − | + | − | − |
| 19 | − | − | − | + | − | − |
| 20 | − | − | − | + | − | − |
| 21 | − | − | − | + | − | − |
GL, granular lymphocytes; LDGL, lymphoproliferative disease of granular lymphocytes.
Isolation of granular lymphocytes
Peripheral blood mononuclear cells were obtained by centrifugation on Ficoll-Hypaque gradient. For functional and molecular analyses (described below), the samples from patients under study were enriched for T and NK GLs using a modification of the method previously described.20 The cell suspensions were initially depleted of adherent cells by incubation for 45 minutes in plastic petri dishes at 37°C in an atmosphere of 95% air and 5% CO2. GLs were further purified by magnetic separations over columns (Mini MACS, Sunnyvale, CA). Briefly, the cell suspensions obtained as above were incubated at 4°C for 30 minutes with magnetic beads coated with anti-CD19 (Leu 12, Becton Dickinson, Sunnyvale, CA) and anti-CD14 (LeuM1, Becton Dickinson) mAbs for patient 11; with anti-CD19, anti-CD14, and anti-CD4 (OKT4, Ortho, Raritan, NJ) mAb in patients 1 to 10; with anti-CD19, CD14, CD4, and CD8 (OKT8, Ortho) in patients 12 to 14; to obtain CD3− GL (patients 15 to 21), cells were incubated with anti-CD3 (OKT3, Ortho), anti-CD14, and anti-CD19 mAbs. The cells rosetting with antibody coated beads were then isolated and removed by applying a magnetic system to the outer wall of the columns. After this multi-step negative selection procedure, more than 97% of the cells were viable when tested by the trypan blue exclusion test and 92% to 97% showed GL morphology.
Monoclonal antibodies
The commercially available mAbs used belong to the Ancell (Bayport, MN), Becton Dickinson (Sunnyvale, CA), Dako (Denmark), Immunotech (Marseille, France), PharMingen (San Diego, CA), and Serotec (Oxford, UK) series, and included CD3 (Leu4), CD4 (Leu3), CD8 (Leu2a), CD16 (Leu11c), CD40 (5C3), CD40 ligand (CD40L, 24.31), CD27(M-T271), CD70 (BU69), CD30 (Ki1), CD95 (UB2), CD95L (NOK-1), and isotype-matched control reagents. The following mAbs were also used: LD6 mAb, recognizing CD70 antigen, kindly provided by Dr S. Ferrini (IST, Genova, Italy) 21; CD30 (M44) mAb and CD30 ligand (CD30L, M81) mAb, kindly provided by Dr A. Troutt (Immunex, Seattle, WA). A type V Annexin mAb (R&D Systems, Minneapolis, MN) was used to detect apoptotic cells, according to the manufacturer's instructions. In preliminary experiments, Annexin V staining was performed on freshly isolated cells and after 24, 48, 72, and 96 hours of culture. Our data indicate that the percentage of Annexin V+/Propidium Iodide− and Annexin V+/ Propidium Iodide+ cells increased after 48 hours, remaining unchanged after 72 hours (data not shown). These data were in accordance with those obtained in dexametasone-induced apoptosis of normal peripheral blood stem cells (PBMCs).22 The results after 72-hour stimulation are reported.
Flow cytometry analysis
The expression of the above mentioned antigens on GLs was assessed by flow cytometry analysis using direct or indirect immunofluorescence assay, as previously described.20 Analysis was performed on freshly recovered GLs and after cell culture with medium alone, rIL-2 (Glaxo Institute for Molecular Biology, Geneva, Switzerland) (10 ng/mL) and rIL-15 (100 ng/mL) kindly provided by Dr A. Troutt (Immunex, Seattle, WA). A gate on CD16+ cells was defined for each case and the positivity for the antigen of interest was analyzed only on CD16+ cells. In patients with CD3+ LDGL, NK cells were excluded from the gate, taking advantage of the lower density of CD16 antigen expressed on pathologic CD3+CD16+ GLs.3 To evaluate the expression of CD30L antigen, an indirect immunofluorescent technique was performed as previously reported.5 After incubation of cells with the above mentioned mAbs or the control matched mAb, cells were washed, and a fluorescein isothiocyanate (FITC)-conjugated antimouse IgG mAb (Technogenetics, Turin, Italy) was added. In these cases, CD16 mAb was added after blocking the free binding site of goat antimouse with normal mouse serum. Intracellular staining for CD95L was also performed, according to the method of Zipp et al.23Briefly, the expression of cytoplasmic cytokines was evaluated after permeabilization of cell membranes using 1:2 diluted PermeaFix (Ortho) for 40 minutes. After the permeabilization procedure, FITC anti-FasL mAb was added.
For FACS analysis, 1 × 10−4 cells were acquired and the analysis was determined by overlaying the histograms of the samples stained with the different reagents. Cells were scored using a FACScan analyzer (Becton Dickinson) and data were processed using CELLQuest (Becton Dickinson). Mean fluorescence intensity (MFI) values were obtained by subtracting the MFI of isotype controls from the MFI of positively stained samples. To evaluate whether the differences between the peaks of cells were statistically significant with respect to controls, the Kolmogorov-Smirnov test for analysis of histograms was used and a D/s ratio greater than or equal to 15 was accepted as significant in the experimental condition used.
RNA extraction, complementary DNA synthesis and polymerase chain reaction amplification of CD30, CD30L, CD40, CD40L, and CD95L
Total cellular RNA was extracted using the Ultraspec-II RNA isolation system (Biotecx Lab, Houston, TX) from enriched GL populations and PHA (5 μg/mL) stimulated PBMCs, as previously reported.24 Complementary DNAs (cDNAs) were prepared from 2 μg of total cellular RNA by reverse transcription (RT) using a kit from Invitrogen Corp (San Diego, CA).
For the amplification of CD30, CD30L, CD40, CD40L, CD95L (FasL), and β-actin, a PCR was conducted in 50 μL reaction using 2 μL of cDNA as template, as previously reported.25 To avoid contamination, appropriate reagent controls were used. Furthermore, monocyte contamination of messenger (mRNA) samples was checked using specific primers for CD14. No detectable messages for these molecules were observed in enriched GL samples (data not shown).
Cytotoxic activity
Cytotoxic activity was assessed by the lysis of51Cr-labeled NK-sensitive K-562 and Fcγ positive P815 in a 4-hour assay, as previously reported.20 Lytic function of GLs was analyzed both at resting conditions and after in vitro activation of lymphocytes (106/mL) with rIL-2 (10 ng/mL) for 24 hours at 37°C in 5% CO2 atmosphere, in the presence or absence of CD40, CD40L, CD70, CD30, and CD30L mAbs. These mAbs were used at 0.5 μg/mL. The above quoted mAbs were added at the beginning of the test, together with effector and target cells. In all instances, target cells were used at a concentration of 10 × 104/mL and the results referred to the 10:1 effector/target ratio.
Proliferative activity
Ninety-six round-bottom well plates (Corning, New York, NY) were precoated with the CDw32 stably transfected L fibroblast cell line (ATTC, Rockville, MD) (2500 cells per well). GLs at the concentration of 1 × 106/mL were added for each well and cultured for 120 hours at 37°C in 5% CO2atmosphere in medium alone or with rIL-2 (Glaxo Institute for Molecular Biology) (10 ng/mL), in the presence of CD40 (5C3), CD40L (24.31), CD70 (L26), CD30 (M44), CD30L (M81), CD95 (DX2), and CD95L (NOK-1) or control isotype-matched IgG. Antibodies were added at the concentration of 1 μg/mL final dilution at the beginning of the culture. Each experiment was carried out in quadruplicate. For the last 18 hours of culture, plates were pulsed with 0.037 MBq (1 μCi) per well of (3H)thymidine (6.7 Ci/mmol, Du Pont, NEN, Boston, MA); cells were then harvested and the radioactivity measured with a β counter. Results are expressed as counts per minute (cpm) ± SEM.
Results
Table 2 summarizes the results of flow cytometry data obtained with different cell suspensions. All the molecules under study showed an unimodal expression on the GL surface. The data were obtained by overlaying histograms of a defined antigen and the related control. The positivity for antigen expression refers to the cases significantly binding to the molecule under study as evaluated using the Kolmogorov-Smirnov analysis, as described in “Patients, materials, and methods.”
Expression of TNF-R and TNF-L in patients under study
| Case number . | CD40 . | CD40L (CD154) . | CD27 . | CD70 (CD27L) . | CD30 . | CD30L . | CD95 (Fas) . | CD95L (FasL) . |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | − | − | + | + |
| 2 | + | + | − | + | − | − | + | −* |
| 3 | + | + | − | + | + | − | + | + |
| 4 | + | + | − | + | + | − | + | + |
| 5 | + | + | − | + | + | − | + | + |
| 6 | + | + | − | + | + | − | + | −* |
| 7 | + | − | − | + | + | − | + | −* |
| 8 | + | − | − | + | − | + | + | + |
| 9 | + | − | − | + | + | − | + | + |
| 10 | − | − | + | + | + | − | + | −* |
| 11 | + | + | − | + | − | − | + | + |
| 12 | − | + | − | + | + | − | + | + |
| 13 | + | + | − | + | + | − | + | + |
| 14 | + | ND | − | − | − | − | + | −* |
| 15 | + | + | − | + | + | + | + | + |
| 16 | + | + | + | + | + | − | + | + |
| 17 | − | + | − | + | + | − | + | + |
| 18 | + | + | − | + | + | − | + | −* |
| 19 | + | − | − | + | − | − | + | + |
| 20 | + | + | − | + | + | − | + | + |
| 21 | + | + | − | + | − | − | + | −* |
| Normal CD3+ lymphocytes | − | − | + | − | − | − | + | − |
| Normal CD16+ lymphocytes | − | − | − | − | − | − | + | −* |
| Case number . | CD40 . | CD40L (CD154) . | CD27 . | CD70 (CD27L) . | CD30 . | CD30L . | CD95 (Fas) . | CD95L (FasL) . |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | − | − | + | + |
| 2 | + | + | − | + | − | − | + | −* |
| 3 | + | + | − | + | + | − | + | + |
| 4 | + | + | − | + | + | − | + | + |
| 5 | + | + | − | + | + | − | + | + |
| 6 | + | + | − | + | + | − | + | −* |
| 7 | + | − | − | + | + | − | + | −* |
| 8 | + | − | − | + | − | + | + | + |
| 9 | + | − | − | + | + | − | + | + |
| 10 | − | − | + | + | + | − | + | −* |
| 11 | + | + | − | + | − | − | + | + |
| 12 | − | + | − | + | + | − | + | + |
| 13 | + | + | − | + | + | − | + | + |
| 14 | + | ND | − | − | − | − | + | −* |
| 15 | + | + | − | + | + | + | + | + |
| 16 | + | + | + | + | + | − | + | + |
| 17 | − | + | − | + | + | − | + | + |
| 18 | + | + | − | + | + | − | + | −* |
| 19 | + | − | − | + | − | − | + | + |
| 20 | + | + | − | + | + | − | + | + |
| 21 | + | + | − | + | − | − | + | −* |
| Normal CD3+ lymphocytes | − | − | + | − | − | − | + | − |
| Normal CD16+ lymphocytes | − | − | − | − | − | − | + | −* |
Positivity was calculated by comparing the mean fluorescence intensity (MFI) of control and sample, as reported in “Patients, materials, and methods.”
Analysis of surface expression only.
ND: not determined.
Flow cytometry analysis of costimulatory molecules on CD3+ CD16+ GL of T-cell type patients with LDGL
As reported in Table 2, all cases studied expressed CD95 (Fas). Interestingly, the density of antigen in patients with CD3+LDGL was significantly higher than that detected on normal T cells (MFI 285 ± 25 vs 143 ± 34; P < .01); in contrast, the surface expression of FasL was usually very faint, and in some cases, it was absent. After intracellular staining, a clear expression of FasL could be demonstrated on GLs in the majority of patients tested (9 of 14). The expression of CD40, CD40L, and CD30 was demonstrated in 12 of 14, 9 of 13, and 9 of 14 cases, respectively; on the contrary, CD30L was not usually expressed by GLs (Table 2). CD27 was negative in 13 of 14 cases, despite the high expression of this antigen on normal CD3+ lymphocytes (Table 2). CD70 was usually found in CD3+ GLs (13 of 14 cases). The pattern observed in 2 representative cases (nos 11 and 13) is show in Figures1 and 2. Resting normal T lymphocytes were found to be negative for CD40, CD40L, CD30, CD30L, and FasL antigen expression (Table 2).
Phenotypic expression, redirected cytoxicity, and proliferative activity.
Phenotypic expression (A), redirected cytotoxicity against P815 target cells (B), and proliferative activity (C) in the presence of mAbs to CD30, CD30L, CD40, CD40L, CD70, CD95, and CD95L in a representative CD3+TCRαβ+ patient (case 11). Because the expression of TNF-R and TNF-L antigens was different among the patients, the relationship among phenotype, cytotoxicity, and proliferation are shown for a correct interpretation of data. The differences between the peaks of cell fluorescence (continuous lines) with respect to controls (dotted lines) were analyzed using the Kolmogorov-Smirnov test for analysis of histograms and a D/s value ≥ 15 was accepted as significant, as reported in “Patients, materials, and methods.” Cytotoxicity and proliferation experiments were performed in triplicate and mean ± SEM are shown.
Phenotypic expression, redirected cytoxicity, and proliferative activity.
Phenotypic expression (A), redirected cytotoxicity against P815 target cells (B), and proliferative activity (C) in the presence of mAbs to CD30, CD30L, CD40, CD40L, CD70, CD95, and CD95L in a representative CD3+TCRαβ+ patient (case 11). Because the expression of TNF-R and TNF-L antigens was different among the patients, the relationship among phenotype, cytotoxicity, and proliferation are shown for a correct interpretation of data. The differences between the peaks of cell fluorescence (continuous lines) with respect to controls (dotted lines) were analyzed using the Kolmogorov-Smirnov test for analysis of histograms and a D/s value ≥ 15 was accepted as significant, as reported in “Patients, materials, and methods.” Cytotoxicity and proliferation experiments were performed in triplicate and mean ± SEM are shown.
Case 13.
Phenotypic expression (A), redirected cytotoxicity against P-815 target cells (B), and proliferative activity (C) in the presence of mAbs to CD30, CD30L, CD40, CD40L, CD70, CD95, and CD95L in a representative CD3+TCRγδ+ patient (case 13). The experimental conditions were the same as in Figure 1.
Case 13.
Phenotypic expression (A), redirected cytotoxicity against P-815 target cells (B), and proliferative activity (C) in the presence of mAbs to CD30, CD30L, CD40, CD40L, CD70, CD95, and CD95L in a representative CD3+TCRγδ+ patient (case 13). The experimental conditions were the same as in Figure 1.
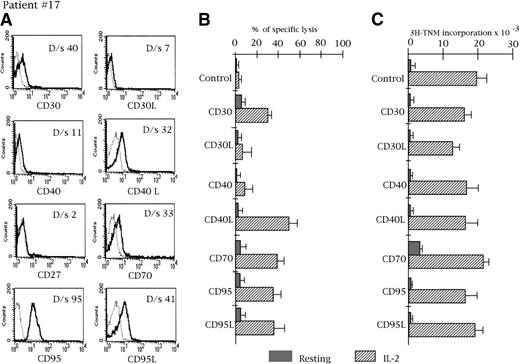
Flow cytometry analysis of costimulatory molecules on CD3− CD16+ GLs of natural killer-type LDGL
As shown in Table 2, GLs of patients with CD3−LDGL were found to express CD95 antigen. As for CD3+ GL, the MFI value of patients' GLs was significantly higher than the MFI of control NK cells (197 ± 21 vs 89 ± 14,P < .01). FasL expression was detected in 4 of 7 cases; however, the negative cases were only tested for the surface expression (Table 2) and a clear signal for FasL mRNA was demonstrated by PCR (discussed below). A significant expression of CD30, CD40, CD40L, and CD70 antigens was observed in most patients, whereas CD30L and CD27 positivity was reported in 1 case each. The pattern of expression in a representative case (no 17) is shown in Figure3. Normal resting CD16+ NK cells usually lack or show a very faint expression of CD30, CD30L, CD40, CD40L, CD27, CD70, and FasL antigens (Table 2).
Case 17.
Phenotypic expression (A), redirected cytotoxicity against P-815 target cells (B), and proliferative activity (C) in the presence of mAbs to CD30, CD30L, CD40, CD40L, CD70, CD95, and CD95L in a representative CD3− patient (case 17). The experimental conditions were the same as in Figure 1.
Case 17.
Phenotypic expression (A), redirected cytotoxicity against P-815 target cells (B), and proliferative activity (C) in the presence of mAbs to CD30, CD30L, CD40, CD40L, CD70, CD95, and CD95L in a representative CD3− patient (case 17). The experimental conditions were the same as in Figure 1.
Flow cytometry analysis of costimulatory molecules after cell culture
In 4 cases (cases 1, 4, 17, and 21), we investigated the expression of CD40, CD40L, CD27, CD70, CD30, CD30L, CD95, and FasL after in vitro culture with rIL-2 and rIL-15. After 24-hour culture in medium alone, the pattern of expression of cells was unchanged, compared with those of freshly isolated cells. In fact, as reported in Table3, rIL-2 stimulation induced up-regulation of CD70 in only 1 of 4 cases tested, the expression of receptors and ligands in the other cases being unmodified. The expression of TNF-R and TNF-L was also evaluated after stimulation with rIL-15 (100 ng/mL for 72 hours). This cytokine has been claimed to play a key role in the pathogenetic events of LDGL. As reported in Table 3, rIL-15 was demonstrated in one case (no 1) to up-regulate CD70, whereas down-modulating CD30L; in 1 case (no 4) to down-modulate CD30 and to have no effect on antigen expression in the remaining 2 cases.
Expression of TNF-R and TNF-L in patients with LDGL after different culture conditions
| Patient number . | Culture conditions . | CD40 . | CD40L . | CD27 . | CD70 . | CD30 . | CD30L . | CD95 (Fas) . | CD95L (FasL) . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Medium alone3-150 | + | + | − | + | − | − | + | + |
| rIL-2 | ns3-151 | ns | ns | 193-152 | ns | ns | ns | ns | |
| rIL-15 | ns | 223-153 | ns | 243-152 | ns | ns | ns | ns | |
| Case 4 | Medium alone | + | + | − | + | + | − | + | + |
| rIL-2 | ns | ns | ns | ns | ns | ns | ns | ns | |
| rIL-15 | ns | ns | ns | ns | 293-153 | ns | ns | ns | |
| Case 17 | Medium alone | − | + | − | + | + | − | + | + |
| rIL-2 | ns | ns | ns | ns | ns | ns | ns | ns | |
| rIL-15 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Case 21 | Medium alone | + | + | − | + | − | − | + | − |
| rIL-2 | ns | ns | ns | ns | ns | ns | ns | ns | |
| rIL-15 | ns | ns | ns | ns | ns | ns | ns | ns |
| Patient number . | Culture conditions . | CD40 . | CD40L . | CD27 . | CD70 . | CD30 . | CD30L . | CD95 (Fas) . | CD95L (FasL) . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Medium alone3-150 | + | + | − | + | − | − | + | + |
| rIL-2 | ns3-151 | ns | ns | 193-152 | ns | ns | ns | ns | |
| rIL-15 | ns | 223-153 | ns | 243-152 | ns | ns | ns | ns | |
| Case 4 | Medium alone | + | + | − | + | + | − | + | + |
| rIL-2 | ns | ns | ns | ns | ns | ns | ns | ns | |
| rIL-15 | ns | ns | ns | ns | 293-153 | ns | ns | ns | |
| Case 17 | Medium alone | − | + | − | + | + | − | + | + |
| rIL-2 | ns | ns | ns | ns | ns | ns | ns | ns | |
| rIL-15 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Case 21 | Medium alone | + | + | − | + | − | − | + | − |
| rIL-2 | ns | ns | ns | ns | ns | ns | ns | ns | |
| rIL-15 | ns | ns | ns | ns | ns | ns | ns | ns |
Positivity was calculated by comparing the mean fluorescence intensity (MFI) of control and sample, as reported in the “Materials and methods” section.
ns: not significant. The histogram obtained at resting conditions was compared with the histogram obtained after rIL-2 and rIL-15 stimulations. The statistical analysis was calculated using the Kolmogorov-Smirnov test and a D/s ratio >15 was accepted as significant.
Indicates that the expression of antigen was up-modulated.
Indicates that the expression of antigen was down-modulated.
Reverse transcriptase-polymerase chain reaction characterization of CD30, CD30L, CD40, CD40L, and CD95L on GLs of T- and natural killer-type LDGL
Because of the low membrane expression of some TNF-R and TNF-L superfamily molecules on CD3+ and CD3−GLs as detected by flow cytometry, the presence of CD30, CD30L, CD40, CD40L, and FasL molecules was further investigated by RT-PCR. Data obtained in 3 representative patients with CD3+ and 3 representative patients with CD3− LDGL patients are reported in Figure 4. Both in patients with CD3+ LDGL (lanes 4-6, cases 3, 8, and 13), and in patients with CD3− LDGL (lanes 1-3, cases 15, 17, and 20), CD30, CD30L, CD40, and CD40L demonstrated the same pattern with flow cytometry. Interestingly, a definite signal for FasL was demonstrated in all patients tested.
RT-PCR analysis of the expression of CD30, CD30L, CD40, CD40L, Fas, FasL, and control β-actin mRNA in GLs from 6 LDGL patients.
Lane 1 indicates molecular weight marker (100 base pairs [bp] DNA ladder); lane 2: control peripheral blood mononuclear cells stimulated for 12 hours with PHA (5 μg/mL) (positive control) for CD30, CD30L, CD40L, and FasL; positive control for CD40 was represented by purified B lymphocytes; lane 3: the negative control (sample without cDNA), lanes 4 to 6: 3 CD3+ LDGL cases (cases 3, 8, and 13 in Table 1), lane 7 to 9: 3 CD3− LDGL (cases 15, 17, and 20 in Table 1).
RT-PCR analysis of the expression of CD30, CD30L, CD40, CD40L, Fas, FasL, and control β-actin mRNA in GLs from 6 LDGL patients.
Lane 1 indicates molecular weight marker (100 base pairs [bp] DNA ladder); lane 2: control peripheral blood mononuclear cells stimulated for 12 hours with PHA (5 μg/mL) (positive control) for CD30, CD30L, CD40L, and FasL; positive control for CD40 was represented by purified B lymphocytes; lane 3: the negative control (sample without cDNA), lanes 4 to 6: 3 CD3+ LDGL cases (cases 3, 8, and 13 in Table 1), lane 7 to 9: 3 CD3− LDGL (cases 15, 17, and 20 in Table 1).
Cytotoxic activity
Redirected cytotoxic activity was evaluated against the Fcγ positive P815 cell line. Cells alone were not cytotoxic for P815 targets; the addition of anti-CD30, CD30L, CD40, CD40L, and CD70 mAbs did not result in significant changes of lytic activity at resting conditions. After incubation of effector cells with rIL-2, a definite increase in cytotoxicity, although to variable degrees, was obtained with anti-CD70, CD30, CD40, CD40L, Fas, and anti-FasL mAbs, when cells expressed these antigens (Figures 1 to 3, panel B). Cytotoxic activity against the NK-sensitive K562 target cells was not modified by the addition of anti-CD30, CD30L, CD40, CD40L, CD70, Fas, and anti-FasL mAbs at resting conditions or after cell activation with rIL-2 (data not shown).
Proliferative activity
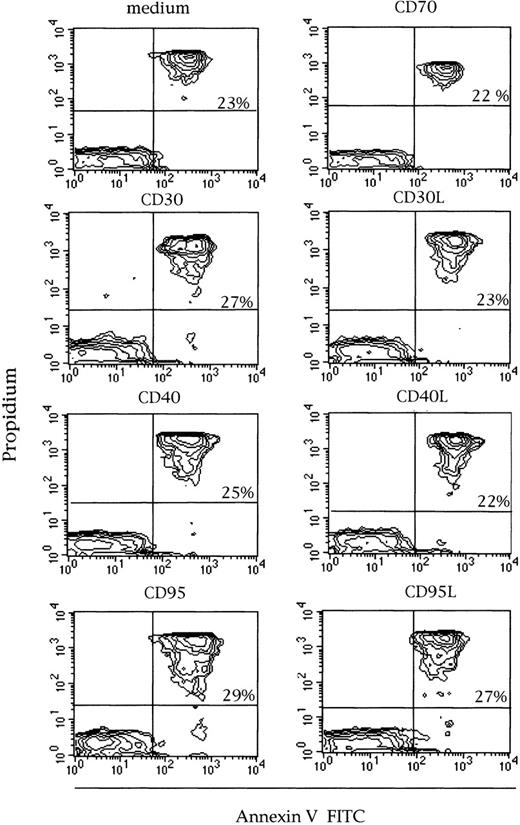
To evaluate whether the proliferative activity could be induced by the molecules under study, the effect of immobilized mAbs to anti-CD30, CD30L, CD40, CD40L, CD70, CD95, and CD95L mAb was evaluated on purified CD3+ and CD3− GLs expressing these antigens. Cells were cultured at resting conditions and after activation with rIL-2. The results obtained in 3 representative cases are reported in Figures 1 to 3, panel C. Antibodies to CD30, CD30L, CD40, CD40L, Fas, and FasL were unable to significantly stimulate GL proliferation using cells alone or when cells were stimulated with rIL-2. A slight proliferative effect was shown using the anti-CD70 LD6 mAb in 6 of 8 cases tested. Annexin V expression by flow cytometry showed consistent results in cells stimulated with rIL-2 alone and with the addition of stimuli. Figure 5 shows 1 representative case (no 4) of the 5 cases tested.
Expression of Annexin V on purified GLs in a representative LDGL case (no 4) of 5 different patients tested.
Purified GLs were stimulated with rIL-2 (“Patients, materials, and methods”) for 72 hours, then cells were stained with FITC Annexin V and propidium iodide. The percentage of double positive cells ranged between 22% and 29%.
Expression of Annexin V on purified GLs in a representative LDGL case (no 4) of 5 different patients tested.
Purified GLs were stimulated with rIL-2 (“Patients, materials, and methods”) for 72 hours, then cells were stained with FITC Annexin V and propidium iodide. The percentage of double positive cells ranged between 22% and 29%.
Discussion
In this study, we demonstrated that both freshly isolated CD3+ and CD3− GL from patients with LDGL express molecules belonging to TNF receptors and ligand families, supporting the notion that GL of patients with LDGL are activated in vivo. The pattern of expression of these antigens is intriguing and may lead to speculations on the pathogenetic mechanisms, leading to the GL proliferation.
Different studies have evaluated the expression of TNF receptors and ligands on normal T lymphocytes and NK cells, demonstrating that virtually all these molecules can be expressed on the cell surface, either constitutionally (eg, CD27 and CD95) or after appropriate triggering.7,25 Controversy exists over the expression of some of these antigens, namely, CD30, CD40L, and FasL by resting lymphocytes.27-29 Several reports demonstrated the presence of TNF-R and TNF-L on neoplastic cells in B-lymphoproliferative disorders,24,25 whereas the role of these molecules on T-cell malignancies is less known. CD70, CD30, CD40L, as well as the CD120a and CD120b, TNF receptors have been reported to be expressed in some T–non-Hodgkin's lymphomas.25,30,31 Recent data on FasL expression by GLs of patients with LDGL suggested a role for this molecule as a marker of disease.32 33 No data are available on the expression of other costimulatory molecules belonging to TNF-R and TNF-L families in LDGL.
The first conclusion from our results is that the phenotypic expression of TNF-R and TNF-L on GLs of patients with LDGL is restricted to some of these antigens. This finding is consistent with our previous data showing that the cells of patients with LDGL express CD120b, but lack CD120a antigen.5 The current results extend the above mentioned data by showing the preferential expression of CD40, CD40L, CD30, and CD70 antigens on the GL surface. This pattern is usually associated with the lack of CD30L and CD27 antigens. This latter finding is particularly intriguing for CD3+ GLs because CD27 is constitutionally expressed by most CD3+CD8+ cells. Interestingly, recent data demonstrate that CD27 antigen is down-regulated on the T-cell surface after prolonged activation,34 this feature possibly accounting for the absence of the antigen on the surface of GLs. The expression of many of these molecules was usually unimodal and weak, consistent with the low antigen density on the cell surface. As a matter of fact, a correspondence between phenotypic expression of antigen and the presence of specific mRNA by PCR was demonstrated.
A second conclusion from our results deals with the fact that, although a preferential pattern of expression was demonstrated in LDGL patients as a homogeneous group, relevant differences in terms of expression of TNF-R and TNF-L molecules by GL could be shown if one considers individual cases. Results reported in Table 2 indicate that many different combinations were possible. For each patient, however, the pattern of antigen expression was shown to be maintained, as demonstrated by repeating immunophenotypic analyses at different times during the follow-up (data not shown). This indicates that the relevant pattern represents an individual specific marker. There is not a clear explanation for this feature of patients with LDGL. Because in these patients, GLs are considered to represent in vivo primed CTLs),1,3 a possible interpretation could be related on the putative inciting antigenic stimulation, which is assumed to be responsible for the selection and further growth of the subset of GLs. Interestingly, many features of CD3+ GLs of patients with LDGL are shared with CTLs, namely, the phenotypic cytotoxic profile, activation through the CD3/TCR receptor, the preferential usage of a restricted Vβ repertoire,24 the constitutive expression of FasL33 and perforin,35 and the demonstration of an antigenic pressure acting on proliferating GLs.36 A second, still unknown event, is supposed to occur to establishing a GL proliferation, finally leading to the monoclonal pattern of growth.1,37 As a matter of fact, all CD3+ cases under study have been demonstrated to show a monoclonal rearrangement on β and γ genes of TCR. Taken together, our results indicate that we are dealing with clonal populations immortalized at a discrete activation stage. It might be speculated that the persistent expression of costimulatory molecules of the TNF superfamily by patients' GLs be referred to the different phases of disease in which clonality was established. This is consistent with the hypothesis that the transforming event responsible for the maintenance of proliferation is expected to take place after the expression of relevant antigens is accomplished. Although clonality is difficult to demonstrate in CD3− GL, the proliferation of a discrete subset of NK cells has been reported for patients with CD3−NK-type proliferation,38 suggesting that a similar mechanism might occur also for CD3− LDGL.
The contribution of TNF-R and TNF-L in mediating activation processes and ultimately leading to cell proliferation or death by apoptosis has been extensively evaluated in normal T lymphocytes and NK cells. In patients with LDGL, Lamy et al39 recently demonstrated that CD3+ GLs are resistant to the lysis mediated through Fas, suggesting that this finding could partially account for the cell accumulation in these patients. We tried to investigate the possible functional role for CD30, CD30L, CD40, CD40L, CD70, Fas, and FasL on cytotoxicity and proliferation using immobilized mAbs. By using the majority of these stimuli, we were unable to show either positive or negative effects in the proliferative activity. This feature was not associated with an increased cell death by apoptosis, as indicated by Annexin binding analysis. The only exception was the CD70 antigen that was demonstrated to induce proliferation in some cases. In most patients, cytotoxic activity was induced by CD30, CD40, CD40L, and by CD70 in a redirected cytotoxic assay against the P815 target cells after rIL-2 activation, whereas lysis of the NK-sensitive K-562 target cells was unaffected. As reported in Table 3, the modulation of the receptors and ligands (both up- or down-regulation) by itself does not explain the mechanisms of rIL-2 induced redirected cytotoxicity in the P815 assay. The coexpression of other molecules, ie, the recently described Killer Activatory Receptors40 or the Natural Cytotoxicity Receptors,41 induced by rIL-2 might be necessary for the release of cytotoxic potential through TNF-R and TNF-L. It is possible that various cytokines could differently modulate antigen expression. Our data on TNF-R and TNF-L expression after stimulation with rIL-15, which has been claimed to play a key role in the pathogenetic events of LDGL,20 seem to suggest a similar mechanism. Studies are in progress to test the role of different cytokines on TNF-R and L expression.
Our data are consistent with a functional role of these molecules on the activation of cytotoxic machinery. Interestingly, cytotoxic activity of GLs against BFU-E has been claimed to play a role in pure red cell aplasia, associated with GL proliferation,42 thus for the first time indicating an in vivo role for the cytotoxic machinery in the pathologic manifestation of disease. Whether our results could be biased by the in vitro experimental conditions is still unclear. It has been reported that the different antigen density, as well as the specific microenvironment, could be crucial for the final effect of a stimulus mediated through these receptors and ligands.8 Because these conditions cannot be strictly reproduced in vitro, the role of these molecules in vivo is still elusive. Alternatively, the expression of some of these antigens could represent a biologic marker of a transformed cell, devoid of functional activity. No correlation could be demonstrated between the clinical status of patients and the pattern of TNF-R and TNF-L expression. Because prognosis of this disease is fairly good,1 3 a long follow-up of patients may contribute to a better definition of whether the pattern of expression of TNF-R and TNF-L could have prognostic significance in LDGL.
In conclusion, we herein provide evidence that the majority of patients with LDGL express different TNF-R and TNF-L antigens, thus indicating a peculiar cell activation condition. Several issues remain to be defined concerning the in vivo activities of these antigens, their ability to modulate the immune response, and their possible role on some clinical manifestations of LDGL, namely, anemia and neutropenia.
Acknowledgments
We thank Dr S. Ferrini (IST, Genova, Italy) for providing LD6 mAb, Dr A. Troutt (Immunex Co, Seattle, WA) for providing M44 mAb and M81 mAb and rIL-15, and Mr Martin Donach for his help in the preparation of the manuscript.
Supported by Associazione Italiana per la Ricerca sul Cancro, Milan, Italy.
Reprints:Gianpietro Semenzato, Università degli Studi di Padova, Dip. Medicina Clinica e Sperimentale, Immunologia Clinica, Via Giustiniani 2, 35128 PADOVA, Italy; e-mail:giansem@ux1.unipd.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 4. RT-PCR analysis of the expression of CD30, CD30L, CD40, CD40L, Fas, FasL, and control β-actin mRNA in GLs from 6 LDGL patients. / Lane 1 indicates molecular weight marker (100 base pairs [bp] DNA ladder); lane 2: control peripheral blood mononuclear cells stimulated for 12 hours with PHA (5 μg/mL) (positive control) for CD30, CD30L, CD40L, and FasL; positive control for CD40 was represented by purified B lymphocytes; lane 3: the negative control (sample without cDNA), lanes 4 to 6: 3 CD3+ LDGL cases (cases 3, 8, and 13 in Table 1), lane 7 to 9: 3 CD3− LDGL (cases 15, 17, and 20 in Table 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.647/5/m_bloo01418004w.jpeg?Expires=1767732094&Signature=vefo6KPidnsIyF7jRhTrxog7-cPjy8ufubSShTkm~p~eCH29lR-BKk-TFU1txpQw5eggKczY7CR9BEQUmhz1FeeDcTbvtffpVfJK8iOOnbD-Hp-3rUslxslD4-NbdC5ZTqWeaS02AMAnqMVUcwl~5NzYc10NFkb0hwCxsD-ZaevVeu8kkZpwsQPUUiWbc1mUZIToRWRtMTPnMDURcd4b96lhH5PCzDAOknsWkg9mCFL0b3fSiPVKH4VFy8KCvXtUxMfEVYLy-aWSnCyARUKBkA~LHq-7b2QiR5e18-rSzt6lKiYJvabG4Xz9dbnzxLg~DBTot~DNY0ls9R2oq7XpAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal