Abstract
Transgenic mouse lines were created that express FcγRIIA on platelets and macrophages at human physiologic levels, and they were used to explore the consequences in vivo of activating antiplatelet antibodies. Anti-CD9 antibody activated platelets of FcγRIIA transgenic (tg) mice and, following injection in vivo, caused more rapid severe thrombocytopenia than nonactivating antiplatelet antibody. Anti-CD9 injected into FcγRIIA tg crossed with FcR γ-chain knockout (γ-KO) mice caused thrombosis and shock in all mice, and death in 16 of 18 mice. The shock depended on platelet Fc receptor density and antibody dose. On histologic examination, the lung vasculature of anti-CD9–treated FcγRIIA tg × γ-KO mice contained extensive platelet-fibrin thrombi. Thrombosis and shock in FcγRIIA tg mice in the context of the FcR γ-chain knockout suggested the importance of the interplay of intravascular platelet activation and splenic clearance. Reduction of splenic clearance surgically (splenectomy) or functionally (monoclonal antibody treatment) also facilitated anti-CD9–mediated shock in FcγRIIA tg mice. The spleen, which clears nonactivating antibody-coated platelets leading to thrombocytopenia, appears to play a protective role in the thrombosis and shock observed with activating antiplatelet antibody. The data indicate that antibodies, which activate platelets in an FcγRIIA-dependent manner, can lead to thrombosis, shock, and death. Furthermore, antibody titer, platelet Fc receptor density, and splenic clearance are likely important determinants of the outcome.
Introduction
The immune-mediated thrombocytopenias are an important group of clinical disorders that occur at any age. Immune-mediated thrombocytopenia is caused by antibodies directed against platelet surface glycoproteins, antibodies against drug-containing complexes on the platelet surface, or by antibody-coated cells or immune complexes that interact with the platelet surface. Immune-mediated platelet clearance has often served as a model system for exploring a wide range of autoimmune disorders.
A number of antiplatelet antibodies has been demonstrated to activate platelets in a manner dependent on the functional expression of the human platelet Fc receptor for immunoglobulin G (IgG), FcγRIIA.1-7 The clinical consequences of antibody-induced platelet activation in vivo can be profound. For example, the antibodies in heparin-induced thrombocytopenia, which are directed against the heparin-PF4 complex, can activate human platelets in vitro via FcγRIIA and can be associated with fatal thrombosis in vivo.8-13 Platelets also release potent mediators of vascular function and inflammation following activation via FcγRIIA. To dissect the consequences and critical determinants of antibody-induced platelet activation in vivo, we have taken a genetic approach, using transgenic and knockout mice.14
In humans, FcγRIIA is expressed on platelets, neutrophils, monocytes, and macrophages and activates these cells following the binding of IgG-coated cells or IgG-containing immune complexes.15,16Mice lack the genetic equivalent of human FcγRIIA and, in fact, do not express a platelet Fcγ receptor. Instead, the Fcγ receptors present in wild-type mouse strains that have been studied in immune-mediated thrombocytopenia are FcγRI and FcγRIII present on spleen macrophages. Absence of the functional expression of both FcγRI and FcγRIII, which is produced when their accessory FcR γ-chain is knocked out, leads to no detectable thrombocytopenia in mice when antiplatelet antibodies are injected.14,17 We previously generated and characterized human FcγRIIA transgenic (tg) mice in which FcγRIIA was expressed on mouse platelets and macrophages at levels equivalent to that in human cells.14We demonstrated a critical role for FcγRIIA expression in antiplatelet antibody-induced thrombocytopenia in vivo. However, the antiplatelet antibody 4A5 used in those studies was not platelet activating.
We have performed a series of experiments with an antiplatelet antibody against mouse CD9, because higher density antigens such as GPIIb/IIIa and CD9 are more often associated with FcR-dependent activating antiplatelet antibodies.2,5 Such antibodies are representative of a large group of activating antiplatelet antibodies demonstrated in several types of human immune thrombocytopenia.1-3,5 6 In this report, we demonstrate for the first time in vivo that activating antiplatelet antibodies can induce not only thrombocytopenia but also thrombosis and shock that is worsened by the absence of a functional spleen. By use of defined lines of transgenic and knockout mice, we also identify for the first time 3 independent critical determinants for the occurrence of thrombosis and shock: antibody titer, platelet Fc receptor density, and splenic clearance.
Materials and methods
FcγRIIA tg mice were created, using methods previously described.14 One transgenic line (tg line 11) of mice was used throughout this study because we have previously demonstrated that the receptor density on platelets and macrophages is on the high end of the physiologic range for these cells in humans. In addition, a second mouse line with lower receptor density (tg line 32) was used in selected experiments. In the descriptions that follow, the FcγRIIA tg line being discussed is FcγRIIA tg line 11 unless otherwise specified. The FcR γ-chain knockout (γ-KO) mice were provided generously by Dr Jeffrey Ravetch (Rockefeller University). C57Bl/6 × SJL F1 mice served as wild-type controls (The Jackson Laboratory, Bar Harbor, ME). The FcγRIIA tg line 11 mice were crossed with the FcR γ-chain KO mice and bred to be homozygous for the γ-chain KO gene and hemizygous for the FcγRIIA transgene (FcγRIIA tg × γ-KO). Comparable crossed lines were created for tg line 32. All studies were approved by the Institutional Animal Care and Use Committee of the A. I. duPont Hospital for Children and Thomas Jefferson University.
Rat antimouse CD9 monoclonal antibody (Pharmingen, San Diego, CA; clone KMC8, rat IgG2a) was selected for use in this study for its potential to bind and activate mouse platelets via the FcγRIIA isoform R131 present in our tg mice.14 Multiple independent lots of anti-CD9 were tested, and we observed no difference in outcome based on the lot used. Additional antibodies included a rat monoclonal antibody directed against mouse FcγRIIb/III (2.4G2, rat IgG2b; Pharmingen) and mouse monoclonal antibody to human FcγRIIA (IV.3, mouse IgG2b) isolated from hybridoma supernatant (ATCC, Manassas, VA).
FcγRIIA receptor density on platelets and macrophages
Platelet and macrophage surface receptor density was quantified, using Scatchard analysis, as we previously reported for tg line 11. Platelets were prepared from whole blood from 5 wild-type mice or 5 FcγRIIA tg line 32 mice, isolated using cardiac puncture in 3.8% sodium citrate (1:10 vol/vol) to prevent coagulation. The samples for each type of mouse were pooled, then spun at 100g in a Sorvall RT6000B centrifuge (Kendro Lab Products, Newtown, CT) to isolate the platelet-rich plasma (PRP), counted, and resuspended in 1× Tyrode buffer. 125I-IV.3 binding to tg mouse platelets and human platelet controls was performed to measure FcγRIIA receptor density on the platelet surface as previously reported.14Peritoneal macrophages were induced with thioglycollate, following standard procedures.18125I-IV.3 binding to transgenic mouse macrophages, wild-type mouse macrophages, and human FcγRIIA-positive human erythroleukemia (HEL) cell line control was performed to measure FcγRIIA receptor density as previously reported.19
Platelet aggregation in vitro
Effects of the anti-CD9 antibody were tested in platelet aggregation in vitro, using blood from FcγRIIA tg mice or strain-matched wild-type control mice. The mice were anesthetized with an intraperitoneal (IP) injection of ketamine (80 mg/kg; Abbott Laboratories, N. Chicago, IL) and xylazine (16 mg/kg; Phoenix Pharmaceuticals, St. Joseph, MO). Whole blood from 5 wild-type mice or 5 FcγRIIA tg mice was isolated, using cardiac puncture in 3.8% sodium citrate to prevent coagulation. The samples for each type of mouse were pooled, then spun at 100g in a Sorvall RT6000B centrifuge to isolate the PRP. The remaining plasma was spun at 1900g to create platelet- poor plasma (PPP) as the control. The PRP and PPP (250 μL total volume) were placed in an aggregometer (Chronolog Corporation, Havertown, PA), warmed to 37°C, and when samples had equilibrated varying concentrations of anti-CD9 antibody were added. ADP (Chronolog), a known agonist, was added at a final concentration of 10 μmol/L as a positive control. As a negative control, 10 μg of 4A5, a rat antimouse platelet antibody known not to activate platelets in vitro, was added. The extent of aggregation over time was measured.
IP antibody injection
Effects of anti-CD9 antibody in vivo were tested by injection of anti-CD9 IP into FcγRIIA tg mice, B6SJL F1 wild-type mice, γ-KO mice, and FcγRIIA tg × γ-KO mice. The initial series of experiments used a dose of anti-CD9 of 50 μg; subsequently, it was varied from 25 to 200 μg. Control mice from each line were injected with an equivalent volume of phosphate-buffered saline or with isotype-matched control antibody. Platelet counts were obtained before and at timed intervals after injection of the antiplatelet antibody. Mice were anesthetized, using inhaled isoflurane (Ohmeda PPD, Liberty Corner, NJ). Whole blood (200 μL) was collected into heparinized tubes by puncture of the retro-orbital sinus. Platelet counts were obtained, using a Coulter Z1 counter with a 50-μm aperture tube (Coulter, Miami, FL). Upper and lower threshold values for cell counting were adjusted for mouse platelets according to the manufacturer's instructions. The platelet counts are reported in number/μL.
Intravenous antibody injection
Intravenous (IV) injections of anti-CD9 were given to mice from both FcγRIIA tg × γ-KO mouse lines as well as wild-type controls. The mice were anesthetized by inhaled isoflurane. Anti-CD9 antibody was injected IV into the tail vein of each mouse at a concentration of 50 μg and observed for 30 minutes. In some experiments, anti-CD9 was also injected IV to FcγRIIA tg mice and their wild-type controls that had been pretreated 24 hours earlier with 50 μg antimouse FcγRIIb/III antibody 2.4G2 by IP injection. Finally, in some experiments, anti-CD9 was injected IV to FcγRIIA tg mice that had recovered from surgical splenectomy (see below).
Histologic analysis
Three mice from the FcγRIIA tg × γ-KO mouse line and 3 wild-type controls were killed 15 minutes after IV injection of anti-CD9 for autopsy of brain, lung, liver, heart, spleen, and kidney tissues (Pathology Associates International, Frederick, MD). The tissues selected were processed through paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). H&E-stained sections were then evaluated for histopathologic changes.
Surgical splenectomy
Four FcγRIIA tg mice underwent surgical splenectomy, using aseptic techniques. The animals were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg) given IP. A 10-mm left flank incision was made to visualize and exteriorize the spleen. The splenic vessels were cauterized and cut to remove the spleen intact. The peritoneum and skin were closed separately, using 3-0 monofilament suture. The mice recovered over the next 3 days and then were treated with anti-CD9 or isotype control antibody IV following recovery.
Statistical analysis
Statistical analysis of nadir platelet counts between groups was performed with analysis of variance with P < .05 considered significant.
Results
Activating anti-CD9 antibody in FcγRIIA tg mice results in more rapid severe thrombocytopenia
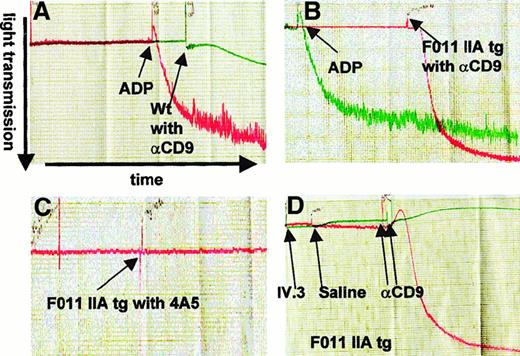
We first established that the anti-CD9 antibody, a rat IgG2a that binds the R131 isoform of human FcγRIIA expressed by our tg mice, activates platelets in an FcR-dependent manner. We observed that 40 μg/mL of anti-CD9 antibody activated the tg mouse platelets from the FcγRIIA tg mice in an in vitro aggregation assay (Figure1B). In contrast, the anti-CD9 antibody at the same concentration was unable to activate platelets of wild-type mice (Figure 1A). Anti-CD9–mediated activation of tg mouse platelets was blocked by preincubation of the platelets with IV.3 monoclonal antibody (20 μg/mL) that blocks FcγRIIA (Figure 1D). Both wild-type and tg mouse platelets did aggregate in response to treatment with ADP (10 μmol/L final concentration), a known agonist that served as a positive control. Neither platelets from wild-type nor those from FcγRIIA tg mice were activated by 40 μg/mL 4A5 antibody (Figure1C), in agreement with prior observations with this nonactivating antibody. In subsequent experiments, we observed activation of FcγRIIA tg mouse platelets at concentrations of anti-CD9 as low as 1.3 μg/mL, indicating that this antibody is a potent platelet activator.
Anti-CD9 antibody activates FcγRIIA tg mouse platelets in an FcγRIIA-dependent manner.
Tracings from in vitro platelet aggregometry assays are shown. Platelet aggregation is measured as light transmission over time. (A) Platelet-rich plasma (PRP) from wild-type mice treated with either anti-CD9 antibody (αCD9) at a final concentration of 40 μg/mL, in green, or treated with ADP (positive control, red) at a final concentration of 10 μmol/L shows no activation by anti-CD9. (B) PRP from FcγRIIA tg mice (F0 11) treated with 40 μg/mL αCD9, in red, or 10 μmol/L final concentration ADP (green), shows activation by anti-CD9. (C) PRP from FcγRIIA tg mice treated with 40 μg/mL antiplatelet antibody 4A5, in red, shows no activation. (D) PRP from FcγRIIA tg mice treated with 20 μg/mL αCD9 following pretreatment with 20 μg/mL IV.3, an anti-FcγRIIA antibody (green), or saline (red), shows blockade of activation by IV.3 but not saline.
Anti-CD9 antibody activates FcγRIIA tg mouse platelets in an FcγRIIA-dependent manner.
Tracings from in vitro platelet aggregometry assays are shown. Platelet aggregation is measured as light transmission over time. (A) Platelet-rich plasma (PRP) from wild-type mice treated with either anti-CD9 antibody (αCD9) at a final concentration of 40 μg/mL, in green, or treated with ADP (positive control, red) at a final concentration of 10 μmol/L shows no activation by anti-CD9. (B) PRP from FcγRIIA tg mice (F0 11) treated with 40 μg/mL αCD9, in red, or 10 μmol/L final concentration ADP (green), shows activation by anti-CD9. (C) PRP from FcγRIIA tg mice treated with 40 μg/mL antiplatelet antibody 4A5, in red, shows no activation. (D) PRP from FcγRIIA tg mice treated with 20 μg/mL αCD9 following pretreatment with 20 μg/mL IV.3, an anti-FcγRIIA antibody (green), or saline (red), shows blockade of activation by IV.3 but not saline.
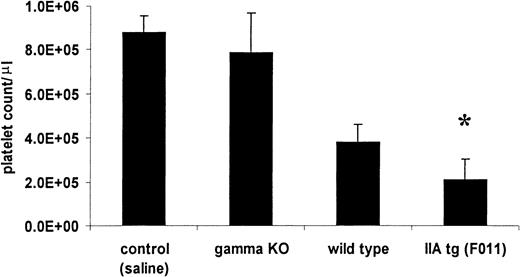
We then tested the response to the injection of anti-CD9 antibody in vivo. We injected 50 μg IP into wild-type mice and FcγRIIA tg mice. All mice became thrombocytopenic within 24 hours. The wild-type mice experienced moderate thrombocytopenia, reaching a nadir count of 0.38 ± 0.08 × 106, a 60% decrease. The FcγRIIA tg mice experienced a more profound thrombocytopenia, reaching a nadir count of 0.21 ± 0.09 × 106, a 78% drop (n = 6 per group; P < .05) (Figure2). In comparison with the injection of 75 μg of 4A5, the nonactivating antiplatelet antibody, injection of 50 μg of anti-CD9 into FcγRIIA tg mice led to a platelet nadir that was more rapid (24 hours for anti-CD9 versus 72 hours for 4A5) and as severe (average 0.21 × 106 versus 0.19 × 106 platelets/μL, respectively) (Figure3).
FcγRIIA tg mice experience more severe anti-CD9–mediated thrombocytopenia.
The bar graph depicts average nadir counts (± SD) from the FcγRIIA tg mice, strain-matched wild-type controls, and FcR γ-KO mice all treated with 50 μg rat antimouse CD9 antibody by IP injection. Control counts represent the combined nadir counts from all lines given identical injections with an equal volume of sterile saline. The FcγRIIA tg mouse nadir counts were significantly different from control, γ-KO, and wild-type mouse nadir counts by analysis of variance (*, P < 0.05).
FcγRIIA tg mice experience more severe anti-CD9–mediated thrombocytopenia.
The bar graph depicts average nadir counts (± SD) from the FcγRIIA tg mice, strain-matched wild-type controls, and FcR γ-KO mice all treated with 50 μg rat antimouse CD9 antibody by IP injection. Control counts represent the combined nadir counts from all lines given identical injections with an equal volume of sterile saline. The FcγRIIA tg mouse nadir counts were significantly different from control, γ-KO, and wild-type mouse nadir counts by analysis of variance (*, P < 0.05).
Activating antiplatelet antibody anti-CD9 causes more rapid thrombocytopenia than nonactivating antiplatelet antibody 4A5.
Average platelet counts (± SD) over time of FcγRIIA tg mice following injection of 50 μg anti-CD9 (▪) or 75 μg 4A5 (▴).
Activating antiplatelet antibody anti-CD9 causes more rapid thrombocytopenia than nonactivating antiplatelet antibody 4A5.
Average platelet counts (± SD) over time of FcγRIIA tg mice following injection of 50 μg anti-CD9 (▪) or 75 μg 4A5 (▴).
Activating anti-CD9 antibody in FcγRIIA tg × γ-KO mice causes thrombosis and shock
In prior studies, we demonstrated that injection of the nonactivating antibody 4A5 caused no evident thrombocytopenia in mice in which the FcR γ-chain was knocked out (γ-KO), but severe thrombocytopenia in FcγRIIA tg × γ-KO mice. This finding is consistent with a major role in vivo for FcγRIIA by itself in immune-mediated thrombocytopenia. We used these FcγRIIA tg × γ-KO mice to investigate their response to anti-CD9. We injected 50 μg IP, as we had done with wild-type and FcγRIIA line 11 tg mice. We first observed that γ-KO mice showed no change in baseline platelet count or in physical well-being when injected with anti-CD9 antibody. This result clearly indicates that binding of the antibody to CD9 in the absence of Fcγ receptors causes neither thrombocytopenia nor cellular activation. To our surprise, 5 of the 6 initially treated FcγRIIA tg × γ-KO transgenic mice died within 20 hours after IP injection of anti-CD9, a result not seen in control mice (Table 1).
Difference in degree of thrombocytopenia and presence of shock phenotype following treatment with 50 μg anti-CD9 antibody in different defined mouse lines
| Mouse line . | Degree of thrombocytopenia* . | Shock phenotype . |
|---|---|---|
| Wild type | Moderate | No |
| IIA tg | Severe | No |
| γ-KO | None | No |
| IIA tg × γ-KO | Severe | Yes (fatal 16/18) |
| IIA tg × γ-KO, lower dose antibody | Moderate | No |
| IIA tg low expressors (± γ-KO) | Moderate | No |
| IIA tg after 2.4G2 spleen FcγR blockade | Severe | Yes |
| IIA tg after splenectomy | ND† | Yes |
| Mouse line . | Degree of thrombocytopenia* . | Shock phenotype . |
|---|---|---|
| Wild type | Moderate | No |
| IIA tg | Severe | No |
| γ-KO | None | No |
| IIA tg × γ-KO | Severe | Yes (fatal 16/18) |
| IIA tg × γ-KO, lower dose antibody | Moderate | No |
| IIA tg low expressors (± γ-KO) | Moderate | No |
| IIA tg after 2.4G2 spleen FcγR blockade | Severe | Yes |
| IIA tg after splenectomy | ND† | Yes |
tg indicates transgenic; KO, knockout.
Moderate thrombocytopenia is defined as a platelet count between 0.3 and 0.6 × 106/μl, whereas severe thrombocytopenia is defined as less than 0.3 × 106/μl.
ND, not determined, as mice died of shock before platelet count could be obtained.
To investigate the mechanism of this reaction further, we performed IV injections of 50 μg anti-CD9 antibody into the FcγRIIA tg × γ-KO tg mice. All these mice experienced a dramatic phenotype, shock characterized by rapid shallow breathing, pale extremities and ears, marked tactile hypothermia, a hunched posture, and decreased locomotor activity. Five of the 6 injected mice died within 30 minutes. Control FcγRIIA tg × γ-KO mice injected at the same time with sterile saline solution were well. Furthermore, wild-type mice injected IV at that same time with 50 μg of the same lot of anti-CD9 antibody were also unaffected with respect to shock.
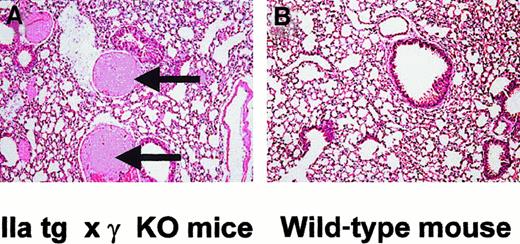
We performed histologic analysis of treated mice to identify what tissues were affected and responsible for the phenotype. Three FcγRIIA tg × γ-KO and 3 wild-type mice were killed within 30 minutes of the IV antibody injection. The FcγRIIA tg × γ-KO mice were killed while in shock but before death. Platelet counts at the time of sacrifice were on average 0.1 × 106/μL in FcγRIIA tg × γ-KO mice. The FcγRIIA tg × γ-KO mice were found to have thrombosis with cellular and fibrin aggregates deposited in the lung vasculature after treatment with the activating antibody (Figure 4). The pulmonary arterial and capillary circulation was involved. There was no intracranial or intestinal hemorrhage evident, and no other organs examined (brain, kidneys, liver, spleen, heart) were affected. In contrast, antibody-treated wild-type mice had normal lungs and lung vasculature, as well as normal histology of the other organs (Figure 4). The histologic findings were consistent throughout the vasculature of the entire lung and for each mouse of a given group.
FcγRIIA tg × γ-KO mice show abnormal lung pathology when compared with wild-type controls.
Lung sections (H&E, 100 ×) of FcγRIIA tg × γ-KO mice show intravascular fibrin precipitation and thrombus formation on the arterial side of the pulmonary circulation (A, arrows), whereas wild-type mouse lung sections show no thrombus formation (B).
FcγRIIA tg × γ-KO mice show abnormal lung pathology when compared with wild-type controls.
Lung sections (H&E, 100 ×) of FcγRIIA tg × γ-KO mice show intravascular fibrin precipitation and thrombus formation on the arterial side of the pulmonary circulation (A, arrows), whereas wild-type mouse lung sections show no thrombus formation (B).
Because the shock phenotype and thrombosis were observed initially in FcγRIIA tg × γ-KO mice, but not in γ-KO or in FcγRIIA tg mice, the genetic knockout of endogenous mouse spleen macrophage receptors FcγRI and FcγRIII likely resulted in an altered balance of splenic clearance and intravascular platelet activation mediated by platelet FcγRIIA. One interpretation of these data is that, when splenic clearance is robust, the balance favors removal of the antibody-coated platelets before significant amounts of FcγRIIA-mediated intravascular activation by platelet activating antibody occurs. Conversely, when splenic clearance is less robust, the intravascular platelet activation by platelet activating antibody proceeds with the highly detrimental consequences of shock and thrombosis. We proceeded to investigate the determinants of thrombosis and shock, using additional transgenic mouse lines with different platelet Fc receptor densities, varied doses of the antibody, and other experimental manipulations to alter splenic clearance.
Determinants of thrombosis and shock
Role of receptor density.
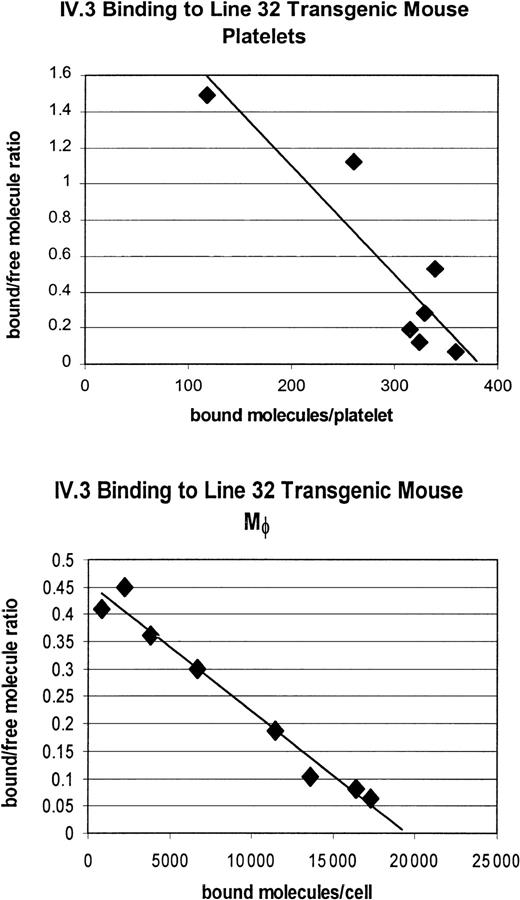
We had previously determined the receptor density on the platelets and macrophages of our line 11 tg mice (1550 receptors/platelet and 65 000 receptors/macrophage) and found it to be at the high end of the physiologic range for human platelets (500-2000 receptors/platelet, as defined by binding of antihuman FcγRIIA antibody IV.3).14,20 We generated a second independent FcγRIIA tg line, line 32, and characterized its receptor expression level, using125I-IV.3 antihuman FcγRIIA binding to quantify receptor density. The density of FcγRIIA receptors on the platelets of these mice were approximately 25% to 30% of the density of the line 11 mice. Specifically, the line 32 FcγRIIA tg mice express 450 receptors/platelet and 18 000 receptors/macrophage (Figure5). For platelets, this receptor density is at the low end of the human physiologic range.20
Scatchard analysis of FcγRIIA on platelets and macrophages of FcγRIIA tg line 32 shows lower receptor density than tg line 11.
Measurement of 125I-labeled IV.3 antibody binding to FcγRIIA on platelets indicates approximately 450 receptors/cell, about 25% to 30% of the receptor density of the FcγRIIA tg line 11 mice. The receptor density on macrophages of FcγRIIA tg line 32 mice is approximately 18 000/cell, about 30% of the receptor density of FcγRIIA tg line 11.
Scatchard analysis of FcγRIIA on platelets and macrophages of FcγRIIA tg line 32 shows lower receptor density than tg line 11.
Measurement of 125I-labeled IV.3 antibody binding to FcγRIIA on platelets indicates approximately 450 receptors/cell, about 25% to 30% of the receptor density of the FcγRIIA tg line 11 mice. The receptor density on macrophages of FcγRIIA tg line 32 mice is approximately 18 000/cell, about 30% of the receptor density of FcγRIIA tg line 11.
We found that 10 μg/mL of anti-CD9 antibody was able to activate the transgenic mouse platelets from both the high (tg line 11) and low (tg line 32) expressing lines in an in vitro aggregation assay. We then varied the concentration of antibody to determine the dose-response relationship for platelet activation. The threshold concentration for the low expressing line 32 was 3.0 μg/mL, below which the platelets were not activated. In contrast, the threshold concentration for the high expressing line 11 was less, 1.3 μg/mL. These results are in agreement with the known dependence in human platelets in vitro on FcγRIIA density for activation by antiplatelet antibodies. Thus, our 2 tg lines provided us with an opportunity to dissect the contribution of receptor density in vivo.
Line 32 FcγRIIA tg mice did not manifest shock or respiratory distress after injection of anti-CD9 at doses of 50 and 75 μg IV, or up to 200 μg IP. For technical reasons, it was not possible to inject more than 75 μg IV using the tail vein. The same observation of a benign to absent systemic reaction was seen for tg line 32 × γ-KO mice. In fact, comparison of line 32 mice with line 32 × γ-KO mice was quite interesting. The line 32 FcγRIIA tg mice experienced platelet counts after IP antibody injection similar to those of wild-type mice, the average nadir count being 0.34 ± 0.11 × 106 platelets/μL). The line 32 FcγRIIA × γ-KO tg mice, by comparison, not only were free of shock but actually experienced a milder thrombocytopenia than FcγRIIA tg line 32 mice on a wild-type background (0.62 ± 0.08 versus 0.34 ± 0.11 × 106 platelets/μL;P < .05). Thus, in the setting of no platelet activation, as in wild-type mice, reduction of splenic clearance by the γ-KO ameliorated the severity of the thrombocytopenia. Line 32 FcγRIIA × γ-KO tg mice were injected IV with the same concentration of antibody and, as with the IP injections, did not experience the shock symptoms. The platelet counts in these mice were similar to those after IP injection, 0.568 ± 0.22 × 106 platelets/μL.
We concluded that the line 32 tg mice received an antibody dose in vivo that did not activate the platelets because of their lower receptor density and thus did not induce the shock phenotype. This was true even in the background of line 32 FcγRIIA tg × γ-KO mice in which splenic clearance was impaired, indicating an important role for platelet activation when the systemic reaction is seen.
Role of antibody titer.
For the FcγRIIA tg line 11, we varied the dose of injected antibody to vary the antibody titer in vivo. When 25 μg of anti-CD9 antibody was injected IV into FcγRIIA tg mice and in FcγRIIA tg × γ-KO mice, no shock or respiratory distress was observed. Thrombocytopenia was induced, although to a lesser degree than with the higher dose of 50 μg IV. These observations in vivo are consistent with the observation of a threshold dose needed for platelet activation in vitro.
Experimental reduction of splenic clearance.
We were interested in understanding if the predominant effect of platelet activation seen in FcγRIIA line 11 tg × γ-KO mice could be recapitulated when the FcR γ-chain (and thus FcγRI and FcγRIII) was present, but splenic clearance was reduced. One approach to alter splenic clearance is to inhibit endogenous mouse macrophage Fcγ receptors.21 22 We injected 2.4G2 directed against mouse spleen macrophage FcγRIIb/III as described in the “Materials and methods” section into FcγRIIA tg mice 24 hours before IV injection of 50 μg anti-CD9. As a control, we performed the same protocol in wild-type mice. For the FcγRIIA tg mice (n = 5) pretreated with 2.4G2, the average nadir platelet count was 0.268 ± 0.141 × 106/μL, whereas for the wild-type mice (n = 6) pretreated with 2.4G2 the average nadir platelet count was 0.633 ± 0.255 × 106/μL 4 hours after IV injection (platelet counts significantly different,P < .05). Once again, all FcγRIIA-expressing mice showed a shock phenotype in the 30 to 60 minutes after anti-CD9 injection, although none died. No shock was observed in similarly treated wild-type mice.
Another approach for altering splenic clearance is surgical splenectomy. Four FcγRIIA tg mice underwent total splenectomy as described in the “Materials and methods” section. After recovery, anti-CD9 antibody was injected at 50 μg IV. All 4 mice injected with anti-CD9 developed the shock phenotype within 30 minutes. One died, and the other 3 recovered. These 3 mice were treated 2 days later with isotype control antibody and experienced no shock or signs of distress. Thus, surgical splenectomy facilitated anti-CD9–mediated shock in FcγRIIA tg mice.
These 3 independent methods of inhibiting splenic clearance in platelet FcγRIIA-expressing tg mice, (1) genetic (γ-KO), (2) functional (antimouse macrophage FcγRIIb/III), and (3) surgical (splenectomy), demonstrate the importance of the balance of splenic clearance and intravascular platelet activation in facilitating thrombosis and shock in vivo (Table 1).
Discussion
Mouse model studies of antibody-induced thrombocytopenia in the past have not included the presence of FcγRIIA on platelets and macrophages, although this receptor is clearly present in humans. We have observed that FcγRIIA is important for the thrombocytopenia induced by nonactivating antiplatelet antibodies.14 We now demonstrate that activating antiplatelet antibodies have different pathologic consequences in vivo than nonactivating antibodies, including thrombosis and shock. In human FcγRIIA tg mice with physiologic levels of expression of FcγRIIA on platelets and macrophages, we observed more rapid severe thrombocytopenia with activating antiplatelet antibody than that which occurred with nonactivating antiplatelet antibody. Thrombocytopenia induced by this activating antibody was more severe in human FcγRIIA tg mice than in wild-type mice. Most importantly, we observed thrombocytopenia, thrombosis, and shock in FcγRIIA tg mice treated with activating antiplatelet antibody under 3 different circumstances, each of which alters the balance of splenic clearance and intravascular platelet activation. Genetic cross of the FcγRIIA tg mice with the FcR γ-chain knockout that lack endogenous mouse spleen Fcγ receptors showed a profound phenotype, including shock and extensive pulmonary thrombosis that was fatal. Independently, blockade of mouse spleen FcγRIIb/III with 2.4G2 antibody resulted in a shock phenotype in FcγRIIA tg mice. Surgical splenectomy also induced the shock phenotype in FcγRIIA tg mice. The accumulation of platelet/fibrin thrombi on the arterial side of the lung vasculature may reflect the fact that these relatively young, previously healthy mice have normal systemic vasculature and that the activated platelets are trapped in the pulmonary circulation, a finding similar to that recently observed in a baboon model.23 It will be of interest to examine the effects of activating antiplatelet antibodies in the setting of inflamed, damaged, or atherosclerotic peripheral vasculature. These results clearly indicate that the functional presence of FcγRIIA on platelets has a major effect on the pathologic consequences in vivo of antiplatelet antibodies.
In addition to experimental manipulation of splenic clearance, we used our mouse models to systematically vary antibody titer and platelet Fcγ receptor density to identify other major determinants of the thrombosis and shock. Our results indicate that antibody titer is important. This finding in vivo was consistent with our observation in vitro of a threshold dose for antibody-induced platelet activation and aggregation, as has been noted by others.4 20 Our second line of FcγRIIA tg mice, line 32, with lower FcγRIIA expression, allowed assessment of the role of receptor density. In these FcγRIIA tg line 32 mice, whether they were crossed with the γ-KO background or not, anti-CD9 antibody injection did not induce the shock phenotype, a result compatible with our observation of requirement for a higher dose of anti-CD9 to achieve an aggregation response in vitro in platelets from tg line 32 mice (low FcγRIIA density) in comparison with platelets from tg line 11 mice (high FcγRIIA density). Thus, an important determinant of platelet activation in vitro and in vivo in our model is the platelet FcγRIIA density.
Platelet Fcγ receptor density is important in human immune thrombocytopenia, as platelets with higher receptor density are more readily activated in vitro and have been associated with a more severe course in heparin-induced thrombocytopenia.11 The stable interindividual variation in healthy people for platelet Fcγ receptor density can extend over a similar 3-fold to 5-fold range as that manifested by our transgenic lines.19 20 The molecular basis for the difference in expression levels in humans remains unexplained.
Our mouse model findings have major implications for understanding human immune-mediated thrombocytopenic disorders. We present a framework in Table 2 that places both our experimental findings and the clinical observations in the context of the pathologic mechanisms. In our model, with nonactivating antiplatelet antibody, the shock syndrome is not observed, and the spleen is largely responsible for the thrombocytopenia. This is likely the case in typical autoimmune thrombocytopenic purpura. However, in the presence of activating antibody, thrombosis and shock were observed, and the spleen plays a second, protective role in removing the antibody-coated platelets from the circulation, decreasing a source of mediator production. Our observations have implications for several immune thrombocytopenia disorders, including the presence or absence of thrombosis in heparin-induced thrombocytopenia (HIT), the presence or absence of disseminated intravascular coagulation (DIC) in bacterial sepsis–associated thrombocytopenia in which opsonized bacteria have been observed to bind to and activate platelets,24 25 and the varied thrombotic manifestations in the antiphospholipid syndromes (APLSS). Although the thrombosis in HIT, bacterial sepsis–associated thrombocytopenia, or APLS is likely multifactoral, the balance of platelet activation and splenic clearance previously has not been addressed. An appreciation of the interplay among the antibody titer, the receptor density, intravascular platelet activation, and splenic clearance may lead to a more effective understanding of prognosis and individualization of treatment.
Framework for understanding the pathologic effects of immunoglobulin G antiplatelet antibodies in immune-mediated thrombocytopenia
| Nonactivating IgG antibody . | Activating IgG antibody . |
|---|---|
| Splenic clearance | Interplay of splenic clearance and intravascular platelet activation |
| Thrombocytopenia | Thrombocytopenia |
| Thrombosis and/or mediator release | |
| Local and/or systemic effects |
| Nonactivating IgG antibody . | Activating IgG antibody . |
|---|---|
| Splenic clearance | Interplay of splenic clearance and intravascular platelet activation |
| Thrombocytopenia | Thrombocytopenia |
| Thrombosis and/or mediator release | |
| Local and/or systemic effects |
IgG indicates immunoglobulin G.
Genetic factors addressed with our model may be important determinants of the interindividual variability seen clinically, as polymorphisms of the Fcγ receptors profoundly influence the binding of the individual IgG subclasses (reviewed in Lehrnbecher et al26). The FcγRIIA-R131 isoform binds hIgG1 and hIgG3 well, but it does not bind hIgG2 well. The FcγRIIA-H131 isoform binds all 3 of these subclasses well. In the spleen, there are additional polymorphisms of human Fcγ receptor expression and ligand binding, notably FcγRIIIA-F/V158, which may be a physiologic determinant of the extent of splenic clearance.26,27 An additional role of the IgG subclass of the antiplatelet antibody in the clearance by other organs, such as liver, has been addressed in a baboon model of antibody-induced thrombocytopenia23; our data indicate, for the IgG we tested, spleen function was the primary determinant of the clearance.
We have focused on the importance of the effector process in the pathophysiology of immune platelet disorders. The processes that regulate the production of the antibodies are also important in the pathophysiology. Progress in understanding T-cell stimulation and T-cell/B-cell interaction in antiplatelet antibody production has been considerable.28 29 Together, new insights into antibody production and into the pathologic consequences of the antibodies in cellular activation and immune clearance will likely continue to lead to improved therapy of immune-mediated thrombocytopenic disorders.
Acknowledgments
We thank Drs Diana Cassel, Saul Surrey, and Morty Poncz for helpful comments and Dr Jean Richa and the staff of the Transgenic Mouse Core Facility of the University of Pennsylvania.
Supported in part by grants R01HL61865, P01HL40387, and AI22193 from the National Institutes of Health and by the Nemours Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven E. McKenzie, Hematology/Oncology Research, A. I. duPont Hospital for Children, 1600 Rockland Rd, Wilmington, DE 19899; e-mail: smckenz@nemours.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal