Abstract
Conflicting findings regarding proadhesion and antiadhesion in cell-to-cell interactions were previously reported for CD43. We examined possible differences in the role of the 130-kd glycoform and the 115-kd glycoform of CD43 in cellular adhesion in vitro. We generated a monoclonal antibody (MFT3) that discriminates between helper and nonhelper murine T-cell clones. Characterization of MFT3 with use of biochemical analysis and complementary DNA (cDNA) transfection experiments showed that it is specific for the 130-kd glycoform of CD43. T-cell clones that expressed the 130-kd CD43 glycoform showed decreased homocytic aggregation and decreased adhesion to spleen cells, B-lymphoma cell lines, and fibroblastic cell lines compared with T-cell clones negative for the 130-kd glycoform. Expression of core 2 β-1, 6-N-acetylglucosaminyltransferase (C2GnT) cDNA together with CD43 cDNA resulted in expression of both the 130-kd CD43 glycoform and the 115-kd CD43 glycoform in fibroblastic cell lines. Using these cell lines, we showed that the 130-kd glycoform but not the 115-kd glycoform of CD43 has an antiadhesive function in cellular interactions. Our findings suggest that the antiadhesive function of CD43 is primarily carried out by the 130-kd glycoform.
Introduction
CD43 (also known as sialophorin or leukosialin) is a cell-surface glycoprotein expressed in a variety of hematopoietic lineage cells, including T lymphocytes (T cells).1,2 The extracellular domain of CD43 contains more than 80 serine or threonine residues, most of which are O-link glycosylated.1,3,4 CD43 has a rodlike structure that is thought to extend 45 nm from the lipid bilayer.5 Its structural characteristics, together with anionic charges due to extensive sialylation of attached O-glycans, may produce the antiadhesive function of CD43 observed in various experimental and physiologic cellular interactions.6-11 On the other hand, several reports suggested that CD43 has a proadhesive function.12-15
CD43 has been reported to have 2 glycoforms, 1 with a molecular mass of 115 kd that is expressed on all T cells and 1 with a mass of 130 kd that is expressed only on activated T cells.16-18 Because the murine and human CD43 gene consists of one exon for the protein-coding sequences,19,20 the 2 different glycoforms are thought to reflect the differences in the attached O-glycan structure.16 Indeed, biochemical analysis showed that CD43 on resting T cells carries tetrasaccharides (115-kd glycoform), whereas activated T cells carry branched hexasaccharide core 2 O-glycans (130-kd glycoform) that are directed by core 2 β-1, 6-N-acetylglucosaminyltransferase (C2GnT).21Expression of the 130-kd glycoform of CD43 on all T cells in transgenic mice showed a reduction in primary T-cell immune responses22 that was probably due to the antiadhesive function of this glycoform.
Previously, we analyzed the autoimmunity of a first-filial-generation (F1) hybrid between New Zealand Black (NZB) and New Zealand White (NZW) strains of mice (the B/WF1 strain) as an animal model of human systemic lupus erythematosus. Analysis of autoreactive T-cell clones derived from B/WF1 mice showed 2 groups of CD4 T-cell clones.23One group was pathogenic in that it induced IgG anti-DNA antibody production in preautoimmune young B/WF1 mice in adoptive cell-transfer experiments. The other group did not show pathogenicity in spite of having the same αβ T-cell receptor (TCR) sequences as pathogenic T-cell clones. In this study, we further examined the differences between these pathogenic and nonpathogenic T-cell clones and found that only the nonpathogenic T-cell clones expressed the 130-kd glycoform of CD43. We also found that the 130-kd CD43 glycoform functioned as an antiadhesion molecule in cellular interactions between T cells and B cells as well as those between B-lymphoma cell lines and transfectant fibroblastic cell lines. Our findings suggest that the antiadhesive function of CD43 in cellular interactions in vitro is carried out primarily by the 130-kd glycoform and that the decrease in or absence of helper activity in vivo may be due to expression of this glycoform.
Materials and methods
Mice and rats
NZB, NZW, and BALB/c mice were obtained from Japan SLC, Inc (Hamamatsu, Japan). F1 B/WF1 mice (NZB crossed with NZW mice) were generated by mating female NZB with male NZW mice in our animal breeding facilities. Wistar rats were purchased from Charles River Japan, Inc (Tokyo, Japan). All animal experiments were done in accordance with Saga Medical School guidelines for the care and treatment of animals used in experimentation.
Cells
The establishment and characterization of T-cell lines and clones were described previously.24 M12.4.5 and M12.C3 B-lymphoma cell lines25 were cultured in RPMI 1640 medium with 10% fetal calf serum (FCS) and 50 μmol/L 2-mercaptoethanol (2-ME). A BALB/3T3 fibroblastic cell line was obtained from the Human Science Research Resources Bank (Osaka, Japan). A human kidney 293 cell line was obtained from the American Type Culture Collection (ATCC; Rockville, MD). These cell lines were grown in Dulbecco modified Eagle medium (Gibco, Gaithersburg, MD) containing 10% FCS and 50 μmol/L 2-ME.
Monoclonal antibodies
A rat antimouse monoclonal antibody (mAb) for 130-kd-glycoform CD43 (1B11) was purchased from PharMingen (San Diego, CA). The hybridoma S7 cell line secreting rat antimouse 115-kd-glycoform CD43 was obtained from the ATCC. Hybridoma FD441.8 (anti-lymphocyte function–associated antigen 1 [LFA-1]), M1/69 (anti-CD24), M1/70 (anti-Mac-1), and MEL14 (anti-L-selectin) cell lines were obtained from the ATCC. Hybridoma RA3-6B2 (anti-B220) and 2C11 (anti-CD3) cell lines were provided by Dr S. Murakami (Osaka University Dental School, Osaka, Japan). Purified KAT-1 mAb against intracellular adhesion molecule 1 (ICAM-1) was purchased from Seikagaku Corporation (Tokyo, Japan). Purified GoH3 mAb against very late antigen (VLA) 6 was purchased from Serotec Ltd (Oxford, UK). Hybridoma PS/2 (anti-VLA-4), M/K-2 (anti-vascular cell adhesion molecule 1), and KM201 (anti-CD44) cell lines were described previously.26 27
mAbs against T-cell clones
A female Wistar rat was immunized intraperitoneally with 107 autoreactive T-cell clones emulsified in 1 mL complete Freund adjuvant. The rat was given a booster with 107T-cell clones in incomplete Freund adjuvant and the final booster in phosphate-buffered saline (PBS). Three days after the final immunization, the spleen was harvested for fusion with the murine myeloma cell line SP2/0 (ATCC). Hybridoma supernatants were screened by means of differential immunofluorescence staining against pathogenic and nonpathogenic T-cell clones. We obtained a mAb (MFT3) that specifically recognized nonpathogenic T-cell clones. We also obtained, by cross-blocking screening with MFT3, a mAb (FY14) that recognized both pathogenic and nonpathogenic T-cell clones. The isotype of the mAbs was determined by Ouchterlony immunodiffusion analysis (ICN ImmunoBiologicals, Lisle, IL). Antibodies were purified with use of Abx plus column chromatography (Yamazen Co, Osaka, Japan) from ascites fluid isolated from severe combined immunodeficient mice (Clea Laboratories, Inc, Tokyo, Japan).
Immunofluorescence staining
T-cell clones obtained more than a week after restimulation were purified on a Ficoll-Paque device (Pharmacia LKB Biotechnology, Uppsala, Sweden) to remove feeder cells and cell debris. In some experiments, cells were treated with 100 mU/mL neuraminidase fromArthrobacter ureafaciens (Nakarai Tesque, Inc, Kyoto, Japan) for 1 hour at 37°C to remove sialic acids from the cell surface. Neuraminidase treatment was confirmed by staining with fluorescein isothiocyanate conjugated (FITC)–wheat germ agglutinin (Sigma Chemical Co, St Louis, MO). T-cell clones and transfectant cell lines were incubated separately with appropriately diluted mAbs for 30 minutes on ice and washed 3 times with staining buffer (PBS containing 5% FCS and 0.1% sodium azide [NaN3]). For cross-blocking staining, cells were first incubated with 10 μg/mL blocking mAb (S7 or 1B11) for 30 minutes on ice, washed 3 times with staining buffer, and stained with biotinylated MFT3 mAb. Cells were then incubated with FITC-mouse antirat κ-chain mAb MAR18.5 (ATCC) or FITC-streptavidin (Gibco) for 30 minutes on ice. Propidium iodide (Sigma) was added for the last 5 minutes to gate out dead cells. Cells were washed 3 times with staining buffer and analyzed on a fluorescence-activated cell-sorter scanner (FACScan; Becton Dickinson Immunocytometry Systems, Mountain View, CA). Results were expressed as mean fluorescence intensity (MFI).
Immunoprecipitation and Western blotting
Cells were lysed in a lysis buffer containing 50 mmol/L Tris–hydrochloric acid (HCl; pH 7.5), 150 mmol/L sodium chloride, 1% Triton X-100, 50 mmol/L iodoacetamide, 2 mmol/L magnesium chloride, 2 mmol/L calcium chloride, and 0.1% NaN3. Soybean trypsin inhibitor (10 μg/mL) and phenylmethylsulfonyl fluoride (Sigma; 1 mmol/L) were added as protease inhibitors. Lysates were cleared with use of centrifugation, and the supernatant was diluted in sample buffer containing 0.125 mol/L Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), and 10% glycerol with 5% 2-ME and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). For immunoprecipitation, the lysates were allowed to react with mAb-coupled, MAR18.5-bound protein A Sepharose 4B (Zymed, South San Francisco, CA) for 2 hours at 4°C, with rotation. After 3 washes in lysis buffer, the bound proteins were released by boiling for 5 minutes in sample buffer and subjected to SDS-PAGE. Proteins were then blotted on a nitrocellulose membrane (Bio-Rad Laboratories, Richmond, CA). The membranes were incubated with appropriately diluted mAbs in PBS containing 1% skim milk and 0.05% Tween 20 and then incubated with peroxidase-conjugated, affinity-isolated goat antirat immunoglobulin (BioSource International, Camarillo, CA). In some experiments, alkaline phosphatase–conjugated, affinity-isolated goat antirat IgG antibody (Cappel, Durham, NC) was used as a second reagent. The membranes were incubated with enhanced chemiluminescence reagent (Pierce Chemical Co, Rockfold, IL) and exposed to x-ray film or developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium reagent.
DNA-mediated gene transfer
A complementary DNA (cDNA) encoding murine CD43 in pM5neo retrovirus vector28 was provided by Dr Toshitada Takemori (Department of Immunology, NIH of Japan, Tokyo, Japan). The CD43 cDNA was subcloned into pME18S (provided by Dr Kazuo Maruyama, Department of Hygiene and Oncology, Tokyo Medical and Dental University School of Medicine, Tokyo, Japan). The pME18S/CD43 was transfected into 293 cells with pBK-CMV (Stratagene, La Jolla, CA) carrying a neomycin-resistance gene by using the standard calcium phosphate method.29After selection by G418 (Wako Chemicals, Osaka, Japan), transfectant cell lines were selected by staining with anti-CD43 mAb S7. For cell-adhesion assays, we generated BALB/3T3 cell lines that express the 115-kd glycoform of CD43 alone or both the 115-kd glycoform and the 130-kd glycoform. A cDNA fragment coding the full length of murine core 2 β-1, 6-N-acetylglucosaminyltransferase (C2GnT) protein was amplified from the total RNA of a T-cell clone (KGU13923) positive for the 130-kd CD43 glycoform. Amplification was accomplished with reverse transcriptase–polymerase chain reaction (RT-PCR) using sense primer 5′-CGCCTCGAGATGCTGAGAAACTTGTTTCGG-3′ and antisense primer 5′-CGGGACCTCTTGAATCTTGTGTCTAGACGC-3′. These primers contain artificial recognition sequences forXhoI or BglII (underlined).
The cDNA was subcloned into the XhoI/BamHI site of a vector derived from a mammalian expression vector (pEF-BOS30) modified to express a fusion protein with the flag epitope tag at the carboxyl terminal. The insert coding for the C2GnT-flag fusion protein was subcloned into pBK-EF (a gift from T. Fujimoto, Hiroshima University, Hiroshima, Japan) derived from pBK-CMV. The plasmid pME18S/CD43 alone or pME18S/CD43 and pBK-EF/C2GnT-flag were transfected into BALB/3T3 fibroblastic cell lines by means of the calcium phosphate method and selected using G418. Transfectant cell lines were screened by staining with anti-CD43 mAbs (S7 and MFT3). As a control, we generated BALB/3T3 cell lines that express C2GnT alone. The pBK-EF/C2GnT-flag was transfected and selected by using G418. The transfectant cell lines were screened by Western blotting of cell lysates using the anti-flag M2 mAb (Sigma).
RT-PCR analysis of expression of core 2 β-1, 6-N-acetylglucosaminyltransferase (C2GnT)
Total RNA was extracted from T-cell clones, 293 cells, and BALB/3T3 cells by using Isogen (Wako), and expression of C2GnT was detected by RT-PCR. Reverse transcription of 5 μg total RNA was done with the SuperScript preamplification system (Gibco) and random hexamers. A 2-μL aliquot of a 1:50 dilution of the resulting cDNA solution was used for PCR amplification with Gene-Taq (Nippon Gene, Tokyo, Japan). For detection of C2GnT transcripts, PCR was performed for 30 seconds at 94°C, 30 seconds at 58°C, and 1 minute at 72°C for 40 cycles, using the oligonucleotide primers used for isolation of C2GnT cDNA. As an internal control for the RT-PCR analysis, mouse β-actin transcripts were amplified from the same cDNA samples. For detection of β-actin mRNA, PCR was performed for 30 seconds at 94°C, 30 seconds at 55°C, and 1 minute at 72°C for 25 cycles, using the murine β-actin primers 5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-GTCCTTAATGTCACGCACGATTTC-3′. PCR products were visualized with 1% agarose gel electrophoresis and stained with ethidium bromide.
In vitro homocytic aggregation of T-cell clones
T-cell clones were stimulated more than a week before the aggregation assay. After purification on a Ficoll-Paque device (Pharmacia), 1 × 105 T-cell clones were resuspended in 0.2 mL complete medium, plated in individual wells of a 96-well culture plate (Corning Glass Works, Corning, NY), and cultured with or without 10 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma) for 16 hours. The degree of T-cell aggregation was observed by using inverted microscopy.
Cell-adhesion assays
T-cell clones or M12.C3 B-lymphoma cell lines were radiolabeled by incubating 1 × 107 cells in 1 mL culture medium with 3.7 MBq sodium chromate (with chromium 51 [51Cr]; Amersham, Tokyo, Japan) for 1 hour at 37°C. They were then washed 3 times in culture medium and added to adhesion target-cell layers, which were prepared as follows: BALB/3T3 cells or their transfected cell lines were plated in wells of a 24-well plate (Corning) at 5 × 104 cells/well and allowed to grow overnight. For M12.4.5 or M12.C3 B-lymphoma cell lines and spleen cells, the plates were precoated with 10 μg/mL poly-L-lysine hydrobromide (Wako) in PBS for 30 minutes at room temperature and washed 3 times with PBS. Then, 2 × 106 B-lymphoma cells or 1 × 107 spleen cells were plated and fixed with 0.25% glutaraldehyde in PBS. After 10 minutes, wells were washed 3 times with PBS, and 100 mmol/L glycine (Wako) and 1% bovine serum albumin (Sigma) in PBS were added to block the functional glutaraldehyde group. After 3 washes with culture medium, the 51Cr-labeled cells (2 × 105/well) were added to the target-cell layers and incubated for 30 minutes at 37°C. The nonadherent cells were removed by 3 cycles of gentle washing in prewarmed medium. Adherent cells were lysed with water, and the radioactivity of cell-associated51Cr was determined with a γ counter (Hewlett Packard Co, Palo Alto, CA). The percentage of adhered cells was calculated as the counts per minute of adhered cells divided by the counts per minute of input cells times 100. Results are expressed as the mean counts per minute of triplicate samples ± SD.
Results
Generation of a mAb (MFT3) that discriminates between nonhelper and helper T-cell clones
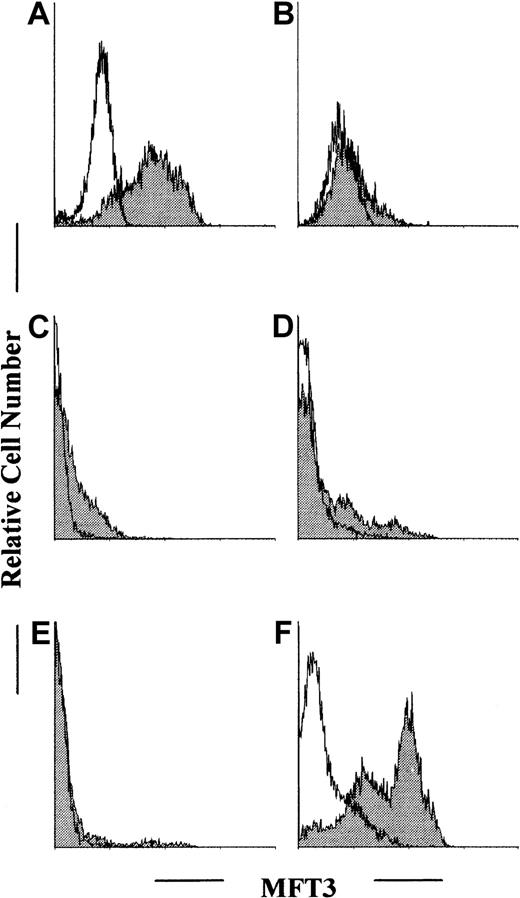
During the analysis of autoimmunity in B/WF1 mice, we found that some but not all autoreactive CD4-positive T-cell clones derived from these mice showed helper activities in that they induced IgG anti-DNA antibody production on transfer to preautoimmune young B/WF1 mice.23 Subsequent analysis showed that these T-cell clones had the same α (AV5S2, RGDYANKM of CDR3; Jα39 as expressed by the amino acid single-letter code) and β (BV4S1, SQDLSSYEQY of CDR3; Jβ2.6) chains as the TCR (unpublished data), indicating that they are in fact derived from the same clone. We decided to examine the differences between these T-cell clones by making discriminating mAbs, and we isolated a hybridoma cell line (MFT3) that secreted a mAb specific to nonhelper T-cell clones. Thus, MFT3 mAb discriminated between nonhelper T-cell clones and helper T-cell clones. Representative stainings of T-cell clones positive and negative for MFT3 antigen are shown in Figure 1A and 1B.
Flow cytometric analysis of MFT3 antigen expression in T-cell clones and in cells from various lymphoid tissues.
Autoreactive T-cell clones KGU139 (A) and KGU140 (B) derived from B/WF1 mice were stained with MFT3 monoclonal antibody (mAb; IgG2a) followed by fluorescein isothiocyanate conjugated (FITC)–MAR18.5 and analyzed on a fluorescence-activated cell-sorter scanner (FACScan). Cells from the thymus (C), spleen (D), lymph nodes (E), and bone marrow (F) of BALB/c mice were stained and analyzed as above. The shaded histogram shows results from staining with MFT3 mAb; and the open histogram, results from staining without MFT3 mAb.
Flow cytometric analysis of MFT3 antigen expression in T-cell clones and in cells from various lymphoid tissues.
Autoreactive T-cell clones KGU139 (A) and KGU140 (B) derived from B/WF1 mice were stained with MFT3 monoclonal antibody (mAb; IgG2a) followed by fluorescein isothiocyanate conjugated (FITC)–MAR18.5 and analyzed on a fluorescence-activated cell-sorter scanner (FACScan). Cells from the thymus (C), spleen (D), lymph nodes (E), and bone marrow (F) of BALB/c mice were stained and analyzed as above. The shaded histogram shows results from staining with MFT3 mAb; and the open histogram, results from staining without MFT3 mAb.
Characterization of MFT3 antigen as the 130-kd glycoform of murine CD43
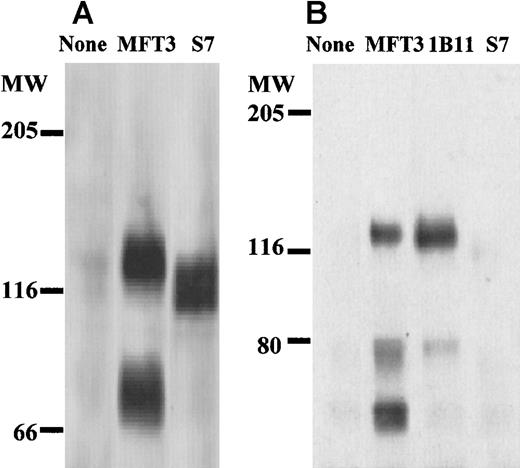
The tissue distribution of MFT3 antigen indicated by FACScan analysis is shown in Figure 1 (C-F). Stimulation of spleen cells and lymph node cells by concanavalin A slightly increased the percentage and intensity of positive staining (data not shown). The molecular mass of MFT3 antigen on assessment by Western blotting using bone marrow cell lysates was about 130 kd (Figure2A). A signal that corresponds to 70 kd, probably the degradative product of the 130-kd molecule,18was also observed.
Biochemical characterization of MFT3 antigen as the 130-kd CD43 glycoform.
(A) Lysates of bone marrow cells from BALB/c mice were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 6% polyacrylamide gels), and Western blotting was performed with MFT3 or S7 (anti-115-kd CD43 glycoform) mAb. (B) MFT3-positive T-cell clones (KGU139) were lysed and immunoprecipitated with MFT3 mAb, subjected to SDS-PAGE (6%), and transferred to a nitrocellulose membrane. The membrane was probed with MFT3, 1B11 (anti-130-kd CD43 glycoform), or S7 mAb.
Biochemical characterization of MFT3 antigen as the 130-kd CD43 glycoform.
(A) Lysates of bone marrow cells from BALB/c mice were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 6% polyacrylamide gels), and Western blotting was performed with MFT3 or S7 (anti-115-kd CD43 glycoform) mAb. (B) MFT3-positive T-cell clones (KGU139) were lysed and immunoprecipitated with MFT3 mAb, subjected to SDS-PAGE (6%), and transferred to a nitrocellulose membrane. The membrane was probed with MFT3, 1B11 (anti-130-kd CD43 glycoform), or S7 mAb.
Because the tissue distribution and molecular weight of MFT3 antigen were similar to those of the 130-kd CD43 glycoform, we examined the specificity of MFT3 mAb by immunoprecipitation followed by Western blotting using anti-CD43 mAbs. Cell lysates from T-cell clones positive for MFT3 (KGU139) were immunoprecipitated with MFT3 mAb, subjected to SDS-PAGE, and probed with mAb 1B11, which is specific for the 130-kd CD43 glycoform.18 As shown in Figure 2B, blotting with 1B11 clearly showed a signal at 130-kd that was identical to that probed by MFT3. Blotting with S7 mAb, which is specific for the 115-kd glycoform of CD43, did not show any signal in the immunoprecipitates. These findings indicate that the antigen recognized by MFT3 mAb is the 130-kd glycoform of murine CD43. They also suggest that the S7 mAb and MFT3/1B11 mAbs recognize separate glycoforms of CD43. The protein size recognized by MFT3 mAb ranged from 120 to 130 kd in various cells (P815 mastocytomas, T-cell clones, 293 cell lines transfected with CD43 cDNA, and BALB/3T3 cell lines dual transfected with CD43 and C2GnT) on Western blotting (see below) and immunoprecipitation (data not shown). The band corresponding to about 70 kd was observed in many cells but was absent in one T-cell clone (KGX114) in the immunoprecipitation studies. It was also not observed with Western blotting in BALB/3T3 cell lines dual transfected with CD43 and C2GnT. Although we believe that this 70-kd band is a degradative product of the 130-kd glycoprotein, it is possible that a 70-kd glycoform of CD43 is expressed in some cells.
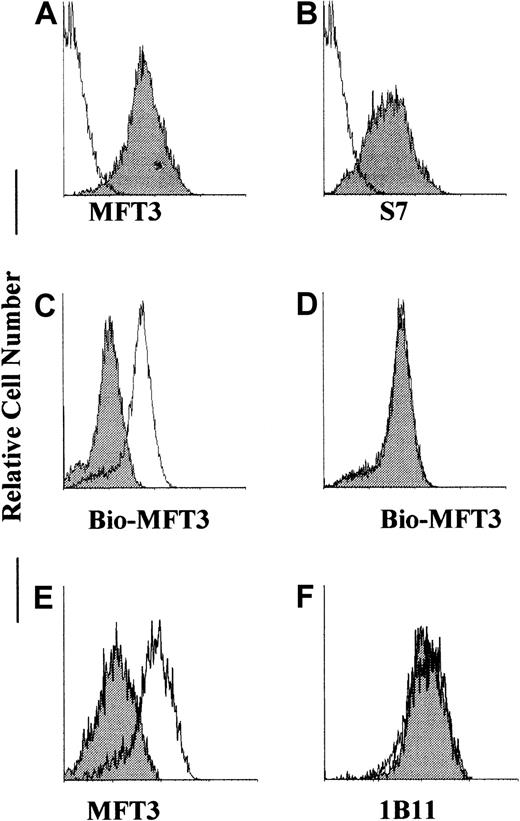
To confirm that MFT3 mAb recognizes CD43 gene products, we transfected CD43 cDNA into 293 cells and isolated stable transfectant cell lines that expressed CD43, as assessed by S7 mAb (Figure3B). This cell line showed positive staining against MFT3 mAb (Figure 3A), indicating that MFT3 mAb reacts with CD43 gene products. Cross-blocking staining done by treating the transfectant cell line with 1B11 and staining it with biotinylated MFT3 and FITC-streptavidin decreased the staining intensity (Figure 3C). Cross-blocking with S7 did not produce any effects on MFT3 staining (Figure 3D). These findings confirmed that MFT3 mAb reacts with the 130-kd glycoform of CD43. Although 1B11 and MFT3 mAb recognize the same glycoform of CD43 and similar epitopes, sialidase treatment of transfected cell lines removed the MFT3 but not the 1B11 epitope from CD43 (Figure 3E and 3F), indicating that the epitopes recognized by these mAbs are not identical. We also observed, using FACScan analysis (data not shown), that sialidase treatment removed the S7 epitope from CD43-transfected cell lines, as was reported previously of EL4 cells.18 These findings suggest that anti-CD43 mAbs MFT3, S7, and 1B11, as discussed by Jones et al,18 recognized the epitope on the carbohydrate structure of CD43.
MFT3 mAb reacted with CD43 gene products.
293 cells were stably transfected with CD43 complementary DNA (cDNA), stained with MFT3 (A) or S7 (B) mAb followed by FITC-MAR18.5, and analyzed on a FACScan. The shaded histogram shows results from staining with the first mAb; and the open histogram, results from staining without the first mAb. CD43 transfectant 293 cell lines were pretreated with 1B11 (C) or S7 (D), washed, stained with biotinylated MFT3 mAb followed by FITC-streptavidin, and analyzed on a FACScan. The shaded histogram shows results after pretreatment with mAbs; and the open histogram, results with no treatment. CD43 transfectant 293 cell lines were treated with 100 mU/ml neuraminidase, washed, and stained with MFT3 (E) or 1B11 (F) followed by FITC-MAR18.5. The shaded histogram show results after neuraminidase treatment; and the open histogram, results without neuraminidase treatment.
MFT3 mAb reacted with CD43 gene products.
293 cells were stably transfected with CD43 complementary DNA (cDNA), stained with MFT3 (A) or S7 (B) mAb followed by FITC-MAR18.5, and analyzed on a FACScan. The shaded histogram shows results from staining with the first mAb; and the open histogram, results from staining without the first mAb. CD43 transfectant 293 cell lines were pretreated with 1B11 (C) or S7 (D), washed, stained with biotinylated MFT3 mAb followed by FITC-streptavidin, and analyzed on a FACScan. The shaded histogram shows results after pretreatment with mAbs; and the open histogram, results with no treatment. CD43 transfectant 293 cell lines were treated with 100 mU/ml neuraminidase, washed, and stained with MFT3 (E) or 1B11 (F) followed by FITC-MAR18.5. The shaded histogram show results after neuraminidase treatment; and the open histogram, results without neuraminidase treatment.
C2GnT expression in T-cell clones and fibroblastic cell lines
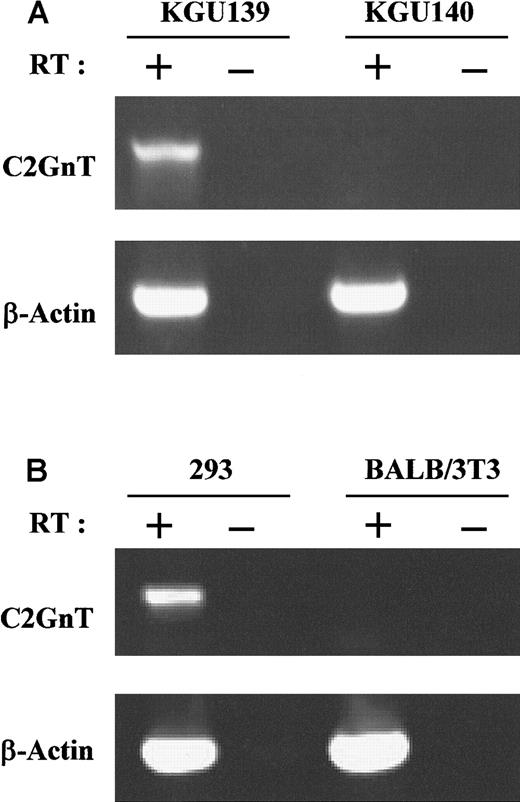
It was previously reported that expression of the 130-kd CD43 glycoform depends on C2GnT activity.21 We used RT-PCR to examine expression of C2GnT transcripts in T-cell clones positive (KGU139) and negative (KGU140) for the 130-kd glycoform. As shown in Figure 4A, KGU139 but not KGU140 T-cell clones expressed C2GnT transcripts. As the internal control, RT-PCR products of β-actin from the same transcripts showed signals almost identical to those of KGU139 and KGU140. This finding suggests that the absence of expression of the 130-kd glycoform is due to the lack of C2GnT activity in KGU140 T-cell clones. The enzymatic activities of C2GnT in KGU139 cells might result in formation of core 2 O-glycans, thus converting the molecular mass from a 115-kd to a 130-kd glycoform of CD43.16 21
C2GnT transcripts were detected in MFT3-positive but not MFT3-negative cells on analysis with RT-PCR.
(A) Total RNA from MFT3 antigen–positive (KGU139) and MFT3 antigen–negative (KGU140) T-cell clones was subjected to RT and amplified by PCR using C2GnT-specific primers or β-actin primers. (B) Total RNA from 293 cells and BALB/3T3 cells that were used as host cells in transfection experiments was subjected to RT-PCR analysis using the primers as in Figure 4A. PCR products were electrophoresed in agarose gels and stained with ethidium bromide. RT (+) indicates RNA reverse transcribed; and RT (−), RNA not reverse transcribed.
C2GnT transcripts were detected in MFT3-positive but not MFT3-negative cells on analysis with RT-PCR.
(A) Total RNA from MFT3 antigen–positive (KGU139) and MFT3 antigen–negative (KGU140) T-cell clones was subjected to RT and amplified by PCR using C2GnT-specific primers or β-actin primers. (B) Total RNA from 293 cells and BALB/3T3 cells that were used as host cells in transfection experiments was subjected to RT-PCR analysis using the primers as in Figure 4A. PCR products were electrophoresed in agarose gels and stained with ethidium bromide. RT (+) indicates RNA reverse transcribed; and RT (−), RNA not reverse transcribed.
We also examined expression of C2GnT in 293 cells and BALB/3T3 cells that were used as host cells in transfection experiments. We considered that because 293 cells expressed the 130-kd glycoform of CD43 after transfection with CD43 cDNA alone, they should be positive for C2GnT activity. RT-PCR analysis (Figure 4B) confirmed this idea. Although 293 cells are of human origin, the primers designed for mouse CD43 worked as well for human CD43. On the other hand, the BALB/3T3 cells used in transfection experiments did not express C2GnT transcripts.
Homocytic aggregation of T-cell clones positive and negative for the 130-kd glycoform
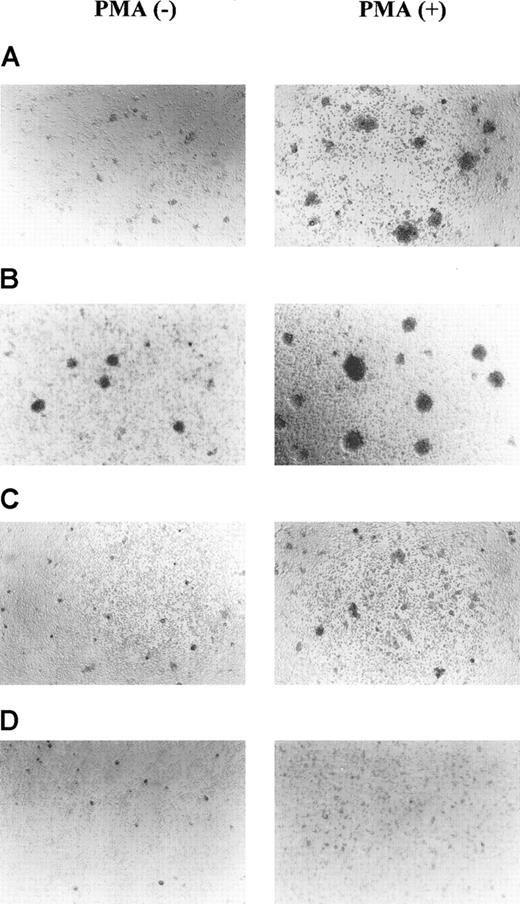
Functional differences in T-cell clones positive and negative for the 130-kd CD43 glycoform were examined using homocytic aggregation assays. T-cell clones positive (KGU139 and KGX114) and negative (KGU140 and KSB2) for the 130-kd glycoform were cultured with or without PMA for 16 hours, and the degree of homocytic aggregation was examined. As shown in Figure 5, KGU140 and KSB2 cells showed increased homocytic aggregation, with large cell clusters, compared with KGU139 and KGX114 cells in both nonstimulated and PMA-stimulated cultures. The differences in adhesive activity were not due to the differential expression of adhesion molecules on each T-cell clone, since the clones expressed similar amounts of adhesion molecules in analyses using various mAbs (Table 1).
MFT3-negative T-cell clones showed increased homocytic aggregation compared with MFT3-positive T-cell clones.
MFT3-negative T-cell clones KGU140 (A) and KSB2 (B) and MFT3-positive T-cell clones KGU139 (C) and KGX114 (D) were cultured in the presence or absence of 10 ng/mL phorbol 12-myristate 13-acetate for 16 hours and observed using inverted microscopy (magnification, ×100).
MFT3-negative T-cell clones showed increased homocytic aggregation compared with MFT3-positive T-cell clones.
MFT3-negative T-cell clones KGU140 (A) and KSB2 (B) and MFT3-positive T-cell clones KGU139 (C) and KGX114 (D) were cultured in the presence or absence of 10 ng/mL phorbol 12-myristate 13-acetate for 16 hours and observed using inverted microscopy (magnification, ×100).
Expression of adhesion molecules on T-cell clones
| Antigen . | Antibody . | T-cell clone . | |||
|---|---|---|---|---|---|
| KGU139 . | KGX114 . | KGU140 . | KSB2 . | ||
| ICAM-1 | KAT-1 | 435 | 190 | 430 | 187 |
| LFA-1 | FD441.8 | 89 | 77 | 82 | 94 |
| VLA-4 | PS/2 | 9 | 9 | 81 | 13 |
| VLA-6 | GoH3 | 7 | 51 | 19 | 5 |
| VCAM-1 | M/K-2 | 61 | 6 | 8 | 4 |
| CD24 | M1/69 | 10 | 6 | 10 | 4 |
| CD44 | KM201 | 56 | 103 | 246 | 111 |
| Mac-1 | M1/70 | 7 | 6 | 10 | 5 |
| L-selectin | MEL14 | 12 | 8 | 14 | 8 |
| CD43 | MFT3 | 133 | 135 | 19 | 28 |
| CD43 | S7 | 141 | 94 | 106 | 143 |
| Antigen . | Antibody . | T-cell clone . | |||
|---|---|---|---|---|---|
| KGU139 . | KGX114 . | KGU140 . | KSB2 . | ||
| ICAM-1 | KAT-1 | 435 | 190 | 430 | 187 |
| LFA-1 | FD441.8 | 89 | 77 | 82 | 94 |
| VLA-4 | PS/2 | 9 | 9 | 81 | 13 |
| VLA-6 | GoH3 | 7 | 51 | 19 | 5 |
| VCAM-1 | M/K-2 | 61 | 6 | 8 | 4 |
| CD24 | M1/69 | 10 | 6 | 10 | 4 |
| CD44 | KM201 | 56 | 103 | 246 | 111 |
| Mac-1 | M1/70 | 7 | 6 | 10 | 5 |
| L-selectin | MEL14 | 12 | 8 | 14 | 8 |
| CD43 | MFT3 | 133 | 135 | 19 | 28 |
| CD43 | S7 | 141 | 94 | 106 | 143 |
Autoreactive T-cell clones from B/WF1 mice were stained with various monoclonal antibodies against adhesion molecules followed by appropriate second reagents and analyzed on a fluorescence-activated cell-sorter scanner. Values are mean fluorescence intensity (arbitrary units) of each staining.
ICAM-1 indicates intracellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen 1; VLA, very late antigen; and VCAM-1, vascular cell adhesion molecule 1.
Antiadhesive effects of the 130-kd CD43 glycoform in T-cell–B-cell and T-cell–fibroblast interactions
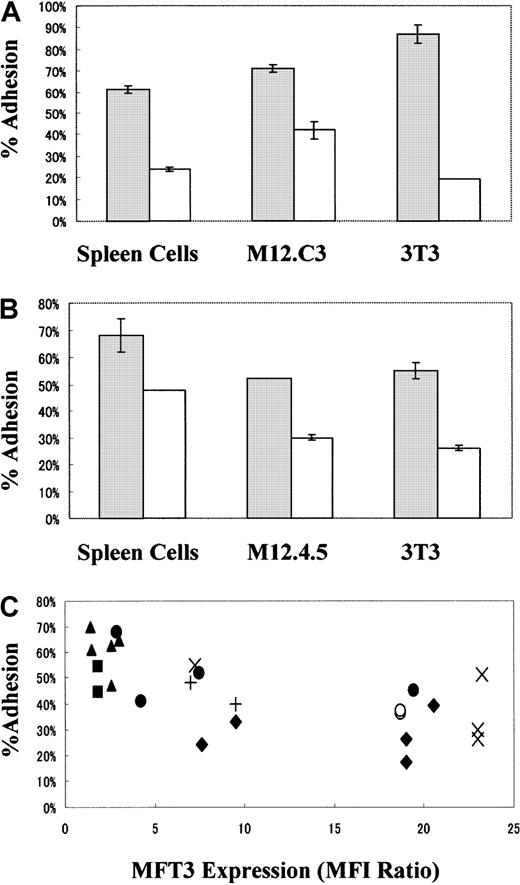
We next examined the effects of expression of the 130-kd CD43 glycoform on T-cell–B-cell and T-cell–fibroblast cellular adhesion using spleen cells, B-lymphoma cell lines (M12.4.5 and M12.C3), and fibroblast cell lines (BALB/3T3) as target cells.51Cr-labeled T-cell clones were added to target-cell monolayers, nonadherent T cells were washed out, and the radioactivity of adhered T-cell clones was determined. As shown in Figure6A, T-cell clones positive for the 130-kd glycoform but not those negative for the 130-kd glycoform showed decreased cell adhesion to spleen cells, B-lymphoma cell lines, and fibroblastic cell lines. Again, this difference was not due to the other adhesion molecules on the T-cell clones (Table 1). The major pathway for T-cell adhesion to spleen cells and B-lymphoma cell lines seemed to be mediated by LFA-1, as determined by mAb blocking experiments. The pathway for T-cell adhesion to fibroblast cell lines could not be determined because none of the mAbs used for blocking studies produced any effects (data not shown).
Antiadhesive function of the 130-kd CD43 glycoform on T cells.
T-cell clones (A) from B/WF1 mice positive for MFT3 antigen (KGU139, ■) and negative for MFT3 antigen (KGU140, ░) and T-cell lines (B) from BALB/c mice positive for MFT3 antigen (SA-3, ■) and negative for MFT3 antigen (line 8-3, ░) were used for adhesion assays. T-cell lines or clones were radiolabeled with chromium 51 (51Cr) and cultured with spleen cells, M12.C3 cells, M12.4.5 cells, or BALB/3T3 (3T3) cells as adhesion target-cell monolayers. After 30 minutes, nonadherent cells were removed and the radioactivity of adherent-cell–associated 51Cr was determined. The percentage of adhesion was calculated as the counts per minute of adhered cells divided by the counts per minute of input cells times 100. (C) Autoreactive T-cell clones KGU139 (♦) and KGU140 (▴) derived from B/WF1 mice and KLH-reactive SA-1 (○), SA-2 (+), SA-3 (×), line 3 (●), line 8-3 (▪) T-cell lines derived from BALB/c mice were stained with MFT3 mAb and analyzed on a FACScan. The T-cell lines and clones were assayed for cell adhesion by using various adhesion target cells at the same time as MFT3 mAb staining. Individual T-cell clones were assayed repeatedly at different times. Results were expressed as the percentage of adhesion compared with MFT3 expression (as a mean fluorescence intensity ratio of MFT3 staining to control staining).
Antiadhesive function of the 130-kd CD43 glycoform on T cells.
T-cell clones (A) from B/WF1 mice positive for MFT3 antigen (KGU139, ■) and negative for MFT3 antigen (KGU140, ░) and T-cell lines (B) from BALB/c mice positive for MFT3 antigen (SA-3, ■) and negative for MFT3 antigen (line 8-3, ░) were used for adhesion assays. T-cell lines or clones were radiolabeled with chromium 51 (51Cr) and cultured with spleen cells, M12.C3 cells, M12.4.5 cells, or BALB/3T3 (3T3) cells as adhesion target-cell monolayers. After 30 minutes, nonadherent cells were removed and the radioactivity of adherent-cell–associated 51Cr was determined. The percentage of adhesion was calculated as the counts per minute of adhered cells divided by the counts per minute of input cells times 100. (C) Autoreactive T-cell clones KGU139 (♦) and KGU140 (▴) derived from B/WF1 mice and KLH-reactive SA-1 (○), SA-2 (+), SA-3 (×), line 3 (●), line 8-3 (▪) T-cell lines derived from BALB/c mice were stained with MFT3 mAb and analyzed on a FACScan. The T-cell lines and clones were assayed for cell adhesion by using various adhesion target cells at the same time as MFT3 mAb staining. Individual T-cell clones were assayed repeatedly at different times. Results were expressed as the percentage of adhesion compared with MFT3 expression (as a mean fluorescence intensity ratio of MFT3 staining to control staining).
The antiadhesive effect of the 130-kd CD43 glycoform was also observed in antigen-specific T-cell lines. Several independent T-cell lines reactive to keyhole-limpet hemocyanin (KLH) were generated from BALB/c mice and maintained without cloning. Expression of the 130-kd glycoform was examined using immunofluorescence staining, and lines positive and negative for that glycoform were selected for adhesion assays. As shown in Figure 6B, lines positive for the 130-kd glycoform showed decreased adhesion to spleen cells, B-lymphoma cell lines, and fibroblast cell lines. The interaction of TCR and major histocompatibility complex class II and antigenic peptides did not seem to be involved in the cellular adhesion or antiadhesion because KLH antigen did not produce any effects (data not shown). These T-cell lines were not subjected to cloning and thus may have contained cell populations that were heterogeneous in terms of adhesion molecule expression and fine TCR specificities; therefore, the difference in adhesion activity between these 2 T-cell lines was attributed primarily to the presence or absence of expression of the 130-kd CD43 glycoform.
To clarify whether there is an inverse relation between the amount of expression of the 130-kd CD43 glycoform and cellular adhesion activity, we tested a battery of independent T-cell lines and clones for adhesion. The amount of 130-kd glycoform expression was examined at the time of the adhesion assays and expressed as a ratio of the MFI on staining with MFT3 mAb to that on staining without MFT3 mAb. In spite of the inevitable daily fluctuations in scores, we found a rough inverse relation between the amounts of expression of the 130-kd glycoform and cellular adhesion activity (Figure 6C). The same tendency was also observed for individual T-cell clones assayed on different occasions.
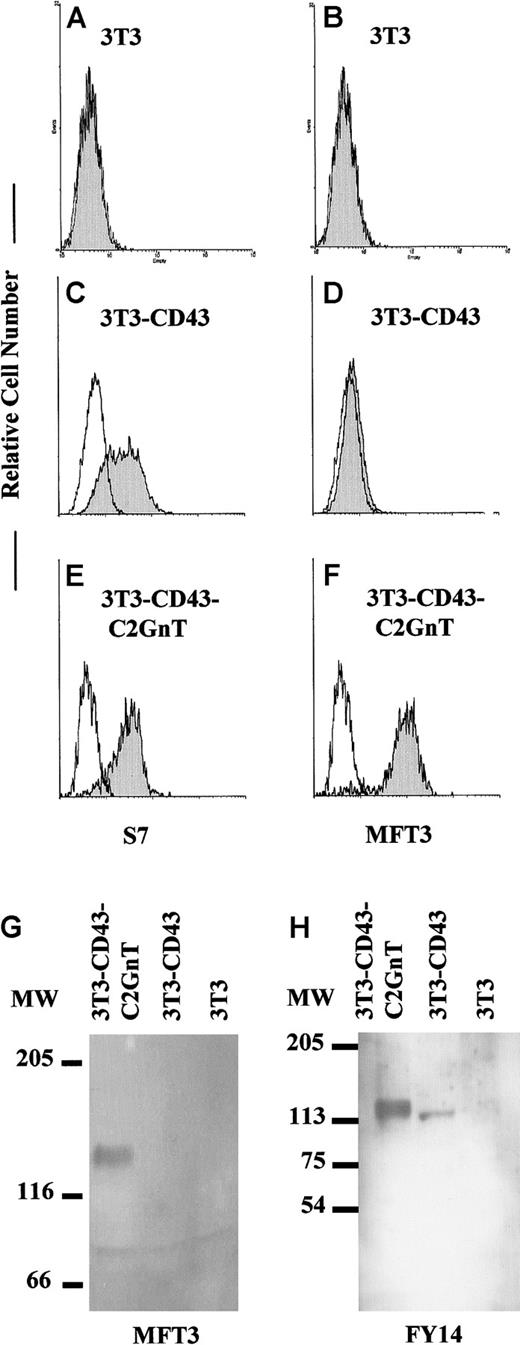
Antiadhesive function is carried out primarily by the 130-kd and not the 115-kd glycoform of CD43
Because our findings indicated an antiadhesive function of the 130-kd CD43 glycoform, we directly compared the antiadhesive functions of the 130-kd and the 115-kd glycoforms. As shown in Figure7A-B, BALB/3T3 fibroblastic cell lines expressed neither the 115-kd nor the 130-kd glycoform of CD43 as assessed by using S7 and MFT3 mAb. Transfection of CD43 cDNA into BALB/3T3 cell lines induced expression of the 115-kd but not the 130-kd glycoform of CD43 (Figure 7C-D). The inability of BALB/3T3-CD43 transfectant cell lines to express the 130-kd glycoform could have been due to the lack of C2GnT expression, since no transcript was detected by RT-PCR analysis (Figure 4B). Cotransfection of C2GnT and CD43 cDNA into BALB/3T3 cell lines induced expression of both the 115-kd and the 130-kd glycoform of CD43 (Figure 7E-F). Western blotting using MFT3 mAb showed that the CD43 protein in cell lines dual transfected with CD43 and C2GnT was 130 kd (Figure 7G). Using cross-blocking screening with MFT3 mAb, we generated another anti-CD43 mAb (FY14) with specificity against both the 115-kd and the 130-kd CD43 glycoform. Western blotting using FY14 mAb confirmed the shift of protein size in a single gel from 115 kd in CD43-transfected cell lines to 130 kd in cell lines dual transfected with CD43 and C2GnT (Figure 7H).
Induction by C2GnT of expression of the 130-kd CD43 glycoform in CD43 transfectant cells.
BALB/3T3 (3T3) fibroblastic cell lines (A,B) were transfected with CD43 cDNA (C,D) or cotransfected with CD43 and C2GnT cDNA (E,F). Stable transfectant cell lines were stained with S7 or MFT3 mAb followed by FITC-MAR18.5 and analyzed on a FACScan. The shaded histogram shows results from staining with the first mAb; and the open histogram, results from staining without the first mAb. Lysate from transfectant cell lines was subjected to SDS-PAGE (6% polyacrylamide gels), and Western blotting was performed with MFT3 mAb or FY14 mAb (IgG2a). The protein signals were detected by alkaline phosphatase–conjugated goat antirat IgG followed by 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium reagent (G) or by peroxidase-conjugated goat antirat immunoglobulin followed by enhanced chemiluminescence reagent (H).
Induction by C2GnT of expression of the 130-kd CD43 glycoform in CD43 transfectant cells.
BALB/3T3 (3T3) fibroblastic cell lines (A,B) were transfected with CD43 cDNA (C,D) or cotransfected with CD43 and C2GnT cDNA (E,F). Stable transfectant cell lines were stained with S7 or MFT3 mAb followed by FITC-MAR18.5 and analyzed on a FACScan. The shaded histogram shows results from staining with the first mAb; and the open histogram, results from staining without the first mAb. Lysate from transfectant cell lines was subjected to SDS-PAGE (6% polyacrylamide gels), and Western blotting was performed with MFT3 mAb or FY14 mAb (IgG2a). The protein signals were detected by alkaline phosphatase–conjugated goat antirat IgG followed by 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium reagent (G) or by peroxidase-conjugated goat antirat immunoglobulin followed by enhanced chemiluminescence reagent (H).
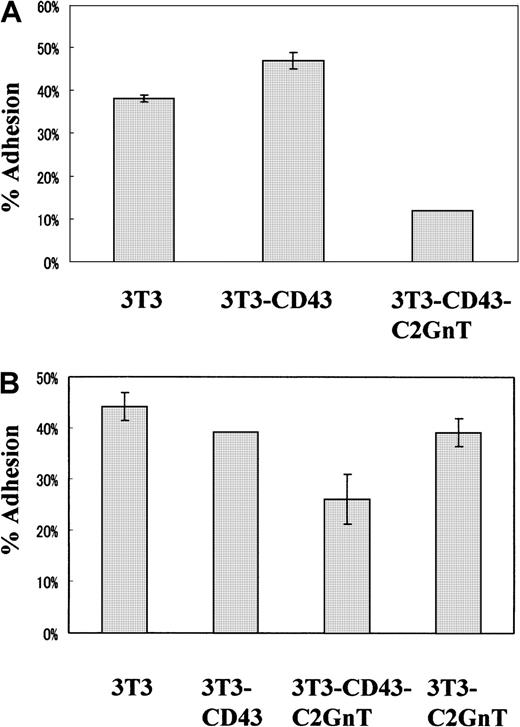
Using these cell lines, we compared the antiadhesive function of the 2 different CD43 glycoforms. 51Cr-labeled M12.C3 B-lymphoma cell lines were added to monolayers of various BALB/3T3 transfectant cell lines. Adhesion assays were performed for 30 minutes, and the adherent-cell–associated radioactivity was measured. As shown in Figure 8A, expression of the 115-kd glycoform in BALB/3T3-CD43 transfectant cell lines did not show antiadhesion to M12.C3 cells. However, expression of the 130-kd glycoform in this transfectant cell line clearly induced antiadhesion to M12.C3 B-lymphoma cell lines. Expression of C2GnT alone in BALB/3T3 cells induced weak antiadhesion effects (Figure 8B) that were probably due to the increased anionic charges imparted by an increase in O-glycans on cell-surface molecules other than CD43, as was suggested previously.22 These findings indicate that the antiadhesive function is carried out primarily by the 130-kd and not the 115-kd glycoform of CD43.
Antiadhesive function was carried out by the 130-kd glycoform of CD43 but not the 115-kd glycoform.
(A,B) BALB/3T3 cell lines stably transfected with no CD43 (3T3), CD43 (3T3-CD43), CD43 plus C2GnT (3T3-CD43-C2GnT), or C2GnT (3T3-C2GnT) cDNA were cultured in 24-well plates overnight and used for adhesion target cells. 51Cr-labeled M12.C3 B-lymphoma cells were added and cultured for 30 minutes, nonadherent cells were removed, and the radioactivity of adherent-cell–associated 51Cr was determined. The percentage of adhesion was calculated as the counts per minute of adhered cells divided by the counts per minute of input cells times 100. Two representative experiments (A,B) are shown.
Antiadhesive function was carried out by the 130-kd glycoform of CD43 but not the 115-kd glycoform.
(A,B) BALB/3T3 cell lines stably transfected with no CD43 (3T3), CD43 (3T3-CD43), CD43 plus C2GnT (3T3-CD43-C2GnT), or C2GnT (3T3-C2GnT) cDNA were cultured in 24-well plates overnight and used for adhesion target cells. 51Cr-labeled M12.C3 B-lymphoma cells were added and cultured for 30 minutes, nonadherent cells were removed, and the radioactivity of adherent-cell–associated 51Cr was determined. The percentage of adhesion was calculated as the counts per minute of adhered cells divided by the counts per minute of input cells times 100. Two representative experiments (A,B) are shown.
Discussion
We generated a mAb, MFT3, that specifically recognizes the 130-kd glycoform of murine CD43. The antigen recognized by MFT3 was similar but not identical to that recognized by the previously described mAb 1B11.18 Using MFT3 mAb, we showed that nonhelper T-cell clones but not helper T-cell clones23 express the 130-kd CD43 glycoform. We also showed that this glycoform functioned as an antiadhesion molecule in cellular interactions between T and B cells as well as interactions between B-lymphoma cell lines and transfectant fibroblastic cell lines. These findings may indicate that the nonpathogenicity and pathogenicity of autoreactive T-cell clones derived from B/WF1 mice in adoptive cell transfer is, as observed previously,23 determined by the presence or absence of expression of the 130-kd CD43 glycoform. T-cell clones that express the 130-kd CD43 glycoform may not be fully activated by poor interactions with antigen presenting cells (APCs) in vivo because of the antiadhesive function.
Neither the reason why we obtained 130-kd-glycoform–positive and 130-kd-glycoform–negative T-cell clones from the same T-cell lines nor the reason why some T-cell lines are rich in 130-kd–positive T cells and others in 130-kd–negative T cells is clear. The procedure followed for establishing the T-cell lines and the clones was essentially the same in these studies. The phenotype of CD43 glycoform expression is relatively stable in long-term culture, but it sometimes changes from 130-kd positive to 130-kd negative (or vice versa) for unknown reasons. In future studies, it will be important to examine the factor or factors that induce expression or suppression of the 130-kd CD43 glycoform in cultured cells.
A variety of functions have been reported to be associated with CD43, including cell migration,10,14,15 thymocyte maturation,31 signal transduction,32-34accessory molecules in T cell activation,35 and inhibition of TCR-CD3–mediated apoptosis.11 Conflicting findings regarding antiadhesion6-11 and proadhesion12-15in cell-to-cell interactions were reported for CD43. One of the reasons for these discrepancies might be the experimental system used. Ardman et al6 reported that expression of CD43 on HeLa cells resulted in inhibition of T-cell adhesion. Gene targeting of CD43 enhanced T-cell adhesion and proliferation7,8 and increased the homing efficiency of lymphocytes to the secondary lymphoid organs.10 Transgenic expression of CD43 on B cells resulted in immunodeficiency in mice that was probably due to the induced antiadhesion of splenic B cells.9 On the other hand, Park et al12 reported that expression of CD43 on T-cell lines resulted in increased adhesion to APCs, suggesting a proadhesive function of CD43. Indeed, the ligand for CD43 was reported to be ICAM-1 in studies in Daudi cells.13 Antibody against CD43 inhibits adhesion of monocytes to endothelial cells15and T-lymphocyte homing.14
The conflicting findings regarding antiadhesion and proadhesion of CD43 might also be explained by the idea proposed by Ostberg et al,36 who speculated that the default function of CD43 is antiadhesion because of its tall, rodlike structure and strong negative charge from abundant O-glycans. Proadhesion of CD43 could be mediated by the association of a specific receptor or receptors as well as by activation of other adhesion molecules or redistribution of CD43 initiated by the signal through CD43 and its receptors.37 38 The final outcome with respect to antiadhesion or proadhesion under various experimental conditions and physiologic and pathologic situations may be the result of a shift in balance between the antiadhesion and proadhesion activities of CD43.
These possibilities, however, do not take into account the possible differences in the role of the 115-kd and 130-kd glycoforms of CD43 in cell adhesion. We found that it is the 130-kd glycoform of CD43 and not the 115-kd glycoform that plays a major role in antiadhesion in cellular interactions. This is consistent with the findings of Tsuboi and Fukuda,22 who reported that C2GnT transgenic mice, in which almost all the lymphocytes express the 130-kd CD43 glycoform, showed reduced T-cell immune responses caused by a decrease in adhesion of lymphocytes. A straightforward interpretation of this finding would be that increased anionic charges due to the attached O-glycans on the 130-kd glycoform exert stronger antiadhesive effects. An alternative but not mutually exclusive interpretation is that the attached O-glycans inhibit efficient interactions of CD43 with its ligand that would otherwise induce activation of proadhesive functions. In any case, the balance between antiadhesion and proadhesion of CD43 glycoforms would play an important role in cellular interactions in various physiologic and pathologic situations. In this context, it should be noted that the 130-kd CD43 glycoform is up-regulated in CD8 effector cytotoxic T lymphocytes and down-regulated when they convert into memory cells39 and that effector CD8 T cells rich in the 130-kd CD43 glycoform are susceptible to apoptotic cell death.40 It is possible that the inability of T-cell clones positive for the 130-kd CD43 glycoform to exert helper functions in our adoptive cell-transfer experiments was due to immunologic incompetence caused by apoptotic cell death, although regulation of CD43 glycoform expression might be different in CD4 and CD8 T lymphocytes.41
In patients with the immunodeficiency associated with Wiskott-Aldrich syndrome (WAS), expression of the 130-kd glycoform of CD43 was shown to be increased with enhanced activity of C2GnT.42-44 It is possible that the mutation in the WAS gene45 could directly or indirectly induce constitutive activation of C2GnT that results in increased expression of core 2 O-glycans in T cells. These T cells might have inefficient interactions with APCs that result in immunodeficiency in patients with WAS. It will be of interest to examine the expression of the 130-kd glycoform of CD43 in patients with other immunodeficiency and autoimmune diseases.
Acknowledgments
We thank Dr Tao Sai and Dr S. Ohta for helpful discussions, Ms N. Yamaguchi for technical assistance, and Ms S. Baba for preparing the manuscript.
Supported in part by a grant-in-aid (10670307) from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masao Kimoto, Department of Immunology, Saga Medical School, Nabeshima, Saga 849-8501, Japan; e-mail:kimoto@post.saga-med.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal