Abstract
Triggering of the tissue factor (TF)-dependent coagulation pathway is considered to underlie the generation of a procoagulant state during endotoxemia. To determine the in vivo pattern of monocytic TF messenger RNA (mRNA) expression during endotoxemia, 10 healthy volunteers were injected with lipopolysaccharide (LPS, 4 ng/kg) and blood was collected before and 0.5, 1, 2, 3, 4, 6, 8, and 24 hours after LPS administration. Total blood RNA was isolated and amplified by NASBA (nucleic acid sequence-based amplification), followed by quantitation of TF mRNA by an electrochemiluminescence (ECL) assay. To compare the pattern of coagulation activation with the kinetics of monocytic TF mRNA expression, we measured plasma levels of markers of thrombin generation, thrombin-antithrombin (TAT) complexes, and prothrombin fragment 1 + 2 (F1 + 2). Baseline value (mean ± SEM) of the number of TF mRNA molecules per monocytic cell was 0.08 ± 0.02. A progressive and significant (P < .0001) increase in TF expression was observed after LPS injection (+0.5 hour: 0.3 ± 0.1, +1 hour: 1.3 ± 0.9, +2 hours: 4.1 ± 0.9), peaking at +3 hours (10 ± 1.9 TF mRNA molecules per monocyte). As TF mRNA levels increased, thrombin generation was augmented. Peak levels of TAT and F1 + 2 were reached later (at t +4 hours) than those of TF mRNA. TF mRNA, TAT, and F1 + 2 levels returned to baseline after 24 hours. In conclusion, we used a NASBA/ECL-based technique to quantify TF mRNA in whole blood during human endotoxemia and observed a 125-fold increase in TF mRNA levels. Our data demonstrate a pivotal role for enhanced TF gene activity in the activation of coagulation after LPS challenge.
Tissue factor (TF) is a membrane-bound glycoprotein that is considered to be the main initiator of the coagulation cascade by acting as a cofactor of activated factor VII.1 In fact, factor VIIa itself bears limited procoagulant activity, but in complex with TF, factor VIIa is capable of proteolytic activation of factors IX and X, which leads to thrombin formation and conversion of fibrinogen into fibrin. It is assumed that TF is not normally expressed on cells in direct contact with blood, but TF expression may become expressed on intravascular cells (mainly monocytes and endothelial cells) by the action of inflammatory stimuli, including lipopolysaccharide (LPS, endotoxin).1 This augmented TF expression is thought to be responsible for the thrombotic manifestations of various inflammatory states.1
Endotoxemia triggered by intravenous injection of Escherichia coli LPS into humans is a powerful model to investigate the pattern of inflammatory responses and hemostatic changes that occur during gram-negative sepsis.2-6 Human endotoxemia is associated with a well-documented state of cytokine activation, transient activation of fibrinolysis, and sustained activation of coagulation, resulting in a net procoagulant state.5,6 There is good evidence for a role of the extrinsic TF-dependent coagulation pathway in eliciting the procoagulant state during endotoxemia and gram-negative sepsis. In particular, the use of antibodies directed against TF or factor VII/VIIa resulted in attenuation of the activation of coagulation in models of endotoxemia and septicemia in primates.7-13
Based largely on in vitro data, both monocytes and endothelial cells are assumed to be the sites of induced intravascular TF expression, but direct experimental evidence for enhanced TF gene activity in these cells in humans is lacking. Endothelial cells are not accessible for TF quantitation. In addition, though some groups have reported increased TF antigen expression in whole blood,14-17 flow cytometric analysis of circulating leukocytes has been negative as reported by others.18 The latter finding contrasts with the relative ease with which it is possible to follow TF expression on human endothelial cells and on leukocytes in ex vivo experiments.19-27 The reason(s) for this discrepancy are poorly understood, but may be related to plasmatic factors that obscure TF epitopes in vivo. To help resolve this issue, we have looked for alternative assessment of TF induction during endotoxemia. We used a sensitive method to accurately quantify TF messenger RNA (mRNA) expression by blood leukocytes, based on the amplification of RNA by a nucleic acid sequence-based amplification (NASBA) system28and precise mRNA quantitation by an electrochemiluminescence (ECL) assay.29 We used this methodology to determine the kinetics of TF mRNA expression in healthy volunteers after exposure to intravenous endotoxin. Additionally, we measured plasma levels of markers of thrombin generation to directly relate the pattern of coagulation activation with the kinetics of monocytic TF mRNA expression during human endotoxemia.
Patients and methods
Patients
Ten healthy male volunteers (mean age, 24 years, range 19-29 years) received an intravenous (IV) injection of E coli LPS (Lot G, 4 ng/kg body weight, United States Pharmacopeial Convention, Rockville, MD). All subjects were in good health, as documented by history, physical examination, and hematologic and biochemical screening. All individuals were admitted to the Clinical Research Unit (Academic Medical Center, University of Amsterdam) and remained under the supervision of trained medical staff during the whole period of the study. Clinical parameters (oral body temperature, arterial blood pressure, pulse rate, score of symptoms) were regularly recorded. Blood (nucleic acid extraction, coagulation activation markers measurements, and leukocyte counts, discussed later) was collected from the antecubital vein immediately before endotoxin injection and at 0.5, 1, 2, 3, 4, 6, 8, and 24 hours thereafter. The institutional Ethics and Research Committees approved this study, and written informed consent was obtained from all study subjects.
Methods
Nucleic acid isolation.
Total nucleic acids were isolated from whole blood according to a solid-phase extraction method described by Boom et al.30 Briefly, 100 μL whole blood was mixed with 900 μL lysis buffer (50 mmol/L Tris-HCl [pH 6.4], 20 mmol/L EDTA, 1.3% [wt/vol] Triton X-100, 5.25 mol/L guanidine thiocyanate). For quantification purposes, in vitro synthesized internal calibrator RNA (Q-RNA) was designed to be identical to the wild-type with only a small region of the sequence replaced with a sequence enabling specific detection of this RNA. Twenty microliters of a Q-RNA solution (prepared by dissolving a freeze-dried Q-RNA sphere in 220 μL elution buffer) was added to each tube containing lysed whole blood. Specifically, 2 × 104 molecules of TF Q-RNA were present in the 20 μL solution that was added to the sample. Next, 50 μL of activated silica suspension (1 g/mL) was added to the lysis mixture. After washing and drying the silica, nucleic acid was eluted with 50 μL elution buffer and stored at −70°C. This material was used as input in the amplification reactions.
Nucleic acid sequence-based amplification.
NASBA reactions were carried out according to Kievits et al31 with modifications. Briefly, 5 μL of nucleic acid solution was added to 10 μL NASBA mixture. The final concentrations in 20 μL reaction mixture were 40 mmol/L Tris-HCl, pH 8.5, 12 mmol/L MgCl2, 70 mmol/L KCl, 15% vol/vol DMSO, 1 mmol/L each dNTP, 2 mmol/L each NTP, 0.2 μmol/L of each TF primers (TF-1 and TF-2), and 2 × 104 molecules of TF Q-RNA. TF oligonucleotide primers were designed for specific amplification of a 213-nucleotide long fragment of TF mRNA. The TF primer sequences were TF-1: 5′-AATTCTAATACGACTCACTATAGGGAGAGGGCTGTCTGTACTCTTCGGTTTA-3′ (T7 promoter part underlined) and TF-2: 5′-GAAGGAACAACACTTTCCTA-3′ (positions 788-765 [TF-1] and 575-594 [TF-2] of TF mRNA sequence, GenBank accession number M16553). The reactions were incubated for 5 minutes at 65°C to destabilize secondary structures in the RNA and then for 5 minutes at 41°C (primer annealing temperature). Subsequently, 5 μL of the NASBA enzyme solution (1.28 U/μL AMV-reverse transcriptase [Seikagagu America, Ijamsville, MD], 0.016 U/μL RNAse H [Pharmacia], 6.4 U/μL T7-RNA polymerase [Amersham Pharmacia Biotech, Rosendaal, Netherlands] and 0.43 g/L bovine serum albumin (BSA) [Roche Diagnostics, Almere, Netherlands]) was added to each reaction tube to initiate amplification, and reactions were incubated for 90 minutes at 41°C. Hence, isothermal nucleic acid amplification was accomplished by the simultaneous activity of the 3 enzymes. To check for possible contamination, we included a tube containing water instead of nucleic acid solution in each set of amplification reactions. NASBA products were visualized on a 2% electrophoresis agarose gel containing ethidium bromide. To exclude the possibility of nonspecific amplification in the initial experiments, separation of amplified RNA on an agarose gel, followed by blotting onto a filter and subsequent hybridization with a labeled oligonucleotide probe was also carried out. These experiments confirmed the presence of specific amplification products for TF mRNA. NASBA reactions were stored at −20°C.
Electrochemiluminescence assay.
The ECL method has been previously adapted for the detection of amplified nucleic acids using ECL-labeled oligonucleotides in sandwich hybridization assays.29 32 Amplified RNA was detected using a 1-step probe hybridization method, followed by detection and quantitation in an ECL reader, which automatically separates free from bound label. The subsequent detection of bound probes uses ECL. The computer linked to the instrument directly calculates results. In our experiments, fresh separate mixtures of TF capture probe with TF wild-type detection probe (1:1 ratio) and TF capture probe with TF Q-ECL detection probe, specific for the internal calibrator RNA, (1:1 ratio) were prepared. Oligonucleotide sequences of the probes were TF capture probe: 5′-[Biotine] GCCTCCGGGATGTTTTTGGCAAGGA-3′ (positions 596-620 in mRNA sequence, GenBank accession number M16553), TF wild-type detection probe: 5′-[ECL label] GTTCAGGAAAGAAAACAGCCA-3′ (positions 656-676 in mRNA sequence, GenBank accession number M16553), and TF Q detection probe: 5′-[ECL label] AAGTAAAGTCGACAAGCACAG-3′ (replaced sequence at the position of the TF wild-type detection probe). Five microliters NASBA reaction (1:5 diluted) was added to 20 μL probe mixture (10 μL capture and 10 μL detection probe; the 2 probe combinations in separate tubes) in ECL tubes. Samples were incubated 15 minutes at 60°C, and tubes were mixed by vortexing every 5 minutes. Three hundred microliters assay buffer (100 mmol/L tripropylamine, pH 7.5, Organon Teknika, Boxtel, The Netherlands) was then added to tube reactions and ECL counts were read in an Origen 1.5 ECL Analyzer (Organon Teknika). During ECL detection, we included a water control for each probe combination tested; these ECL signals were used as background levels for the other reactions analyzed with the same probe combinations. The quantitation of mRNA levels is based on the ECL signals and the amount of Q-RNA spiked per reaction. Specific activities of the wild-type (WT) and Q-ECL probes are known. WT ECL and Q-ECL signals are measured over a range of WT RNA input at a fixed Q-RNA concentration. Therefore, the ratios of WT and Q-NASBA amplificates could be determined from the signal ratios of the respective probes and the initial amount of WT RNA calculated. All ECL signals were corrected for the background before performing the quantitation. Final quantitation of TF mRNA was obtained according to the following formula: log TF = 0.88 × (log TF WT ECL − log TF Q ECL) + 4.57. This formula corrects for differences in amplification rate between WT and internal standard (Q-)RNA, for differences in hybridization efficiencies of nucleotides and for differences in activity of the detection probes (P.v.D. et al, unpublished data).
Markers of coagulation activation.
Blood was drawn from the antecubital vein and collected in tubes containing buffered 3.2% citrate solution. Activation of coagulation was assessed by plasma measurements of markers for thrombin formation, TAT complexes (μg/L) and prothrombin F1 + 2 (nmol/L), with specific enzyme-linked immunosorbent assays (ELISAs) (Behringwercke AG, Marburg, Germany).
Leukocyte responses.
Global and differential white cell counts were performed by flow cytometry in blood samples collected in tubes containing EDTA. Monocyte counts were used to correct the absolute log values of TF obtained in the ECL assay (described below).
In vitro whole blood stimulation with lipopolysaccharide.
We performed in vitro assays involving whole blood stimulation with different concentrations of LPS, followed by both measurement of TF mRNA levels by NASBA/ECL technique and of TF antigen expression on monocytes by flow cytometry (FACscan analysis, described later). Blood samples obtained from several volunteers were collected into tubes containing 100 μL (500 IE) endotoxin-free heparin per 10 mL whole blood. LPS preparations were shaken continuously for 30 minutes before the addition to whole blood samples. Samples were diluted 1:1 with HBSS, and whole blood stimulation was performed in the absence or presence of LPS at 37°C and 5% CO2 for 4 hours. The following concentrations of LPS were added to whole blood obtained from 2 volunteers: 0, 0.01, 0.1, 1, 10, and 100 ng/mL.
One hundred microliters of LPS-stimulated whole blood (diluted 1:1 with HBSS) was mixed with 900 μL lysis buffer, and this material was used for RNA isolation and amplification and ECL detection, as described above. Subsequently, the remaining volume of each LPS-stimulated sample was placed immediately on ice and erythrocytes were lysed for 20 minutes with ice-cold isotonic NH4Cl solution (155 mmol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L EDTA, and pH 7.4). Leukocytes were centrifuged at 600g for 10 minutes at 4°C and residual erythrocytes were lysed for 5 minutes. The remaining cells were washed with phosphate-buffered saline (PBS) and subsequently resuspended in PBS containing 1% BSA (wt/vol) at a final concentration of 107 cells/mL. This suspension was used in FACScan analysis of TF expression.
FACScan analysis.
Fifty microliters cell suspension at a concentration of 107cells/mL was incubated with 50 μL primary antibody (concentration 10 μg/mL) for 60 minutes at 4°C. The mouse monoclonal IgG1 antihuman TF antibodies used were 5G9 (kindly donated by Dr T. S. Edgington, The Scripps Research Institute, La Jolla, CA), 4509(American Diagnostica Inc, Greenwich, CT), and TFE(Kordia/Enzyme Research Laboratories Inc, Leiden, The Netherlands). Thereafter, cells were washed with ice-cold PBS containing 0.1% BSA (wt/vol). Subsequently, a secondary RPE-conjugated rabbit antimouse antibody was diluted in PBS containing 1% BSA and the cells were incubated for 60 minutes at 4°C. After washing, cells were resuspended in PBS containing 1% BSA and analyzed using a FACscan (Becton Dickinson, Mountain View, CA). The monocytes were gated by their specific forward and side-scatter pattern. Furthermore, the monocyte population was identified by high CD14 expression. A total of 20 000 events was recorded for each file. After subtracting control IgG1 mean fluorescence, specific antibody binding was expressed as mean fluorescence intensity (MFI).
Tissue factor antigen levels.
Circulating TF antigen was measured using a commercially available assay at time points −0.5, 0.5, 1, 2, 3, 4, 6, 8, and 24 hours in citrated blood samples according to the instructions of the manufacturer (American Diagnostica).
Statistical analysis
Values are shown as means ± SEM. Changes of variables over time were analyzed using 1-way analysis of variance (ANOVA) (Pvalue over time). A P ≤ .05 was considered statistically significant.
Results
TF mRNA (NASBA/ECL) and TF antigen (FACscan) analysis in LPS-stimulated whole blood cells
In an initial in vitro experiment, we examined whether TF mRNA levels reflect TF protein expression on the cell membrane. This indeed appeared to be the case. LPS concentrations between 0.01 and 100 ng/mL were used. A dose-dependent increase, as shown in Figure1, was found for both flow cytometric antigen levels (using 3 different antibodies) and TF mRNA levels. The increase is particularly impressive for the mRNA levels. Log TF levels (log molecules per milliliter) were 3.7 at baseline and were raised to 6.2, ie, 316-fold increased, after 4 hours incubation with 100 ng LPS. Figure 2 shows a linear plot of TF mRNA levels against MFI as detected with monoclonal antibody 4509. The relationship between antigen and mRNA levels appears to be linear up to a dose of 10 ng/mL LPS. This is well within the range of LPS levels attainable in human volunteer studies, in which 4 ng/kg is administered intravenously.
TF expression by LPS-stimulated whole blood cells in vitro.
(A) FACScan analysis of TF antigen expression on monocytes from 2 volunteers. Values refer to mean fluorescence intensity (MFI). (B) Log values of TF mRNA in 1 volunteer, using LPS concentrations between 0 and 100 ng/mL.
TF expression by LPS-stimulated whole blood cells in vitro.
(A) FACScan analysis of TF antigen expression on monocytes from 2 volunteers. Values refer to mean fluorescence intensity (MFI). (B) Log values of TF mRNA in 1 volunteer, using LPS concentrations between 0 and 100 ng/mL.
TF mRNA and antigen levels in LPS-stimulated whole blood cells.
Antigen levels refer to those detected with monoclonal 4509 in FACScan analysis.
TF mRNA and antigen levels in LPS-stimulated whole blood cells.
Antigen levels refer to those detected with monoclonal 4509 in FACScan analysis.
Clinical and hematologic parameters in human endotoxemia
Administration of LPS was associated with a transient rise in body temperature, peaking after 3 hours (38.3 ± 0.5°C,P < .05). All subjects experienced flu-like symptoms, such as headache, nausea, and myalgia. LPS injection also induced a biphasic change in leukocyte counts, involving early neutropenia, followed by neutrophilia, monocytopenia, and lymphopenia (Table1).
Changes in leukocyte counts and differential after LPS administration to healthy subjects
| Time point (h) . | Leukocytes . | Granulocytes . | Monocytes . | Lymphocytes . |
|---|---|---|---|---|
| −0.5 | 5.2 ± 1.1 | 2.9 ± 0.7 | 0.5 ± 0.1 | 1.6 ± 0.3 |
| +0.5 | 4.9 ± 0.9 | 2.9 ± 0.7 | 0.3 ± 0.1 | 1.5 ± 0.2 |
| +1 | 2.0 ± 0.3 | 0.9 ± 0.3 | 0.05 ± 0.02 | 0.9 ± 0.1 |
| +2 | 5.2 ± 1.4 | 4.4 ± 1.4 | 0.05 ± 0.02 | 0.7 ± 0.1 |
| +3 | 4.9 ± 1.3 | 4.5 ± 1.3 | 0.03 ± 0.02 | 0.4 ± 0.1 |
| +4 | 7.8 ± 1.5 | 7.4 ± 1.4 | 0.07 ± 0.04 | 0.3 ± 0.1 |
| +6 | 11.1 ± 1.4 | 10.5 ± 1.4 | 0.2 ± 0.1 | 0.4 ± 0.1 |
| +8 | 13.0 ± 2.9 | 12.0 ± 2.9 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| +24 | 9.7 ± 2.7 | 7.1 ± 2.6 | 0.6 ± 0.1 | 1.7 ± 0.3 |
| Time point (h) . | Leukocytes . | Granulocytes . | Monocytes . | Lymphocytes . |
|---|---|---|---|---|
| −0.5 | 5.2 ± 1.1 | 2.9 ± 0.7 | 0.5 ± 0.1 | 1.6 ± 0.3 |
| +0.5 | 4.9 ± 0.9 | 2.9 ± 0.7 | 0.3 ± 0.1 | 1.5 ± 0.2 |
| +1 | 2.0 ± 0.3 | 0.9 ± 0.3 | 0.05 ± 0.02 | 0.9 ± 0.1 |
| +2 | 5.2 ± 1.4 | 4.4 ± 1.4 | 0.05 ± 0.02 | 0.7 ± 0.1 |
| +3 | 4.9 ± 1.3 | 4.5 ± 1.3 | 0.03 ± 0.02 | 0.4 ± 0.1 |
| +4 | 7.8 ± 1.5 | 7.4 ± 1.4 | 0.07 ± 0.04 | 0.3 ± 0.1 |
| +6 | 11.1 ± 1.4 | 10.5 ± 1.4 | 0.2 ± 0.1 | 0.4 ± 0.1 |
| +8 | 13.0 ± 2.9 | 12.0 ± 2.9 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| +24 | 9.7 ± 2.7 | 7.1 ± 2.6 | 0.6 ± 0.1 | 1.7 ± 0.3 |
Data shown are mean ± SEM. Values refer to number of cells ×103/μL. Time points refer to intravenous LPS injection (t = 0). LPS, lipopolysaccharide.
TF expression in human endotoxemia
TF antigen levels on monocytes (measured by FACs analysis with the monoclonal antibody 4509) tended to increase in 8 of the 10 subjects (data not shown). However, in accordance with our previous findings,18 it was not possible to establish a significant increase of TF levels after LPS infusion, because of the high interindividual variation in TF surface antigen levels and the differences in time at which peak levels were reached.
In addition to FACscan analysis, we used an ELISA that measures soluble circulating tissue factor as a surrogate marker for intravascular tissue factor expression. As shown in Table2, plasma levels of TF antigen did not change over time after LPS infusion (P > .9).
Circulating tissue factor antigen levels after LPS administration to healthy individuals
| Time point (h) . | TF antigen (pg/mL) . |
|---|---|
| −0.5 | 165 ± 33 |
| +0.5 | 146 ± 23 |
| +1 | 163 ± 38 |
| +2 | 170 ± 27 |
| +3 | 179 ± 28 |
| +4 | 162 ± 13 |
| +6 | 127 ± 9 |
| +8 | 157 ± 23 |
| +24 | 160 ± 26 |
| Time point (h) . | TF antigen (pg/mL) . |
|---|---|
| −0.5 | 165 ± 33 |
| +0.5 | 146 ± 23 |
| +1 | 163 ± 38 |
| +2 | 170 ± 27 |
| +3 | 179 ± 28 |
| +4 | 162 ± 13 |
| +6 | 127 ± 9 |
| +8 | 157 ± 23 |
| +24 | 160 ± 26 |
Data shown are mean ± SEM. Time points refer to intravenous LPS injection (t = 0). LPS, lipopolysaccharide; TF, tissue factor.
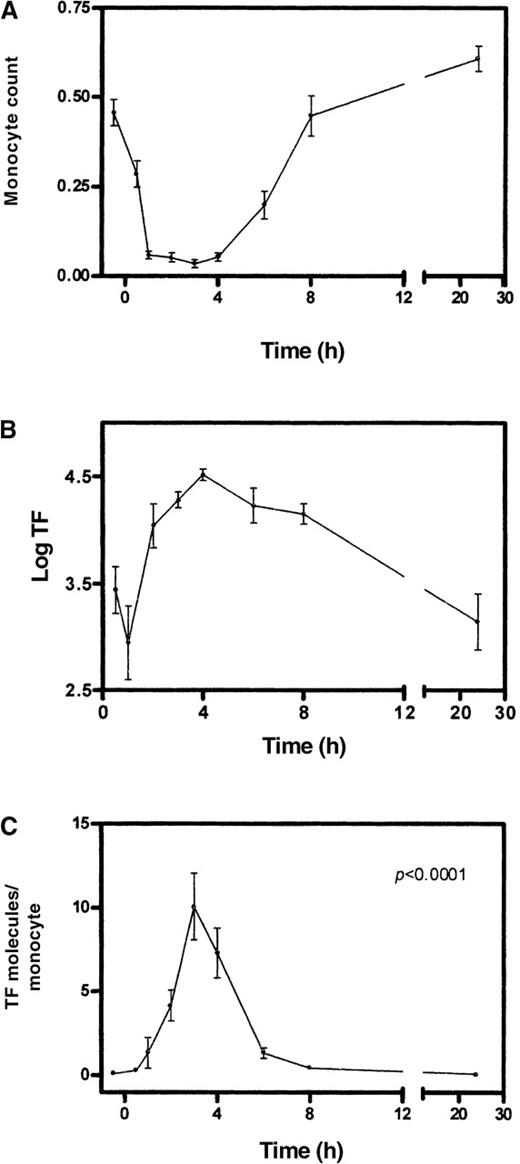
After LPS injection into healthy volunteers, log TF values at t +0.5 hour (3.4 ± 0.7) and +1 hour (3.0 ± 1.0) were similar to baseline value (3.3 ± 0.6). An evident and sustained increase of TF mRNA expression was detected at time points +2 hours (4.0 ± 0.6), +3 hours (4.3 ± 0.2), +4 hours (4.5 ± 0.2), +6 hours (4.2 ± 0.5), and +8 hours (4.2 ± 0.3). Hence, an approximate 10-fold increase in TF mRNA expression was detected between 2 and 8 hours after LPS injection (Figure 3B). At 24 hours after LPS injection, TF levels were reduced to the baseline value (3.1 ± 0.8).
TF mRNA levels in relation to monocyte counts over time in human endotoxemia.
Top: monocyte counts (number of cells × 103/μL). Middle: values of log (mRNA)TF. Bottom: number of mRNA TF molecules/monocyte. Error bars refer to SEM, n = 10.
TF mRNA levels in relation to monocyte counts over time in human endotoxemia.
Top: monocyte counts (number of cells × 103/μL). Middle: values of log (mRNA)TF. Bottom: number of mRNA TF molecules/monocyte. Error bars refer to SEM, n = 10.
It is important to consider the fact that changes in the number of circulating leukocytes over time after LPS injection might bias these levels of TF mRNA. Therefore, we corrected the log TF results for the monocyte count at each time point of the study, because monocytes are the circulating blood cells known to express TF. These analyses (in which the number of TF molecules per monocyte was calculated for each time point) are shown in Figure 3 (lower part). The baseline value of the number of TF mRNA molecules per monocytic cell was 0.08 ± 0.02. A progressive and significant (P < .0001) increase in TF expression was observed after LPS injection (+0.5 hour: 0.3 ± 0.1, +1 hour: 1.3 ± 0.9, +2 hours: 4.1 ± 0.9), peaking at +3 hours (10 ± 1.9 TF mRNA molecules per monocyte), and TF mRNA levels returned to baseline after 24 hours (0.07 ± 0.03). These data also show that the actual increase of TF mRNA expression per monocyte during human endotoxemia is in the order of 125-fold, ie, about 3-fold lower than obtained in vitro with 100 ng LPS.
Coagulation activation markers in human endotoxemia
Although TF mRNA levels increased, augmented thrombin generation (as measured by progressive elevation in TAT and F1 + 2 levels) was observed. As shown in Figure 4, a progressive increase in TAT complexes was detected after LPS injection from baseline plasma concentrations of 7.3 ± 1.1 μg/L (t −0.5 hour) to peak concentrations of 84.5 ± 31.1 μg/L (at t +4 hours), decreasing thereafter and reaching levels of 9.6 ± 2.3 μg/L at time point +24 hours (P < .025 in time). A similar pattern was observed for prothrombin F1 + 2 after LPS injection (Figure 4); plasma levels increased from 0.8 ± 0.1 nmol/L (at t −0.5 hour) to peak values at t +4 hours (10.2 ± 2.8 nmol/L), with subsequent decreased levels being observed thereafter, reaching levels of 1.4 ± 0.2 nmol/L at +24 hours (P < .0001 in time). The kinetics of TF mRNA expression was closely related to the activation of coagulation observed in human endotoxemia; the rise in plasma concentrations of markers of thrombin generation followed the elevation of TF mRNA levels. In general, the peak levels of TAT and F1 + 2 were reached later (at t +4 hours) than peaks of TF mRNA (+3 hours). Interestingly, this was not the case for 2 subjects, who exhibited maximum TAT and F1 + 2 levels at +0.5 hour and +1 hour after LPS injection, whereas peak TF mRNA levels in these individuals were observed at +1 hour and +4 hours, respectively.
Markers of coagulation activation in human endotoxemia.
Top: plasma levels of thrombin-antithrombin complexes (TAT). Bottom: plasma levels of prothrombin fragment 1 + 2 (F1 + 2). Error bars refer to SEM, n = 10.
Markers of coagulation activation in human endotoxemia.
Top: plasma levels of thrombin-antithrombin complexes (TAT). Bottom: plasma levels of prothrombin fragment 1 + 2 (F1 + 2). Error bars refer to SEM, n = 10.
Discussion
We have successfully used a NASBA-based method for RNA amplification, followed by an ECL-based detection system to investigate the kinetics of TF mRNA expression in vivo, in a model of human endotoxemia induced by IV injection of LPS. We observed a maximum 125-fold increase of TF mRNA levels in monocytes that was directly related to activation of the coagulation system. The combined NASBA/ECL technology has been previously used for accurate quantitation of viral copies in blood of HIV-infected patients.30 31 Our data confirm the usefulness of these techniques for mRNA measurements and extend their application to the field of hemostasis. Indeed, this methodology may have numerous applications to accurately quantify TF mRNA expression in clinical situations in which TF is known to play a role, including gram-negative septicemia, atherosclerosis, autoimmune diseases, adult respiratory distress syndrome, and cancer.
The in vivo data show a clear pattern of TF mRNA expression in the absence of detectable TF antigen expression on monocytes in whole blood. This is in contrast with the in vitro data that show detectable TF antigen over a wide range of LPS concentrations. The reason for this discrepancy remains unclear. In this respect, it is important to note that activated monocytes will adhere to the vascular endothelium, as is evident from the monocytopenia that is characteristic of the human endotoxemia model. This does not occur in vitro. If tissue factor expression is related to the adhesive properties of monocytes, preferentially, monocytes with poor TF antigen expression will remain in the blood sample. Such an explanation would also imply that the induction of mRNA expression of tissue factor in the retained monocytes might be even higher than shown in our data and that we are detecting monocytes with a relatively poor response to LPS.
Alternatively, it is possible that flow cytometry did not detect TF antigen because it is retained within the cells and not fully expressed on the monocyte membrane. Another possibility is that conformational changes in TF during endotoxemia prevented it from being detected by antibodies used in FACscan analysis. Alternatively, TF might become rapidly shed from monocytes in the form of microvesicles that are not detected in our FACscan procedure. Finally, it might be speculated that there is indeed increased TF mRNA expression but with no detectable protein, as was recently reported for LDL-induced TF expression in smooth muscle cells.33
The shedding of TF from monocytes in a soluble form does not seem to be a probable explanation for the failure to detect consistent TF expression by flow cytometry. This became evident from measurements of TF antigen levels using an ELISA on citrated plasma. Such antigen levels do not change after LPS administration and thus did not correlate with TF mRNA induction or coagulation activation. This seems to imply that the routing of down-regulating surface expression of TF is through internalization rather than shedding from the surface into the circulation.
The availability of monocyte counts at each time point of the experiment permitted us to estimate the number of TF molecules being expressed per monocyte at different stages after LPS injection (Figure3). The data showed that increased TF mRNA expression on monocytes in vivo is a rapid event that is detectable as early as 30 minutes post-LPS injection. Our findings concerning the pattern of mRNA TF expression agree well with those derived from studies investigating the LPS-induced TF expression on monocytes in vitro: increasing amounts of mRNA TF are quickly generated, peak levels are observed between 2 and 4 hours after exposition to LPS, followed by a progressive decline thereafter.
We took only monocyte numbers into account for these calculations because among white blood cells the monocytes are considered to be the main cell type expressing TF. Recent data raised the possibility that neutrophils are also capable of expressing TF.34 Even if this is the case, neutrophils probably do not importantly contribute to increased TF expression in endotoxemia, because the late neutrophil increase was not associated with increased TF mRNA expression in the current investigation (data not shown).
Several lines of evidence indicated that the TF/VIIa-mediated route drives activation of the coagulation system during endotoxemia and sepsis. Indeed, with a number of different strategies it is possible to prevent activation of the common pathway of coagulation in endotoxemic chimpanzees and septic baboons. These strategies include antibodies directed against TF or factor VII/VIIa, active site inhibited factor VIIa (Dansyl-Glu-Gly-Arg chloromethylketone or DEGR-VIIa) and TFPI.7-9,11,12 35 As predicted by these studies, we found that the kinetics of TF mRNA expression was closely related to the activation of coagulation. This was evident from a rise in plasma concentrations of markers of thrombin generation. As expected, peak levels of TAT and F1 + 2 were reached later (at t +4 hours) than those of TF mRNA (+3 hours). However, it must be emphasized that we measured TF mRNA in circulating cells, and therefore the relative contribution of other cells producing TF, in particular endothelial cells, was not evaluated in the current investigation. In fact, early endothelial TF might explain the finding that, in 2 subjects, the peak values of TAT and F1 + 2 preceded TF peak levels. Alternatively, one could speculate that some degree of intrinsic pathway coagulation activation occurred in these 2 subjects. Finally, one might argue that activation of preformed TF took place.
In summary, we report for the first time the in vivo kinetics of TF mRNA expression after LPS administration to healthy subjects. TF mRNA expression was directly related to biochemical evidence of thrombin formation, as indicated by the elevation of plasma levels of activation coagulation markers. These findings add further evidence to the concept that TF plays a critical role in the activation of coagulation after LPS challenge. The availability of this NASBA method of TF mRNA quantitation may become useful in clinical settings in which enhanced TF expression plays an essential role.
Acknowledgments
We are grateful to Angelique Groot for her help with TF antigen assays, and to Michel de Baar and Suzanne Jurriaans (Department of Human Retrovirology, Academic Medical Center, Amsterdam) for helpful discussions regarding the ECL experiments.
Supported by a FAPESP Grant (98/02821) (R.F.F.); by the E. Dekker program of the Dutch Heart Foundation (H.t.C. and C.A.S.); and by a grant from the Dutch Organization for Scientific Research (J.J.T.). T.v.d.P. is a fellow of the Royal Dutch Academy of Arts and Sciences.
Reprints:Pieter H. Reitsma, Laboratory for Experimental Internal Medicine, G2-135, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: p.h.reitsma@amc.uva.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal