Abstract
Thrombohemorrhagic complications are a major cause of morbidity and mortality in patients with essential thrombocythemia (ET) and polycythemia vera (PV). The pathogenesis of these complications is not completely clarified. Several studies have described abnormalities of red blood cells and platelets in these patients. However, no studies are available on changes in the polymorphonuclear leukocytes (PMNs), which can play an important role in the activation of the hemostatic system. In patients with ET (n = 37) and PV (n = 34), a series of PMN activation parameters (PMN membrane CD11b and leukocyte alkaline phosphatase [LAP] antigen expression, cellular elastase content, plasma elastase, and myeloperoxidase levels) was evaluated simultaneously with the levels of plasma markers of endothelial damage (thrombomodulin and von Willebrand factor antigen) and hypercoagulation (thrombin-antithrombin complex, prothrombin fragment 1 + 2, and D-dimer). The results show the occurrence of PMN activation in both groups of patients compared with a control group of healthy subjects. An increase in CD11b and LAP expression by PMN membrane was observed, together with a significant increase in cellular elastase content, plasma elastase, and myeloperoxidase levels. In addition, patients had high plasma levels of endothelial and hypercoagulation markers compared with controls. For the first time, these data show that in ET and PV, 2 hematologic conditions that place patients at increased risk for thrombosis, an in vivo leukocyte activation occurs and is associated with laboratory signs of endothelium and coagulation system activation.
Introduction
Essential thrombocythemia (ET) and polycythemia vera (PV) are chronic myeloproliferative disorders that share common pathogenetic abnormalities of bone marrow stem cell proliferation. A typical feature of ET and PV is a hemostatic imbalance resulting in increased risk for both thrombotic and hemorrhagic events.1 Arterial and venous thromboembolism are the major causes of morbidity and mortality in these patients (approximately 40%).2 These events have been attributed to quantitative and qualitative abnormalities of red blood cells and platelets arising from the clonal rearrangement of hematopoietic cells. The relevance of uncontrolled polycythemia as a risk factor for thrombosis in these patients has been established. The role of thrombocytosis is still debated,3 though recent evidence shows that a reduction in platelet count is associated with decreased thrombotic risk in ET patients receiving hydroxyurea.4 Numerous platelet defects have also been identified, including abnormal platelet morphology, acquired storage pool disease, platelet membrane abnormalities, and abnormal arachidonic acid metabolism.5 Causal relationships between any of these specific abnormalities and either bleeding or thrombosis have not been clearly established.6In contrast to red blood cells and platelets, no studies have been conducted on white blood cells (WBCs), which can potentially be involved in the thrombotic diathesis of these patients. Increases in WBC count, typical of most patients with ET and PV, can be important in the pathogenesis of a thrombophilic state. As reported by epidemiologic studies in patients with coronary heart and peripheral arterial diseases, high WBC count is associated with enhanced thrombotic risk.7,8 Besides the cell count, numerous polymorphonuclear leukocyte (PMN) functions can be relevant for the hemostatic system activation and are potentially implicated in thrombogenesis and endothelium damage.9 Activated PMNs release reactive oxygen species and intracellular proteases, which can act on endothelial cells and platelets and may modify the hemostatic balance toward a pro-thrombotic state. Indeed leukocyte elastase and cathepsin G can induce detachment or lysis of endothelial cells and can also modify endothelial cell functions involved in thromboregulation10-13—that is, prevent thrombin-induced prostacyclin production, induce plasminogen activator inhibitor release, and proteolyze endothelial surface components such as thrombomodulin. Furthermore, the potential thrombogenic effects of PMN-derived proteases include the direct potent platelet activation elicited by cathepsin G.14 Finally, elastase can directly proteolyze and inactivate natural inhibitors of blood coagulation, including protein C, protein S, tissue factor pathway inhibitor, antithrombin, and heparin cofactor II, thus impairing potent physiological antithrombotic mechanisms.15-18
Therefore, this study was designed to evaluate, in patients with ET and PV, the in vivo activation features of circulating PMNs and the concurrent variations in plasma hemostatic variables. For the first time, we report on measurements of PMN phenotypical changes and degranulation parameters in vivo in these patients9; we also report on a series of parameters reflecting perturbation of the endothelium and activation of blood coagulation in vivo.19
Patients and methods
Patients
Seventy-one consecutive patients with essential thrombocythemia (ET) and polycythemia vera (PV) attending the outpatient clinics at our hematology department entered the study. Of these, 37 patients (13 men, 24 women; age range, 21-75 years) had ET, and 34 patients (22 men, 12 women; age range, 29-87 years) had PV. Diagnoses of ET and PV were made according to commonly accepted clinical and laboratory criteria.20 21 A group of 71 sex-matched healthy subjects of comparable age (35 men, 36 women; age range, 23-79 years) acted as a control group for the laboratory parameters studied. The normal control group was recruited from healthy adults without history of thrombo-hemorrhagic events. Written informed consent was obtained from each person. None had symptoms of active infections or inflammatory diseases, nor had any engaged in physical exercise. Characteristics of the patients and the control subjects are summarized in Table1.
Characteristics of control subjects and patients with essential thrombocythemia and polycythemia vera
| . | Controls . | ET . | PV . |
|---|---|---|---|
| n (M/F) | 71 (35/36) | 37 (13/24) | 34 (22/12) |
| Age, median (range) | 60 (20-77) | 53 (21-75) | 63 (29-87) |
| . | Controls . | ET . | PV . |
|---|---|---|---|
| n (M/F) | 71 (35/36) | 37 (13/24) | 34 (22/12) |
| Age, median (range) | 60 (20-77) | 53 (21-75) | 63 (29-87) |
| Hematologic parameters . | |||
|---|---|---|---|
| . | Controls . | ET . | PV . |
| RBCs (× 1012/L) | 4.63 (4.02-5.27) | 4.28 (2.57-5.8) | 5.88 (3.3-7.21)* |
| WBCs (× 109/L) | 5.1 (3.8-9.8) | 7.24 (4.2-15.6)* | 10.2 (4.6-34)* |
| PMNs (× 109/L) | 3.0 (1.9-6.3) | 4.54 (1.76-11.4)* | 8.2 (3.4-31)* |
| Platelets (× 109/L) | 260.0 (184-391) | 697.0 (360-1,600)* | 328.0 (125-744)* |
| Hemoglobin (g/L) | 13.8 (12.8-16) | 13.5 (9.4-16.1) | 16.2 (13.3-20)* |
| Hematocrit (%) | 40.7 (29.7-48.1) | 39.8 (27.8-48) | 47.0 (37.4-61)* |
| Hematologic parameters . | |||
|---|---|---|---|
| . | Controls . | ET . | PV . |
| RBCs (× 1012/L) | 4.63 (4.02-5.27) | 4.28 (2.57-5.8) | 5.88 (3.3-7.21)* |
| WBCs (× 109/L) | 5.1 (3.8-9.8) | 7.24 (4.2-15.6)* | 10.2 (4.6-34)* |
| PMNs (× 109/L) | 3.0 (1.9-6.3) | 4.54 (1.76-11.4)* | 8.2 (3.4-31)* |
| Platelets (× 109/L) | 260.0 (184-391) | 697.0 (360-1,600)* | 328.0 (125-744)* |
| Hemoglobin (g/L) | 13.8 (12.8-16) | 13.5 (9.4-16.1) | 16.2 (13.3-20)* |
| Hematocrit (%) | 40.7 (29.7-48.1) | 39.8 (27.8-48) | 47.0 (37.4-61)* |
All values are expressed as median (range).
ET indicates essential thrombocythemia; PV, polycythemia vera; RBC, red blood cell; WBC, white blood cell; PMN, polymorphonuclear leukocyte.
P < .01 vs controls (Mann-Whitney test).
Twenty-two patients (12 with ET, 10 with PV) were not receiving any treatment for ET or PV. Twenty-six patients (13 with ET, 13 with PV) were administered hydroxyurea (HU), 12 patients (8 with ET, 4 with PV) were administered HU and aspirin, 5 patients (4 with ET, 1 with PV) were administered aspirin, and 6 patients with PV underwent phlebotomy 3 or more times a year. Five patients (3 with ET, 2 with PV) had had thrombotic manifestations in the past; 3 of those had severe cerebral ischemic attacks.
Samples
Plasma samples for the analysis of circulating markers of activation of either PMN, endothelium, or coagulation system were obtained from venous blood collected into sterile siliconized tubes containing trisodium citrate (0.129 mol/L, 1:9 vol/vol). Plasma was obtained by centrifugation of anticoagulated whole blood at 3000g for 20 minutes at room temperature and was stored in aliquots at −80°C until assays (within 2 months).
Whole blood was used for the cytofluorometric evaluation of CD11b and leukocyte alkaline phosphatase expression by PMN surface. PMNs for the analysis of cellular elastase content were isolated from citrated fresh venous blood immediately after venipuncture by density-gradient centrifugation, as described.10 The PMN fraction separated in the pellet was further purified from erythrocytes by dextrane erythrocyte sedimentation. Isolated PMNs were resuspended (106 cell/mL) in 10 mmol/L HEPES-Tyrode buffer (pH 7.4) containing 129 mmol/L NaCl, 9.9 mmol/L NaHCO3, 2.8 mmol/L KH2PO4, 0.8 mmol/L MgCl2-6H2O, and 1 mmol/L CaCl2. Cell viability was assessed by the trypan-blue exclusion dye test and was found to be greater than 90%. Cell suspensions contained more than 95% PMNs.
Routine hematologic assays
White blood cell (WBC) differential count, hematocrit, hemoglobin, red blood cell (RBC), and platelet counts were determined by automated methods using an NE800 Analyser (Dasit, Milan, Italy).
PMN study
PMN membrane CD11b and LAP.
The expression of CD11b and LAP antigens was measured in whole blood by cytofluorometric analysis. For CD11b analysis, blood samples were incubated with phycoerythrin (PE)–conjugated mouse antihuman CD11b mAb (Becton Dickinson, Mountain View, CA) or with isotype-identical negative control mAb (IgG2a PE; Becton Dickinson). For LAP analysis, samples were incubated with mouse antihuman LAP (mAb 1B12.1) and stained with fluorescein isothiocyanate-conjugated goat-antimouse IgG (10 μg/mL, final concentration; Becton Dickinson).22Samples were analyzed by a FACScan flow cytometer (Becton Dickinson). PMNs were selectively gated using their forward- and side-scatter properties, and 5000 PMN-gated events were measured for each sample. Acquisition and processing of data were performed with the CellQuest software (Becton Dickinson). Results are expressed as mean fluorescence intensity (MFI units), representing the mean level of marker expression/cell, or as percentage positive cells, representing the number of cells positive for the marker. CD11b is preferentially expressed as MFI units because CD11b antigen is constitutively expressed by 100% of the PMNs gated,23 and the number of molecules/cell increases on activation.24 Instead, LAP is expressed as percentage positive cells because LAP antigen is not expressed by all PMNs and a greater number of cells become positive on activation.25
PMN cellular elastase activity assay.
Elastase activity was tested to quantify the enzyme content of PMNs. Activity was measured in cell lysates (106 cell/mL) by monitoring the rate of release of p-nitroanilide from the specific chromogenic substrate N-succinyl-Ala-Ala-Val-p-nitroanilide (Sigma Chemicals, St Louis, MO) at 410 nm.26 The activity of the samples (ng/106 cells) was extrapolated from a standard curve of known concentrations of human purified elastase (Sigma).
Assay of PMN response to in vitro activation by stimulus.
This test evaluates the capacity of PMNs to increase surface CD11b and LAP antigen expression on stimulus. Selected PMN samples from patients with ET (n = 16) and PV (n = 18) and control subjects (n = 20) were stimulated in vitro with N-formyl-Met-Leu-Phe (fMLP; Sigma). Specifically, whole blood was incubated in the aggregometer for 5 minutes at 37°C with 0.1 μmol/L fMLP (final concentration) or control buffer, under constant stirring (1000 rpm). Then samples were stained with anti-CD11b and anti-LAP mAb, as described above. Expression of CD11b and LAP antigens by PMN membranes of stimulated and unstimulated (control) samples was assayed by flow cytometry.
Plasma markers of PMN activation
Elastase and myeloperoxidase antigens were the plasma markers used to test the release of PMN azurophil granule content.27 Elastase coupled to its natural inhibitor α1-antitrypsin (α1-AT) was detected in plasma as elastase–α1-AT complex by an immunoassay procedure (PMN Elastase IMAC; Merck, Darmstadt, Germany). Plasma MPO was measured by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Bioxytech MPO, Enzyme Immunoassay; Oxis-International, Portland, OR).
Plasma markers of endothelial cell activation
The parameters of endothelium damage studied were the plasma concentrations of thrombomodulin (TM) and von Willebrand factor (vWF).28 TM is the endothelial membrane receptor for thrombin.29 vWF is a multimeric protein synthesized and released from endothelial cells.30 Plasma TM antigen was quantitated using an ELISA kit from Diagnostica Stago (Roche Diagnostics, Monza, Italy) according to the manufacturer's instructions. Plasma vWF antigen (vWF:Ag) was determined by an electro-immunodiffusion procedure using reagents from Diagnostica Stago (Assera-Plate vWF; Roche Diagnostics). Results are expressed as percentage vWF, by comparison with a standard curve constructed with known dilutions of pooled normal plasma (made by the plasma samples from 20 healthy persons not receiving any medication). The undiluted plasma pool consisted of 100% vWF; vWF of the control group ranged from 60% to 150%.
Plasma markers of hypercoagulation
As markers of in vivo clotting activation, the plasma levels of prothrombin fragment F1 + 2 (F1 + 2), thrombin-antithrombin (TAT) complex, and D-dimer were measured. These are enzyme-inhibitor complexes or byproducts of the coagulation reactions, liberated during clotting activation, that provide a biochemical tool for the definition of the hypercoagulable state and are modulated by therapy.19 31 Plasma F1 + 2 and TAT complex concentrations were determined by ELISA, using commercially available kits (Enzygnost F1 + 2 and Enzygnost TAT, respectively; DADE Behring, Milan, Italy). Plasma D-dimer levels were determined by a commercial ELISA kit (Asserachrom D-dimer; Roche Diagnostics).
Statistical analysis
The Mann-Whitney U test was used to assess the significance of differences between the mean baseline values of the patient group versus the healthy control group. The Wilcoxon test was used to test the significance of within-group differences.
Differences were considered significant at P < .05. Regression analysis was performed by the least-squares method, and the correlation coefficient (r2) was calculated.
Results
Routine hematologic parameters
As shown in Table 1, WBC, PMN, and platelet counts of patients with ET and PV were significantly greater than those of the control subjects. In patients with PV, RBC counts and hematocrit and hemoglobin levels also were significantly greater than those of the control subjects (P < .01). The lower range limits of some parameters—particularly RBCs, platelets, hemoglobin, and hematocrit—in patients was below the lower limits of normal control range values. These results corresponded to those from patients on cytoreductive therapy. The same was true of some patients with ET, who had normal platelet counts (range, 360-1600 × 109/L). However, no influence of HU therapy was observed in any of the parameters of PMN, endothelial, or clotting activation here studied. One exception was the vWF level, which appeared to be higher in non–HU-treated than in HU-treated subjects in the ET group (non-HU vs HU, 214% ± 16% vs 163% ± 9.4%;P < .05).
PMN study
CD11b and LAP.
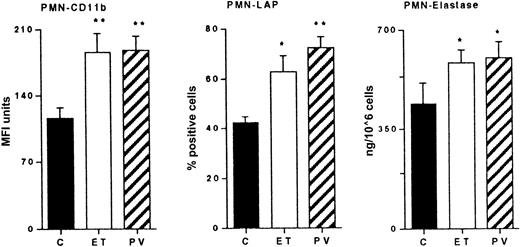
The expression of CD11b by PMNs in patients with ET and PV was significantly greater than control subjects' values (C, 112 ± 11 MFI units; ET, 185 ± 20 MFI units, P < .01; PV, 187 ± 15 MFI units, P < .01). The percentage of LAP-positive PMNs in patients with PV (73% ± 4.4% positive cells) also was significantly greater than that in patients with ET (63% ± 6.7% positive cells) and in control subjects (41% ± 2.3% positive cells) (P < .01) (Figure1).
PMN activation parameters.
Cytofluorometric analysis of the expression of CD11b and of LAP on the PMN cell surface in control subjects (C) and in patients with ET and PV. CD11b results are expressed as MFI arbitrary units. LAP results are expressed as percentage mAb-positive cells. Elastase activity of lysed PMNs isolated from whole blood. Results are mean ± SEM. Statistical analysis using Mann-Whitney U test for nonparametric data. *P < .05; ** = P < .01; both versus C.
PMN activation parameters.
Cytofluorometric analysis of the expression of CD11b and of LAP on the PMN cell surface in control subjects (C) and in patients with ET and PV. CD11b results are expressed as MFI arbitrary units. LAP results are expressed as percentage mAb-positive cells. Elastase activity of lysed PMNs isolated from whole blood. Results are mean ± SEM. Statistical analysis using Mann-Whitney U test for nonparametric data. *P < .05; ** = P < .01; both versus C.
Cellular elastase activity.
To evaluate whether PMN cells also had different elastase contents, the elastase activity of PMN lysates was measured. As shown in Figure 1, there was a significant elevation of this enzyme activity in PMNs isolated from patients with PV (606 ± 52 ng/106 cells;P < .01) and ET (585 ± 45 ng/106 cells;P < .05) compared with controls (442 ± 46 ng/106 cells).
In vitro PMN response.
This assay evaluates the capacity of PMNs to express CD11b and LAP surface antigens after an activating stimulus (Figure2). As demonstrated by preliminary experiments, in vitro stimulation of normal control PMNs with increasing concentrations of fMLP (from 0 to 10 μmol/L) significantly increased the expression of CD11b and LAP by the PMN membrane, peaking at 0.1 μmol/L fMLP (data not shown). Stimulation of patients' PMNs with 0.1 μmol/L fMLP significantly increased CD11b and LAP expression compared with unstimulated PMN counterparts. However, the mean percentage increment ([value of stimulated sample−value of unstimulated sample]/value of unstimulated sample×100) was decreased compared with control PMNs (CD11b: C, 624% ± 89%; ET, 366% ± 41%, P = n.s.; PV, 303% ± 40%,P < .05); LAP: C, 59% ± 15%; ET, 24% ± 10%,P < .05); PV, 8% ± 3%, P < .05). A significant negative correlation existed between CD11b levels of unstimulated PMNs from control subjects, patients, and the respective stimulated PMN counterpart levels (y = −4.01x+1939; r2 = 0.989; P < .01). In summary the finding that the percentage increase in basal levels after fMLP stimulation was lower in patients than in controls, together with the higher basal expression of both markers, strongly supports the conclusion that PMNs from patients underwent degranulation in vivo.
PMN surface CD11b and LAP expression after in vitro stimulation with fMLP.
Whole blood samples obtained from controls (C) and patients with ET and PV were incubated with fMLP and stained with mAb anti-CD11b and anti-LAP, as described in “Patients and methods.” Histograms represent the mean percentage increase (± SEM) of the 2 PMN surface antigens on stimulation compared with the unstimulated counterpart. Mean percentage increase = [value of stimulated sample−value of unstimulated sample]/value of unstimulated sample×100. These values were lower in patients with ET and PV. Statistical analysis as in Figure 1. *P < .05 versus C.
PMN surface CD11b and LAP expression after in vitro stimulation with fMLP.
Whole blood samples obtained from controls (C) and patients with ET and PV were incubated with fMLP and stained with mAb anti-CD11b and anti-LAP, as described in “Patients and methods.” Histograms represent the mean percentage increase (± SEM) of the 2 PMN surface antigens on stimulation compared with the unstimulated counterpart. Mean percentage increase = [value of stimulated sample−value of unstimulated sample]/value of unstimulated sample×100. These values were lower in patients with ET and PV. Statistical analysis as in Figure 1. *P < .05 versus C.
Plasma marker of PMN activation
Plasma elastase and MPO antigens are the markers of PMN azurophil granule content release into the bloodstream. As shown in Figure3 (upper panels), the plasma concentration of these markers was significantly increased (P < .01) in the circulation of both groups of patients compared with control subjects (elastase: ET, 63.7 ± 6.3 μg/L; PV, 86.1 ± 13.3 μg/L; C, 33.8 ± 2.2 μg/L; MPO: ET, 0.9 ± 1.5 μg/L; PV, 11.8 ± 1.5 μg/L; C, 4.2 ± 0.6 μg/L). Because patients and control subjects had different WBC counts, results were also corrected for the WBC count. The ratio (elastase or MPO antigen concentration [μg/L]/WBC count (109/L)]) was calculated for each patient and control subject. After correction, the differences between patients and controls persisted and were statistically significant (Figure 3, lower panels).
Plasma markers of PMN activation.
Concentration of elastase, measured as elastase-α1AT complex, and of myeloperoxidase (MPO) in control subjects (C) and in patients with ET and PV (upper panels). In the lower panels, the ratio (elastase or MPO antigen concentration, [μg/L]/WBC count [109/L]) calculated for ET, PV, and control groups is depicted. Histograms represent mean values ± SEM. Statistical analysis as in Figure 1. *P < .05; **P < .01; both versus C.
Plasma markers of PMN activation.
Concentration of elastase, measured as elastase-α1AT complex, and of myeloperoxidase (MPO) in control subjects (C) and in patients with ET and PV (upper panels). In the lower panels, the ratio (elastase or MPO antigen concentration, [μg/L]/WBC count [109/L]) calculated for ET, PV, and control groups is depicted. Histograms represent mean values ± SEM. Statistical analysis as in Figure 1. *P < .05; **P < .01; both versus C.
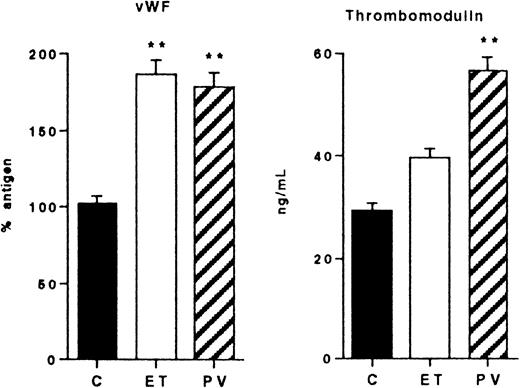
Plasma endothelial cell activation markers
A significant increment of the circulating endothelial cell marker vWF:Ag was observed in the ET and PV groups (Figure4) (ET, 188% ± 15%; PV, 180% ± 12%; C, 105% ± 13%). TM was augmented, though not significantly, in patients with ET (39 ± 5 ng/mL), but it was significantly increased in patients with PV (57 ± 8 ng/mL,P < .01) compared with controls (27 ± 2 ng/mL).
Markers of endothelial cell activation in control subjects (C) and in patients with ET and PV.
Plasma levels of vWF and TM. Mean values and SEM are shown. Statistical analysis as in Figure 1. *P < .05; **P < .01; both versus C.
Markers of endothelial cell activation in control subjects (C) and in patients with ET and PV.
Plasma levels of vWF and TM. Mean values and SEM are shown. Statistical analysis as in Figure 1. *P < .05; **P < .01; both versus C.
Plasma markers of hypercoagulation
As shown in Figure 5, patients with ET and PV showed significantly higher values of plasma F1 + 2 (ET, 1.67 ± 0.14 nmol/L; PV, 1.87 ± 0.19 nmol/L; C, 1.1 ± 0.06 nmol/L) and TAT complex (ET, 5.15 ± 0.34 μg/L; PV, 4.97 ± 0.41 μg/L; C, 3.4 ± 0.31 μg/L) compared with controls. Levels of D-dimer were found significantly (P < .05) elevated only in the PV group (0.30 ± 0.03 μg/mL) compared with both the control (0.24 ± 0.02 μg/mL) and the ET (0.26 ± 0.04 μg/mL) groups.
Markers of hypercoagulation.
Plasma levels of F1 + 2, TAT complex, and D-dimer in control subjects (C) and in patients with ET and PV. Data are expressed as mean values ± SEM. Statistical analysis as in Figure 1. *P < .05; both versus C.
Markers of hypercoagulation.
Plasma levels of F1 + 2, TAT complex, and D-dimer in control subjects (C) and in patients with ET and PV. Data are expressed as mean values ± SEM. Statistical analysis as in Figure 1. *P < .05; both versus C.
Discussion
Recent studies indicate an important role for PMN cells in triggering hemostatic system reactions. In this study, we investigated PMN activation status in patients with ET and PV and the occurrence of concomitant laboratory signs of endothelium or clotting activation, or both. Thirty-seven patients with ET and 34 with PV were studied. Results were compared with those of control subjects. In all patients, median RBC, WBC, PMN, and platelet counts were significantly increased over the values of controls (Table 1), as expected. In addition, median hematocrit and hemoglobin levels were significantly increased in the PV group. However, in some patients, the above parameters (particularly RBC, hematocrit, and hemoglobin) were low because of HU cytoreductive treatment. No influence of HU therapy on any of the parameters included in this study was observed. The only exception was the level of vWF in the ET group, which actually was lower in HU-treated patients. For this reason, we did not separate the results obtained from patients of either group on the basis of treatment.
This study provides the first evidence of a significant in vivo PMN activation in patients with ET or PV. As markers of PMN activation, phenotypical changes (ie, increased CD11b and LAP expression on PMN surface) were measured. An increase in CD11b is currently accepted as a marker of PMN activation in vivo.9,24 25 Increments of LAP can be a feature of cellular activation as well. In fact, in this paper, we show that in vitro stimulation of normal PMNs by fMLP significantly increases the surface expression of LAP antigen. The question may arise as to whether the increments measured are somehow related to the different PMN counts. This is ruled out by the fact that the membrane CD11b and LAP values are expressed as MFI units or as percentage positive cells, respectively, which are calculated on a standard number of cells for each sample. Therefore, the changes detected correspond to activation changes per cell unit.
Further, the elastase content held in the circulating PMNs was increased in the 2 groups of patients compared to the control group. These data are comparable between different subjects because cellular elastase is determined on cell lysates prepared from the same number of cells and is expressed as ng/106 cells. The finding of increased PMN elastase content in patients with ET and PV indicates a cellular abnormality possibly explained by the rapid turnover of these cells, with an increased proportion in the circulation of young PMNs containing more elastase. This interpretation is corroborated by the previous observation of an increased amount of elastase in PMNs from healthy subjects after an initial dose of granulocyte-colony stimulating factor (G-CSF).9 The increased basal levels of CD11b and LAP, together with the lower up-regulation of both markers after fMLP stimulation in vitro, in patients and not in control subjects strongly supports the conclusion that PMNs underwent granule secretion in vivo in patients with ET and PV.
In patients' circulation we measured elevated levels of elastase and MPO, which represent 2 parameters of PMN degranulation and may be considered additional signs of activation of these cells in vivo. Although their concentrations also depend on the number of circulating WBCs,9 31 we found that, after normalization for the WBC count, the values of both elastase and MPO of patients with ET and PV remained greater than those of the control group. This finding supports the hypothesis of the occurrence of a cellular activation.
As mentioned in the “Introduction,” several intracellular PMN enzymes, including elastase, MPO and cathepsin G, have been shown to act on the coagulation and endothelial systems in vitro.10-18,32 In the previously mentioned study of PMN activation in healthy donors receiving G-CSF,9 we found a positive correlation between plasma elastase level and the levels of some markers of endothelial and clotting activation in vivo. In the current study the evaluation of 2 recognized plasma markers of activation/damage of endothelial cells shows that vWF and TM are significantly elevated in patients with ET and PV. It is possible to speculate that in this condition of endothelial perturbation, a role may be played, at least in part, by the abnormal levels of circulating PMN enzymes. In vitro evidences demonstrate that PMNs activated by various agonists (fMLP, bacterial endotoxin, cytokines) are able to damage monolayers of endothelial cells in culture.10,33,34The damage goes from direct cytolysis and cell death, mediated by reactive species of the oxygen produced by activated PMNs, to the retraction and detachment of endothelial cells, mediated by proteases released by PMN granules. Among granule protease, elastase is the most involved in the pathogenesis of endothelial damage.35 The addition of purified elastase to the endothelium in vitro causes the detachment of endothelial cells in a dose-dependent manner. Endothelial cells in culture, incubated with activated PMNs or reactive species of oxygen, release vWF and TM in the culture medium.36 37
Furthermore, in this study, measurements of well-known plasma markers of clotting system activation also indicated concomitant abnormalities of TAT complex, F1 + 2, and D-dimer in the patient population. This finding is in agreement with our previous observation of a hypercoagulable state in a group of patients with ET.38 It could in part be explained by the known hypercoagulable condition associated with malignancy,39 but PMN enzyme involvement is possible. In vitro studies have shown evidence that elastase can proteolytically inactivate several physiological inhibitors of blood coagulation—protein C, protein S, antithrombin, and heparin cofactor II.15-18 This inactivation may contribute to local progression of coagulation reactions at the inflammation sites. In addition, other in vitro studies have shown that both elastase and cathepsin G can cleave and activate coagulation Factor V.40 Although the in vivo conditions are very different, we may speculate that an increased release of PMN proteases may provide a potential mechanism for interference with the hemostatic system. The mechanisms of actions of these enzymes in vivo are complex because of the high levels of proteinase inhibitors available in the circulation. However, leukocyte-bound elastase retains its proteolytic activity41; in addition, the capacity of PMNs to express adhesive properties (ie, surface CD11b) allows the formation of a close microenvironment in which these enzymes are released and protected against inhibitors and their interaction with the substrate is facilitated.14,42 43
Other authors have not confirmed the evidence of abnormalities of the plasma levels of endothelial cell damage markers in patients with ET.44 In that study, TM and vWF levels were increased in patients and not in controls without reaching statistical significance. In our study, we obtained similar results concerning TM levels in the ET group, but the concentration of vWF antigen was confirmed to be statistically greater in patients than in controls. We cannot explain this discrepancy, but published data describe increased vWF antigen levels in association with a history of thrombosis in patients with chronic myeloproliferative disorders.45
In conclusion, in this study, we observed the occurrence of granulocyte activation in association with laboratory abnormalities of clotting activation and endothelium perturbation in patients with 2 hematologic conditions who were at high risk for thrombosis. These results suggest that PMNs may be involved in the pathogenesis of the thrombophilic state in patients with ET and PV. The increase in WBC count, typical of most patients with these disorders, raises the possibility that the number of circulating leukocytes can be involved, as in other clinical conditions (eg, coronary heart and peripheral arterial diseases).7,8 However, the activation status of these cells is important because activated leukocytes have multiple interactions with platelets and vessel walls. In the in vivo model of healthy subjects administered G-CSF,9 the levels of PMN activation markers significantly correlated with the levels of either endothelium or clotting activation markers. We hypothesize that in addition to high hematocrit and blood viscosity levels, as in PV, or thrombocytosis and platelet abnormalities, as in ET, concurrent factors may be required for the onset of a thrombotic event in patients with ET and PV. PMN-related prothrombotic activity may be one of these factors.
We did not find any association between the elevation of any of the markers analyzed and a history of thrombosis. This might likely be due to the limited number of subjects here analyzed. Further studies of patients followed in a prospective manner are needed to establish whether one or more of these parameters can be predictive of thrombosis in these conditions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anna Falanga, Hematology Division, Ospedali Riuniti Bergamo, Largo Barozzi, 1-24128 Bergamo, Italy; e-mail:annafalanga@yahoo.com.

![Fig. 2. PMN surface CD11b and LAP expression after in vitro stimulation with fMLP. / Whole blood samples obtained from controls (C) and patients with ET and PV were incubated with fMLP and stained with mAb anti-CD11b and anti-LAP, as described in “Patients and methods.” Histograms represent the mean percentage increase (± SEM) of the 2 PMN surface antigens on stimulation compared with the unstimulated counterpart. Mean percentage increase = [value of stimulated sample−value of unstimulated sample]/value of unstimulated sample×100. These values were lower in patients with ET and PV. Statistical analysis as in Figure 1. *P < .05 versus C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/13/10.1182_blood.v96.13.4261/5/m_h82400487002.jpeg?Expires=1767702321&Signature=UhTDV2HXT7NnEZIgdmWK8oI6eRQkbMOkrSqoE~u4kLYlf~SWaJSbBzkpQt0IPl5jWs3cRf0D6s2R2zkIORZVjynmZlkt5ATCZ~7JpTYq5bdtOxN5XDi0o0y8yUMFUHQWA274o5vHyMLuQHADXqbovYFR4TKfUjghE8W4Vk4uuZShS6XxYQvxIi8SBWKVpzNGsEhF8-ewC4oQl3~HxUnE5wkLDJ0oQrwrC4eCon3tQ-PyBKn7Eg5Vk8z7xmC3RDIgLu7MxaHOTlSeMSrTCtug-MvVPPWKuqzaZitmLtR5sRfWcyZIKZRYgcECsVyxuGTxJ~KDnOCklf4UJSYzJBxurQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Plasma markers of PMN activation. / Concentration of elastase, measured as elastase-α1AT complex, and of myeloperoxidase (MPO) in control subjects (C) and in patients with ET and PV (upper panels). In the lower panels, the ratio (elastase or MPO antigen concentration, [μg/L]/WBC count [109/L]) calculated for ET, PV, and control groups is depicted. Histograms represent mean values ± SEM. Statistical analysis as in Figure 1. *P < .05; **P < .01; both versus C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/13/10.1182_blood.v96.13.4261/5/m_h82400487003.jpeg?Expires=1767702321&Signature=nVW~ivCIbJFOqYg9ZzwVupCFvh4kIOXYhCq3R7vzsVHtzX2dtVM8Be-tni7t8Fuuu8j0us3KsdShoOCU02jqncViflA60u~Nyz-UAKHlAqFePEF7RDHKqS9O6pcH76H5nrBrcNjP0rnUTisn~YgWVWCprjSAwrkelVORDj59VQE2ANtcStGrQfTppG0JVGICsVk~1v8B15Tjp~ALrV0TZ~P4tuINw9yGUsaSv~6T6eVRAenalxMK8SbYgzf496MEsfTFSeGhhZYvIYeDsqDsD2sfEkImKhqsHfhDRy8IFJ9vMcSqrtPI2zS3kk2sRfDCLAAnZzIwUjd2H--TVm7DDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal