Abstract
Recent studies concerning the numbers of circulating clonal B cells in patients with multiple myeloma (MM) have reported conflicting data regarding the exact level and phenotype of clonal B cells and their response to treatment. In this report we document that the peripheral blood tumor burden at presentation was reduced by induction therapy to a low level, regardless of the initial tumor burden. However, the residual clonal compartment persisted before and after transplant. The level of clonal cells showed no correlation with CD19+cell levels. In a single patient with MM, high numbers of phenotypically aberrant clonal cells with altered CD19 expression were identified.
Introduction
Studies reporting the phenotype and frequency of clonally related B cells and their role in the pathogenesis of multiple myeloma (MM) represent an ongoing controversy in myeloma research. It has been reported that the peripheral blood (PB) of patients with MM has CD19+ cell levels significantly higher than those in healthy donors1 and that the majority of these cells were aberrant lymphocytes clonally related to bone marrow (BM)-localized myeloma plasma cells.2 In contrast, others have found that CD19+ cell levels in patients with MM were not significantly different from healthy donors, although a pronounced heterogeneity was seen in the patients with MM,3 and that only a minor fraction of the CD19+ cells were clonal.4,5 The CD19+ PB-localized clonal cells have also been reported to be insensitive to chemotherapy and thus hypothesized to be responsible for relapse.6 7 To address this controversial area of myeloma research, the level of circulating clonal cells and their responses to treatment were monitored using quantitative real time and single-cell reverse transcriptase-polymerase chain reaction (RT-PCR) assays.
Study design
A total of 9 diagnostic BM aspirates and PB samples from 27 patients with MM and 5 healthy donors were obtained at multiple time points after informed consent. PB samples obtained at diagnosis from the 27 patients were simultaneously analyzed by flow cytometry and CD19 real-time RT-PCR. Further, 5 patients were analyzed by single-cell CD19 RT-PCR.
The isolation of BM mononuclear cells (BMMNCs), PB mononuclear cells (PBMNCs), DNA, RNA, and flow-sorting of single cells was performed as recently reported.8 All patients were treated as previously described.8 PBMNCs were stained with the CD19 monoclonal antibodies (MoAbs) Leu-12 fluorescein isothiocyanate/phycoerythrin (FITC/PE) (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) and B4 FITC/PE (Coulter Clone, Miami, FL) as previously described.9 Generation of allele-specific oligonucleotides (ASOs) IgH PCR assays were performed as previously described.8 For real-time ASO PCR, a forward CDR3 primer was designed to span the V-D junction, and the CDR3 TaqMan probe was designed to span the D-J junction, giving maximum specificity of the assays (Table 1). Real-time ASO IgH PCR and CD19 single-cell RT-PCR assays were performed as previously described.10-12
Results and discussion
Kinetics of polyclonal and clonal cells during treatment
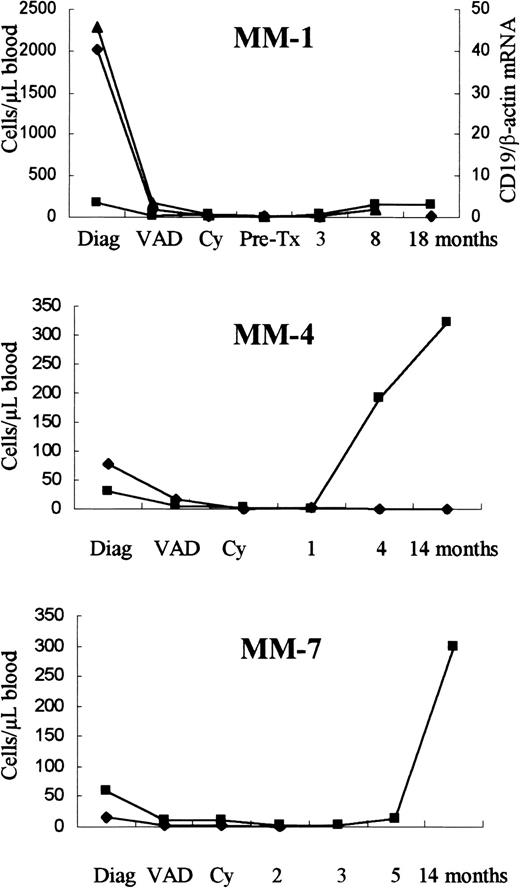
The level of clonal cells in PBMNCs was determined in a cohort of patients with MM at the time of diagnosis and during treatment by a quantitative real-time ASO IgH PCR assay10 (Figure1). At the time of diagnosis, the level of clonally related cells in PB ranged from less than 0.00002 to 2020 clonal cells per microliter blood (n = 9, mean 282, median 15). These findings are very similar to those reported by Billadeau et al13 and Kay et al.3 The proportion of clonal cells (less than 0.00001% to 30.55% of PBMNCs) was reduced to less than 0.5% of PBMNCs in all patients tested (n = 7) after induction therapy, regardless of the initial level of clonal cells (Figure 1). However, the level of clonal cells after induction therapy appeared to be stable before and after PB stem cell (PBSC) transplantation. This is in accordance with the findings of Billadeau et al.14However, the observed initial reduction and low numbers of residual PB-localized clonal cells after chemotherapy is in conflict with recent reports.6 15 The numbers of CD19+ cells differed greatly both between patients at diagnosis (4 to 1790 cells/μL blood, mean 323, n = 27) and in individual patients (0.08 to 324 cells/μL blood, Figure 1, MM-4). In general, low numbers of CD19+ cells were seen during induction and high-dose treatment, followed by a significant increase in CD19+cells after transplant (Figure 1).
Levels of CD19+ and clonal cells among PBMNCs.
The level of CD19+ (▪) and clonal cells (⧫) among PBMNCs as determined by flow cytometry and real-time ASO IgH PCR. In patient MM-1, the level of CD19 mRNA (▴) was measured by quantitative real-time CD19 RT-PCR. The amount of CD19 mRNA is given as the fraction of β-actin mRNA. Serial PB samples were obtained at diagnosis, after 3 cycles of VAD, after cyclophosphamide (Cy), before (pre-Tx) and after PBSC transplantation.
Levels of CD19+ and clonal cells among PBMNCs.
The level of CD19+ (▪) and clonal cells (⧫) among PBMNCs as determined by flow cytometry and real-time ASO IgH PCR. In patient MM-1, the level of CD19 mRNA (▴) was measured by quantitative real-time CD19 RT-PCR. The amount of CD19 mRNA is given as the fraction of β-actin mRNA. Serial PB samples were obtained at diagnosis, after 3 cycles of VAD, after cyclophosphamide (Cy), before (pre-Tx) and after PBSC transplantation.
Clonal cells with aberrant CD19 expression
In patient MM-1, the numbers of CD19+ cells (170 cells/μL blood) and myeloma plasma cells (PCS) (10 cells/μL blood) at the time of diagnosis was far below the level of clonal cells detected in PB (2020 cells/μL blood). By following the levels of CD19 messenger RNA (mRNA), using real-time CD19 RT-PCR (Figure 1), we observed a high level of CD19 mRNA at diagnosis that was markedly reduced after induction therapy, reaching a minimum level at 3 months after transplant. This correlated with the clonal cell levels. In general, numbers of CD19+ cells were not at variance with the level of CD19 mRNA at the time of diagnosis. However, when looking at patient MM-1, a clear discrepancy was observed between the number of CD19+ cells and the level of CD19 mRNA, at the time of diagnosis. After transplant, a simultaneously rise in CD19+cells and CD19 mRNA was observed, and at this time, the frequency of CD19+ cells correlated with the level of CD19 mRNA. To assess whether these cells had cytoplasmic CD19 protein, surface staining was performed with B4 FITC, followed by intracellular staining with Leu-12 PE. At the time of diagnosis both a population of cells with high-scatter properties, having only cytoplasmic CD19 protein (610 cells/μL blood), and a population of lymphoid cells (170 cells/μL blood), having both surface and cytoplasmic CD19 protein, were present. After VAD treatment, no cells with only cytoplasmic CD19 protein was observed. The correct cellular localization of the CD19 MoAbs tested was confirmed by confocal microscopy. In addition, the frequency of CD19 mRNA+ cells in the monocyte gate was determined using a single-cell CD19 RT-PCR assay.12 In both healthy donors and patients with MM (5 each), the frequency of CD19+(mRNA+) cells was between 1:100 to 1:1000 (data not shown), probably due to the presence of activated B cells. Although we had previously observed a patient with MM similar to MM-1 with abundant CD19−/CD19 mRNA+ clonal cells,12the frequency of these patients seems low. Thus, their rare existence does not fully explain the conflicting reports regarding the consistent findings of the high numbers of aberrant CD19 clonal cells.16
In contrast to normal plasma cells, the myeloma cells at one stage lose CD19 surface expression before final differentiation into mature myeloma PCS.17 It has been shown in transfection experiments with myeloma cell lines that surface expression of CD19 introduces growth regulation in myeloma cell lines.18 It is tempting to speculate that the position in the clonal hierarchy of the CD19−/CD19 mRNA+cells is at the point where the myeloma cells select against the CD19 surface expression to gain a growth advantage. These cells may be transient or present in all patients with MM in low numbers, making them difficult to detect.
Supported by the Danish Cancer Society, The Meyer Foundation, and the Desirè & Niels Ydes Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Rasmussen, Department of Hematology L 54P4, Herlev Hospital, University of Copenhagen, DK-2730 Herlev, Denmark; email: thra@herlevhosp.kbhamt.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal