Abstract
Activation of platelets by collagen is mediated by the complex glycoprotein VI (GPVI)/Fc receptor γ (FcRγ chain). In the current study, the role of 2 Src family kinases, Fyn and Lyn, in GPVI signaling has been examined using murine platelets deficient in one or both kinases. In the fyn−/−platelets, tyrosine phosphorylation of FcRγ chain, phopholipase C (PLC) activity, aggregation, and secretion are reduced, though the time of onset of response is unchanged. In the lyn−/−platelets, there is a delay of up to 30 seconds in the onset of tyrosine phosphorylation and functional responses, followed by recovery of phosphorylation and potentiation of aggregation and α-granule secretion. Tyrosine phosphorylation and aggregation in response to stimulation by collagen-related peptide is further attenuated and delayed in fyn−/−lyn−/−double-mutant platelets, and potentiation is not seen. This study provides the first genetic evidence that Fyn and Lyn mediate FcR immune receptor tyrosine-based activation motif phosphorylation and PLCγ2 activation after the ligation of GPVI. Lyn plays an additional role in inhibiting platelet activation through an uncharacterized inhibitory pathway.
Introduction
Platelets interact with subendothelial collagen through several receptors, including glycoprotein VI (GPVI) and the integrin α2β1. Although α2β1 is important for platelet adhesion to collagen, major changes in tyrosine phosphorylation, including activation of phospholipase C γ2 (PLCγ2), are thought to be mediated predominantly by the ligation of GPVI.1,2 A collagen-related peptide (CRP), based on a repeated GPP* sequence (single-letter amino acid code: P*, hydroxyproline), activates GPVI without binding to α2β1.3,4 More recently, the major constituent of the venom of the tropical rattlesnakeCrotalus durissus terrificus, convulxin, has been described as a specific ligand for GPVI.5,6 GPVI is a member of IgG superfamily7 and is associated with the Fc receptor γ (FcRγ) chain, a low-molecular-weight (12-14 kd) homodimer that contains an immune receptor tyrosine-based activation motif (ITAM) in its cytoplasmic tail.7 Tyrosine phosphorylation of the ITAM recruits the cytoplasmic tyrosine kinase p72syk (Syk) and initiates a signaling cascade, leading to the activation of phosphoinositide 3′kinase (PI 3′kinase)8 and PLCγ2.9 This sequence of events closely parallels those for many immune receptors, indicating a divergence of function of this signal transduction pathway in hematopoietic cells.
The Src family of tyrosine kinases is believed to phosphorylate the ITAM after the activation of immune receptors. Available evidence suggests that this is also the case for GPVI. Structurally distinct Src-family kinase inhibitors PP1 and PD173956 inhibit platelet activation by collagen, CRP, and convulxin.10,11 In contrast, they have a weak inhibitory effect against activation induced by the G-protein–coupled receptor agonist thrombin.10,11Two independent studies in human platelets have proposed a role for Fyn and Lyn in mediating phosphorylation of the FcRγ chain, including the observation that both kinases coimmunoprecipitate with FcRγ chain.10,11 In contrast, similar interactions with the FcRγ chain were not observed for other platelet Src-family kinases, namely Src, Yes, Fgr, Lck, or Hck.10,11 Fyn and Lyn were also found to associate with a number of other tyrosine phosphorylated proteins in GPVI-stimulated platelets,10 several of which have since been characterized, including the adapter SLP-76,12 the tyrosine kinase Btk,13 and PLCγ2.12 However, little is known of the functional roles of Fyn and Lyn in collagen-mediated platelet activation. Furthermore, there have been no comparative studies of the roles of Fyn and Lyn in platelet signaling.
Our results show that lack of Fyn or Lyn results in reduced or delayed phosphorylation of the FcRγ chain, respectively, in response to CRP. Further, in lyn−/− platelets, the initial delay is followed by a recovery of FcRγ chain phosphorylation and potentiation of activation. A double deficiency of both Fyn and Lyn causes a greater impairment of tyrosine phosphorylation and functional responses of platelets to CRP, and recovery of potentiation is lost.
Materials and methods
Materials
Source of mice.
Fyn−/− breeding pairs, engineered on an Sv129 background,14 were from Jackson Laboratories (Bar Harbor, ME). Controls for fyn−/− experiments werefyn+/− mice raised from crosses betweenfyn−/− and C57Bl6J mice and wild-type C57Bl6J and CD1 mice. Lyn−/− mice15 were initially bred from a population of heterozygotes kindly supplied by Dr V. Tybulewicz (Institute of Medical Research, London, England). Controls were either wild-type littermates or CD1 mice.Fyn−/−lyn−/− double-knockout mice were bred on a mixed-strain background. These mice were raised from mixed breedings of fyn−/−background14 and lyn−/−mice.16 Controls for the double-knockout mice were double-positive wild-type mice from a mixed background and C57Bl6J mice. Fgr−/− mice were as described.17 Mice were genotyped by PCR amplification of mouse tail genomic DNA, and lack of expression of protein was confirmed by Western blot platelet lysates. There were no significant differences in platelet responses between littermates and the various strains of mice.
Materials for functional assays.
CRP (GCP*[GPP*)]10GCP*G; single-letter amino acid code where P* represents hydroxyproline) was cross-linked as described.4 PP1 was dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in platelet samples did not exceed 0.1%. Flow cytometric analysis (FACS) was performed using FACScalibur apparatus (Becton Dickinson, Oxfordshire, United Kingdom), and the results were analyzed using Cell Quest software (Becton Dickinson). Materials for FACS analysis of P-selectin surface expression were purchased from sources described previously.18 Fura-2-am, a cell-permeant ratiometric calcium reporter, was purchased from Molecular Probes (Leiden, The Netherlands). The fluorometer used was supplied by PerkinElmer (Norwalk, CT). Data were analyzed using FWinlab software (PerkinElmer). [32P]-Orthophosphate and Hyperfilm (Amersham Pharmacia Biotech, Amersham, United Kingdom) used for autoradiography were obtained as described previously.3 Anti-Btk (BL7), anti-Syk (BR15), and anti-PLCγ2 (DN84) rabbit polyclonal antiserum were the kind gifts of Drs M. G. Tomlinson and J. Bolen (DNAX Research Institute, Palo Alto, CA). Anti–SLP-76 (H3) monoclonal antibody was the kind gift of Dr G. Koretzky (Abramson Family Cancer Research Institute, Philadelphia, PA). Other antibodies and reagents were obtained from sources previously described.8 13
Methods
Isolation of murine platelets.
Blood was drawn from terminally CO2-anesthetized mice by cardiac puncture using acid citrate dextrose as an anticoagulant (1:9 vol/vol). Preparation of murine platelets has been previously described.18 Platelets were resuspended to the appropriate platelet concentration in acidified HEPES-Tyrode's buffer (20 mmol/L HEPES, 135 mmol/L NaCl, 3 mmol/L KCl, 0.35 mmol/L Na2HPO4, 12 mmol/L NaHCO3, and 1 mmol/L MgCl2, pH 7.30). Typically, 1.0 mL whole blood from an adult male wild-type mouse yields 5 to 8 × 108 platelets.
Stimulation of murine platelets.
Aggregation and flow cytometric analysis studies were carried out at a concentration of 1 × 108 platelets/mL. Aggregation was measured using a 300-μL sample in a Born aggregometer (Chronolog, Havertown, PA) with high-speed stirring (1200 rpm) at 37°C.
FACS analysis of P-selectin surface expression in murine platelets.
For measurements of P-selectin cell surface expression, murine platelets were stimulated at a concentration of 8 × 107/mL in aliquots of 50 μL, with mixing but without stirring. Platelets were stimulated by various agonists for 10 minutes in the presence of 10 μmol/L indomethacin and 1 mmol/L CaCl2 unless stated otherwise. Two microliters primary antimouse P-selectin (CD62P) IgG and 5 μL secondary fluorescein isothiocyanate-conjugated rat antimouse IgG were used per platelet sample to detect exposed P-selectin. Samples were incubated at room temperature for 10 minutes with primary and secondary antibodies and then diluted by the addition of 500 μL HEPES-Tyrode's buffer and analyzed immediately.
Calcium fluorometry.
Platelets isolated from PRP were resuspended in HEPES-Tyrode's buffer (pH 7.3) to a concentration of 5 × 108/mL. Murine platelets were loaded with the calcium reporter Fura-2 by incubation with 10 μmol/L Fura-2-am for 1 hour at 30°C. Platelets were stimulated with fast stirring at 37°C in a fluorometer with an excitation wavelength of 530 nm and emission wavelengths at 330 and 380 nm. The platelet concentration used was 8 × 107/mL. The ratio of Fura-2 emissions were measured and analyzed using phycoerythrin FWinlab software. Ratiometric analysis was converted to a concentration of Ca++ by taking measurements of Rmax (maximal fluorescence in lysed, labeled platelets in the presence of 5 mmol/L CaCl2) and Rmin(minimum fluorescence in lysed, labeled platelet suspension in the presence of 5 mmol/L EGTA).
[32P]-Labeling of murine platelets and preparation of lysates for SDS-PAGE and for thin-layer chromatography of phospholipids.
Platelets were labeled with 500 μCi [32P]-orthophosphate per milliliter platelet suspension for 60 minutes at 37°C. Washed, labeled platelets were stimulated at a concentration of 2 × 108/mL in the presence of 10 μmol/L indomethacin and 1 mmol/L EGTA in 100 μL sample volumes in an aggregometer, as described above. Twenty-microliter aliquots of platelets were removed and added to equal volume 2× sample buffer at various time points of stimulation and resolved by 10% SDS-PAGE. Gels were stained with Coomassie blue before drying to check for equal loading of protein. Pleckstrin was detected as a phosphorylated band at 47 kd. Autoradiographs were taken using Hyperfilm. The remaining 80-μL sample was lysed using 4 × volume of lipid extraction buffer (CHCl3:methanol, 1:1 vol/vol) and treated with 1 mmol/L EDTA and 0.4 mol/L HCl. Each lysed sample was vortexed at high speeds for 30 seconds to ensure thorough mixing. Phases were separated by fast centrifugation. Lower-phase phospholipids were dried under N2 with gentle heating (30°C) and resuspended in 30 μL CHCl3:methanol (1:1, vol/vol). Silica thin-layer chromatography plates were pretreated with a priming solution (0.5 mol/L oxalic acid in 70% methanol and 30% water) before the separation of phospholipids using a solution of acidified CHCl3:methanol (87:13; vol/vol) as running buffer. Phosphatidic acid appears as the major separated band using pretreated plates and is visualized by autoradiography using Hyperfilm. The phosphatidic acid band of silica was then scraped off the thin-layer chromatography plate into scintillant for scintillation counting.
Preparation of murine platelet lysates.
Platelets were stimulated in the presence of 10 μmol/L indomethacin and 1 mmol/L EGTA, at 37°C under stirring conditions in an aggregometer. Whole-platelet lysates and platelet lysates for immunoprecipitation were carried out at a concentration of 1 × 108 platelets/mL, using no more that 100 μL platelet suspension per sample. Immunoprecipitation was carried out as previously described.10 13
Analysis of data.
Results are shown as mean ± SEM. Statistical significance was tested using the Student t test. All experiments were performed at least 3 times.
Results
Selective inhibitor of Src-family kinases PP1 inhibits activation of murine platelets by CRP
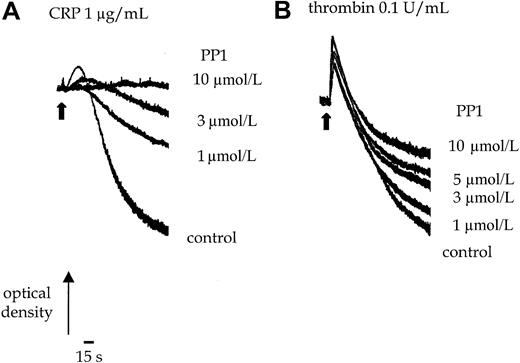
PP1 (10 μmol/L) completely inhibits shape change and aggregation of murine platelets stimulated by an intermediate concentration of CRP (1.0 μg/mL) (Figure 1A). Shape change represents the transition from a resting discoid morphology to an optically more dense spherical form. Aggregation of platelets is detected as a decrease in optical density. The EC50 value for inhibition by PP1 is approximately 3 μmol/L. Increasing the concentration of CRP to a maximally effective concentration of 10 μg/mL does not overcome the inhibition (not shown). In contrast, PP1 (10 μmol/L) has no significant effect on thrombin (0.1 U/mL)-induced shape change or the early phase of aggregation, though it inhibits late-phase aggregation by up to 50% (Figure 1B). This suggests that, as reported in human platelets, Src-family kinases are involved in GPVI-mediated signaling and have a minor role in regulating later phases of the thrombin pathway in murine platelets.
Effect of PP1 on CRP and thrombin-stimulated aggregation.
Platelets were incubated with 0.1% DMSO or PP1 for 5 minutes before the addition of the agonist. (A) Shape change and aggregation of wild-type murine platelets was measured in a Born aggregometer after stimulation with CRP (1.0 μg/mL) in the absence and presence of PP1 (1-10 μmol/L). (B) Aggregation of wild-type murine platelets to thrombin (0.1 U/mL) was measured in the absence or presence of PP1 (1-10 μmol/L). Arrowhead indicates the time of agonist addition. The experiment is representative of 3 experiments with similar results.
Effect of PP1 on CRP and thrombin-stimulated aggregation.
Platelets were incubated with 0.1% DMSO or PP1 for 5 minutes before the addition of the agonist. (A) Shape change and aggregation of wild-type murine platelets was measured in a Born aggregometer after stimulation with CRP (1.0 μg/mL) in the absence and presence of PP1 (1-10 μmol/L). (B) Aggregation of wild-type murine platelets to thrombin (0.1 U/mL) was measured in the absence or presence of PP1 (1-10 μmol/L). Arrowhead indicates the time of agonist addition. The experiment is representative of 3 experiments with similar results.
Independent expression of Fyn and Lyn in murine platelets
Immunoblots of whole-cell lysates from fyn−/−platelets show that though there is no detectable Fyn expression, Lyn is expressed at levels equivalent to those in wild-type platelets (Figure 2A). A complementary set of observations was made in lyn−/− platelets. Immunoblotting of platelet lysates from mice deficient in both kinases confirms their lack of expression, whereas the expression of Tec family kinase Bruton tyrosine kinase (Btk) was normal (Figure 2C). Therefore, we found no evidence of compensatory changes in the expression of Lyn and Fyn in fyn−/− andlyn−/− mice.
Immunoblots of lysates fromfyn−/−,lyn−/−, andfyn−/−lyn−/−platelets.
Murine whole-platelet lysates were immunoblotted with anti-Fyn, anti-Lyn, and anti-Btk antisera after 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). (A)Fyn−/−platelets express no detectable Fyn but normal levels of Lyn. (B) Conversely, lyn−/−platelets express normal levels of Fyn but not Lyn. (C)Fyn−/−lyn−/−express neither Fyn nor Lyn but normal amounts of Btk. The traces are representative of 5 to 20 experiments.
Immunoblots of lysates fromfyn−/−,lyn−/−, andfyn−/−lyn−/−platelets.
Murine whole-platelet lysates were immunoblotted with anti-Fyn, anti-Lyn, and anti-Btk antisera after 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). (A)Fyn−/−platelets express no detectable Fyn but normal levels of Lyn. (B) Conversely, lyn−/−platelets express normal levels of Fyn but not Lyn. (C)Fyn−/−lyn−/−express neither Fyn nor Lyn but normal amounts of Btk. The traces are representative of 5 to 20 experiments.
Tyrosine phosphorylation in fyn−/−platelets
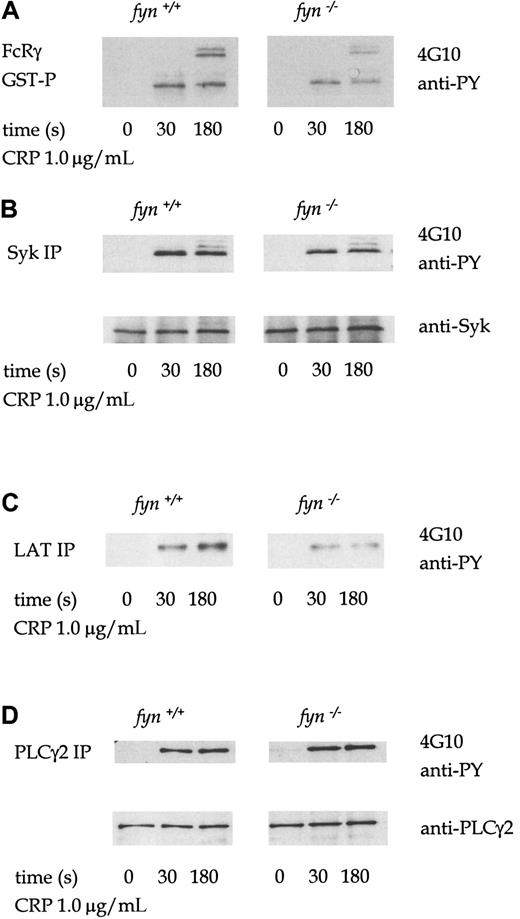
The profile of tyrosine phosphorylation in whole-cell lysates infyn−/− platelets stimulated by collagen, CRP, or thrombin was not significantly different from that of control cells (not shown). However, FcRγ chain precipitated from CRP-stimulatedfyn−/− platelet lysates using a GST fusion protein of the tandem SH2 domains of Syk (GST-(SH2)2-Syk) is less phosphorylated than in control platelets (Figure3A). FcRγ chain appears as several bands in the region of 12 to 14 kd, which are likely to represent different tyrosine-phosphorylated forms.19 Tyrosine phosphorylation of immunoprecipitated Syk and the adapters LAT and SLP-76 from CRP-stimulated fyn−/− platelets were also similarly reduced compared to control platelets (Figure3B-C). However, CRP-induced tyrosine phosphorylation of PLCγ2 was not decreased in fyn−/− platelets (Figure 3D). Wild-type and fyn−/− samples were prepared in parallel and resolved on the same gel. The separate panels shown for each protein precipitation in the figure were from the same exposure. A similar set of phosphorylation results was obtained in collagen-stimulated platelets (not shown).
Tyrosine phosphorylation in CRP-stimulated wild-type andfyn−/−platelets.
Murine platelets were treated with 1 mmol/L EGTA and 10 μmol/L indomethacin for these experiments. Antiphosphotyrosine immunoblots show that CRP (1.0 μg/mL)-induced tyrosine phosphorylation of FcRγ chain (A), Syk (B), LAT (C), and PLCγ2 (D) in wild-type andfyn−/−platelets. FcRγ chain was precipitated using GST fusion protein of the tandem SH2 domains of Syk. We were unable to obtain a reprobe for LAT from LAT immunoprecipitations because the antibody could not be readily used for Western blotting for murine platelets. The results are representative of 3 to 5 experiments. Wild-type and knockout samples were obtained from experiments run in parallel. Results shown in each panel in A are from samples resolved on the same gel and are from the same exposure on the same film. This also applies to B-D.
Tyrosine phosphorylation in CRP-stimulated wild-type andfyn−/−platelets.
Murine platelets were treated with 1 mmol/L EGTA and 10 μmol/L indomethacin for these experiments. Antiphosphotyrosine immunoblots show that CRP (1.0 μg/mL)-induced tyrosine phosphorylation of FcRγ chain (A), Syk (B), LAT (C), and PLCγ2 (D) in wild-type andfyn−/−platelets. FcRγ chain was precipitated using GST fusion protein of the tandem SH2 domains of Syk. We were unable to obtain a reprobe for LAT from LAT immunoprecipitations because the antibody could not be readily used for Western blotting for murine platelets. The results are representative of 3 to 5 experiments. Wild-type and knockout samples were obtained from experiments run in parallel. Results shown in each panel in A are from samples resolved on the same gel and are from the same exposure on the same film. This also applies to B-D.
Parameters of PLC activity are reduced infyn−/−platelets
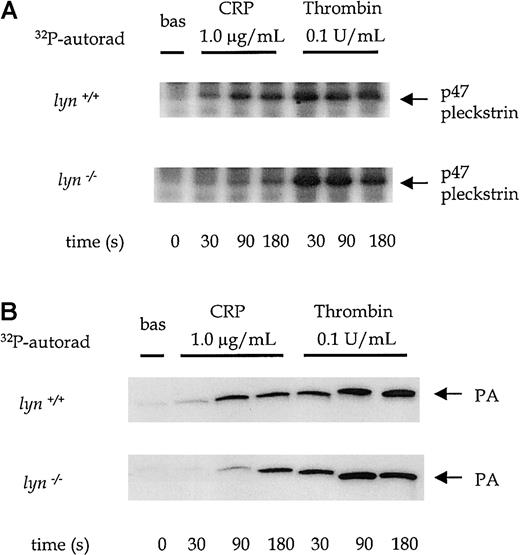
The degree of tyrosine phosphorylation of PLCγ2 does not necessarily correlate with its activity because other pathways are also involved in the regulation of the phospholipase—for example, the PI 3′kinase pathway.8,20 It was, therefore, important to measure PLC activity. Although inositol 1,4,5-triphosphate (InsP3) is a direct product of PLC, it cannot be measured in the small number of platelets obtained from mice using standard techniques. As an alternative, we measured levels of phosphatidic acid (PA), a metabolite of 1,2-diacylglycerol, in [32P]-labeled platelets and the formation of [32P]-pleckstrin, a substrate of protein kinase C.8 18 Activation of phospholipase D does not contribute significantly to the increase in [32P]-PA because of the low turnover of the inositol head group, which means that the phosphate group in the 1-position is minimally radiolabeled during short incubations. A reduction in PA formation of approximately 50% was seen in response to CRP in fyn−/− platelets compared to controls (Figure 4A). The basal level of [32P]phosphatidic acid was similar in both wild-type and fyn−/− platelets. In contrast, thrombin-induced [32P]phosphatidic acid production was slightly increased in fyn−/− platelets (Figure4A), though this was not statistically significant. A shorter exposure of the autoradiogram is shown for the more powerful agonist, thrombin, compared with CRP in the figure to enable differentiation between PA levels induced by different concentrations of thrombin. Pleckstrin phosphorylation was also reduced in fyn−/−platelets stimulated by low concentrations of CRP but reached normal levels after stimulation with higher concentrations (Figure 4B). This recovery is in contrast to the results for PA and can be explained by the difference in concentration-response relations for these 2 responses in that only submaximal concentrations of CRP are required for the maximal phosphorylation of pleckstrin. In contrast, pleckstrin phosphorylation was not significantly altered infyn−/− platelets in response to thrombin compared to controls (Figure 4B).
PLC activation in fyn−/−platelets.
Pleckstrin phosphorylation (an indicator of PKC activation) and phosphatidic acid (PA) production (a metabolite of diacylglycerol) were measured in [32P]-labeled platelets in the presence of 1 mmol/L EGTA and 10 μmol/L indomethacin. (A) The autoradiographs show pleckstrin phosphorylation stimulated by a range of concentrations of CRP (0.3-3.0 μg/mL) and thrombin (0.1-1.0 U/mL) in wild-type andfyn−/−platelets. The results are representative of 3 to 5 experiments. (B) Autoradiographs show phosphatidic acid production stimulated by a range of concentrations of CRP (0.3-3.0 μ/mL) and thrombin (0.1-1.0 U/mL) in wild-type andfyn−/−platelets. The results are representative of 3 to 5 experiments. Wild-type andfyn−/−samples were run in parallel, and the same exposure times are shown. A lighter exposure of the autoradiogram from representative samples of the more powerful agonist thrombin is shown to enable differentiation between PA levels stimulated by different concentrations of thrombin. (C) Ca2+ mobilization was measured in Fura-2–labeled murine platelets in the presence of 1 mmol/L EGTA. Wild-type and fyn−/−platelets (i) and wild-type and lyn−/−platelets (ii) were stimulated by 0.3 μg/mL CRP over 200 seconds. The arrow indicates the point of addition of an agonist. Results are representative of 5 experiments.
PLC activation in fyn−/−platelets.
Pleckstrin phosphorylation (an indicator of PKC activation) and phosphatidic acid (PA) production (a metabolite of diacylglycerol) were measured in [32P]-labeled platelets in the presence of 1 mmol/L EGTA and 10 μmol/L indomethacin. (A) The autoradiographs show pleckstrin phosphorylation stimulated by a range of concentrations of CRP (0.3-3.0 μg/mL) and thrombin (0.1-1.0 U/mL) in wild-type andfyn−/−platelets. The results are representative of 3 to 5 experiments. (B) Autoradiographs show phosphatidic acid production stimulated by a range of concentrations of CRP (0.3-3.0 μ/mL) and thrombin (0.1-1.0 U/mL) in wild-type andfyn−/−platelets. The results are representative of 3 to 5 experiments. Wild-type andfyn−/−samples were run in parallel, and the same exposure times are shown. A lighter exposure of the autoradiogram from representative samples of the more powerful agonist thrombin is shown to enable differentiation between PA levels stimulated by different concentrations of thrombin. (C) Ca2+ mobilization was measured in Fura-2–labeled murine platelets in the presence of 1 mmol/L EGTA. Wild-type and fyn−/−platelets (i) and wild-type and lyn−/−platelets (ii) were stimulated by 0.3 μg/mL CRP over 200 seconds. The arrow indicates the point of addition of an agonist. Results are representative of 5 experiments.
Ca++ mobilization from intracellular stores was measured in Fura-2–labeled platelets in the presence of 1 mmol/L EGTA and 10 μmol/L indomethacin. CRP (0.3 μg/mL) stimulated a rapid Ca++ peak that declined to a plateau by 300 seconds in control platelets. In fyn−/− platelets, there was a marked reduction in the initial peak, though the plateau was not altered (Figure 4Ci). Similar results were seen for other concentrations of CRP (not shown). In contrast, there was no change in peak response to thrombin, though the duration of the plateau was slightly increased in the fyn−/− platelets (not shown).
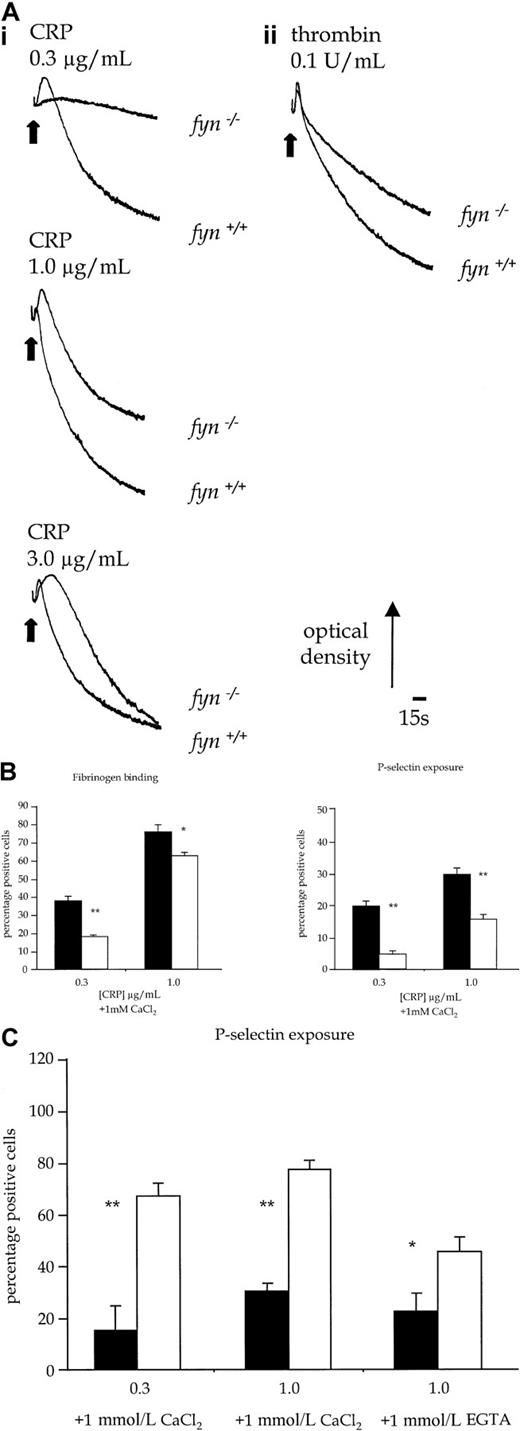
Fyn−/− platelets exhibited a reduced aggregation response over the length of the concentration response curve to CRP (Figure 5Ai). This was associated with an apparent prolongation of shape change to intermediate concentrations of CRP, which was the consequence of the reduction in aggregation. Similar results were obtained with collagen (1.0-10.0 μg/mL, not shown). In contrast, shape change and aggregation of fyn−/− platelets in response to submaximal concentrations of thrombin (0.1 U/mL) were not decreased at early time points when compared to controls. However, there was a reduction in the late-phase aggregation response to thrombin (Figure5Aii). In comparison, there was no alteration in aggregation induced by CRP in platelets deficient in the Src-family kinase, Fgr (not shown), which is also expressed in platelets.17 Activation of platelets by CRP is also associated with the exposure of P-selectin, a constituent of α-granule membrane. P-selectin expression infyn−/− platelets was partially inhibited in response to CRP (0.1-1.0 μg/mL) (Figure 5B), whereas the response to thrombin was not significantly altered (not shown).
Functional responses of control and kinase-deficient platelets.
(A) Platelet shape change and aggregation stimulated with CRP or thrombin. (i) Wild-type and fyn−/−platelets stimulated with CRP (0.3-3.0 μg/mL). (ii) Wild-type andfyn−/−platelets stimulated with thrombin (0.1 U/mL). Results are representative of 5 experiments. (B) Fibrinogen binding and P-selectin exposure in fyn−/−platelets stimulated by CRP in the presence of 1mmol/L CaCl2. The results are mean ± standard error of mean of 4 experiments. ▪ indicates fyn+/+; ■,fyn−/−; *, P < .05; **,P < .01). (C) P-selectin exposure inlyn−/− platelets stimulated by CRP in the presence of 1 mmol/L CaCl2 or 1 mmol/L EGTA. The results are mean ± standard error of mean of 4 experiments. ▪ indicateslyn+/+; ■, lyn−/−; *,P < .05; **, P < .01.
Functional responses of control and kinase-deficient platelets.
(A) Platelet shape change and aggregation stimulated with CRP or thrombin. (i) Wild-type and fyn−/−platelets stimulated with CRP (0.3-3.0 μg/mL). (ii) Wild-type andfyn−/−platelets stimulated with thrombin (0.1 U/mL). Results are representative of 5 experiments. (B) Fibrinogen binding and P-selectin exposure in fyn−/−platelets stimulated by CRP in the presence of 1mmol/L CaCl2. The results are mean ± standard error of mean of 4 experiments. ▪ indicates fyn+/+; ■,fyn−/−; *, P < .05; **,P < .01). (C) P-selectin exposure inlyn−/− platelets stimulated by CRP in the presence of 1 mmol/L CaCl2 or 1 mmol/L EGTA. The results are mean ± standard error of mean of 4 experiments. ▪ indicateslyn+/+; ■, lyn−/−; *,P < .05; **, P < .01.
Protein tyrosine phosphorylation in lyn−/−platelets
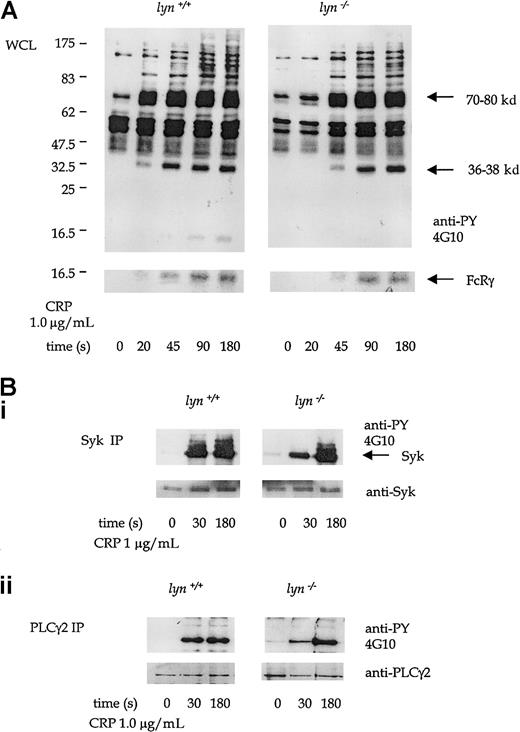
Protein tyrosine phosphorylation induced by CRP (1.0 μg/mL) was significantly delayed in lyn−/− platelets compared to controls. A time-course of CRP-stimulated whole-platelet phosphorylation was performed to pinpoint this delay in initiation of the GPVI-dependent tyrosine phosphorylation. There was little detectable increase in phosphorylation observed after 20 seconds of stimulation by 1.0 μg/mL CRP (Figure 4A). Recovery of CRP-induced protein tyrosine phosphorylation in the lyn−/−platelets was seen in the whole-cell lysates by 45 sec and was complete by 90 seconds (Figure 6A). FcRγ chain, Syk, LAT, and PLCγ2 were among the proteins that show an initial reduction in phosphorylation at 30 seconds, with full recovery at 180 seconds (Figure 6A-B). A similar set of results was seen in platelets stimulated with collagen (not shown).
Tyrosine phosphorylation in CRP-stimulated wild-type andlyn−/−platelets.
(A) Antiphosphotyrosine immunoblots show whole-platelet tyrosine phosphorylation stimulated by CRP (1.0 μg/mL) from 20 to 180 seconds in wild-type and lyn−/−platelets. Regions known to comigrate with Syk (70-80 kd), LAT (36-38 kd), and FcRγ chain (14-16 kd) are indicated. (B) Immunoprecipitates of Syk (i) and PLCγ2 (ii) immunoblotted with antiphosphotyrosine mAb in wild-type and lyn−/−platelets at 30 and 180 seconds. Results are representative of 4 experiments. Wild-type and knockout samples were obtained from experiments run in parallel. Results shown in both panels are from samples resolved on the same gel and are from the same exposure on the same film.
Tyrosine phosphorylation in CRP-stimulated wild-type andlyn−/−platelets.
(A) Antiphosphotyrosine immunoblots show whole-platelet tyrosine phosphorylation stimulated by CRP (1.0 μg/mL) from 20 to 180 seconds in wild-type and lyn−/−platelets. Regions known to comigrate with Syk (70-80 kd), LAT (36-38 kd), and FcRγ chain (14-16 kd) are indicated. (B) Immunoprecipitates of Syk (i) and PLCγ2 (ii) immunoblotted with antiphosphotyrosine mAb in wild-type and lyn−/−platelets at 30 and 180 seconds. Results are representative of 4 experiments. Wild-type and knockout samples were obtained from experiments run in parallel. Results shown in both panels are from samples resolved on the same gel and are from the same exposure on the same film.
Parameters of PLC activity are reduced inlyn−/−platelets
There was a significant impairment of CRP-stimulated pleckstrin phosphorylation and production of [32P]PA inlyn−/− platelets at time points up to 90 seconds, which was restored to control levels by 180 seconds (Figure7A-B). For example, the level of PA in response to 1.0 μg/mL CRP was reduced by 54% ± 5% at 90 seconds in lyn−/− platelets (P < .05). In contrast, thrombin-induced pleckstrin phosphorylation and PA formation were similar in lyn−/− and control platelets at all time points (Figure 7A-B). Lyn−/−platelets also show a delay of up to 45 seconds in Ca++ mobilization in response to 1.0 μg/mL CRP (Figure4Cii). The initial response was followed by a sustained plateau inlyn−/− platelets. It is notable that the plateau response of wild-type platelets declined toward baseline levels more rapidly than in lyn−/− platelets (Figure 4Cii).
PLC activation in wild-type andlyn−/−platelets.
Phosphorylation of pleckstrin and formation of phosphatidic acid were measured over time from 0 to 180 seconds after the addition of an agonist in platelets treated with 1 mmol/L EGTA and 10 μmol/L indomethacin. The autoradiographs show pleckstrin phosphorylation (A) and phosphatidic acid production (B) stimulated by CRP (1.0 μg/mL) and thrombin (0.1 U/mL) in wild-type and lyn−/−platelets compared with wild type. Results are representative of 4 experiments.
PLC activation in wild-type andlyn−/−platelets.
Phosphorylation of pleckstrin and formation of phosphatidic acid were measured over time from 0 to 180 seconds after the addition of an agonist in platelets treated with 1 mmol/L EGTA and 10 μmol/L indomethacin. The autoradiographs show pleckstrin phosphorylation (A) and phosphatidic acid production (B) stimulated by CRP (1.0 μg/mL) and thrombin (0.1 U/mL) in wild-type and lyn−/−platelets compared with wild type. Results are representative of 4 experiments.
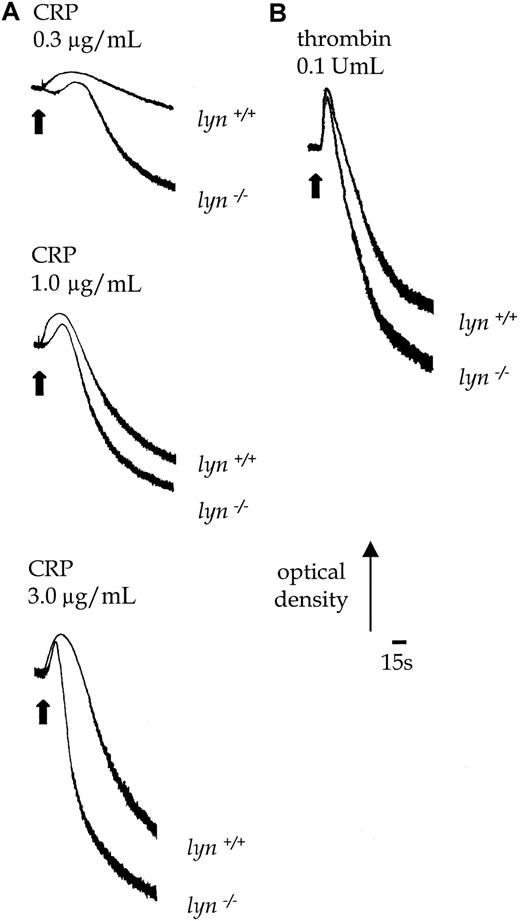
Lyn−/− platelets exhibited a delay of up to 30 seconds in the onset of shape change in response to CRP, which was followed by potentiation of the rate and magnitude of aggregation (Figure 8B). Similar results were obtained with collagen (1.0-10.0 μg/mL, not shown). In contrast, the initial shape change response of lyn−/−platelets to thrombin (0.1 U/mL) was not altered relative to controls, though aggregation was slightly enhanced in terms and rate and magnitude (Figure 8B). Expression of P-selectin in response to CRP was also significantly increased in lyn−/−platelets compared with controls when measured after 120 seconds, either in the absence or the presence of extracellular Ca++(Figure 5C). In contrast, the response to thrombin was not altered (not shown).
Aggregation responses in lyn−/−compared with wild-type platelets.
Shape change and aggregation of wild-type andlyn−/−platelets in response to CRP (A, 0.3-3.0 μg/mL) and thrombin (B, 0.1 U/mL) were measured in a Born aggregometer. Other conditions are as in the legend to Figure 1. Results are representative of 3 experiments.
Aggregation responses in lyn−/−compared with wild-type platelets.
Shape change and aggregation of wild-type andlyn−/−platelets in response to CRP (A, 0.3-3.0 μg/mL) and thrombin (B, 0.1 U/mL) were measured in a Born aggregometer. Other conditions are as in the legend to Figure 1. Results are representative of 3 experiments.
GPVI-dependent tyrosine phosphorylation in fyn−/−lyn−/−platelets
The partial inhibition of ITAM tyrosine phosphorylation and platelet activation in fyn−/− andlyn−/− platelets suggests functional redundancy within this pathway between Src-family kinases. Additionally, the participation of Lyn in inhibitory pathways indicates that Src-family kinases have distinct signaling roles. The responses infyn−/−lyn−/− platelets were investigated to characterize further the roles of the 2 Src family kinases in GPVI signaling.
Whole-cell protein tyrosine phosphorylation was severely reduced in platelets deficient in Fyn and Lyn in response to CRP. There was minimal tyrosine phosphorylation of the 12- to 14-kd bands that comigrated with FcRγ chain in the whole-cell lysates, and the phosphorylation of Syk and of PLCγ2, measured after immunoprecipitation, was severely attenuated at times up to 180 seconds (Figure 9).
CRP-stimulated tyrosine phosphorylation in wild-type andfyn−/−lyn−/−platelets.
Platelets in this experiment were treated with 1 mmol/L EGTA and 10 μmol/L indomethacin. (A) Antiphosphotyrosine immunoblots show tyrosine phosphorylation at 0, 30, and 180 seconds in CRP (1.0 μg/mL)-stimulated wild-type andfyn−/−lyn−/−platelets. The 12- to 14-kd band corresponds to the FcRγ chain. Phosphorylation of Syk (B) and PLCγ2 (C), measured after immunoprecipitation from platelets stimulated as in panel A. Results are representative of 3 experiments. Wild-type and knockout samples were obtained from experiments run in parallel. Results shown in each panel of A are from samples resolved on the same gel and are from the same exposure on the same film. This also applies to B-D.
CRP-stimulated tyrosine phosphorylation in wild-type andfyn−/−lyn−/−platelets.
Platelets in this experiment were treated with 1 mmol/L EGTA and 10 μmol/L indomethacin. (A) Antiphosphotyrosine immunoblots show tyrosine phosphorylation at 0, 30, and 180 seconds in CRP (1.0 μg/mL)-stimulated wild-type andfyn−/−lyn−/−platelets. The 12- to 14-kd band corresponds to the FcRγ chain. Phosphorylation of Syk (B) and PLCγ2 (C), measured after immunoprecipitation from platelets stimulated as in panel A. Results are representative of 3 experiments. Wild-type and knockout samples were obtained from experiments run in parallel. Results shown in each panel of A are from samples resolved on the same gel and are from the same exposure on the same film. This also applies to B-D.
Responses of fyn−/−lyn−/−platelets to CRP are severely impaired
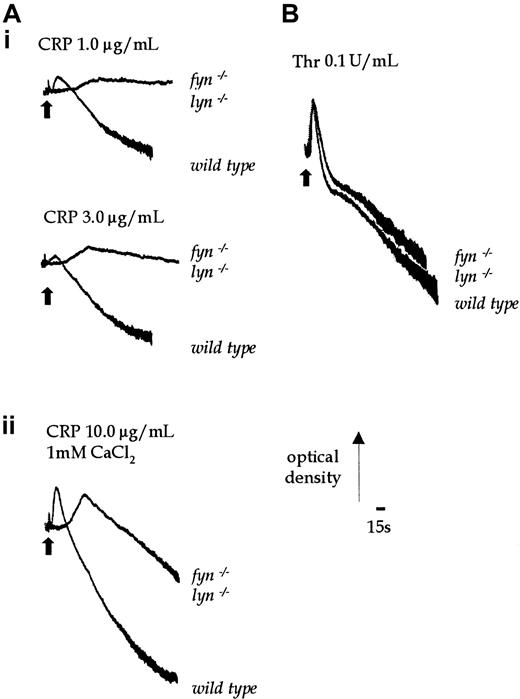
Shape change and aggregation offyn−/−lyn−/− platelets in response to CRP was severely delayed and, in the case of aggregation, markedly inhibited. Shape change was only elicited in response to relatively high concentrations of CRP (1.0-3.0 μg/mL) after a delay of 60 to 80 seconds (Figure 10Ai). A weak aggregation response was only seen in response to a maximal concentration of CRP (10.0 μg/mL), with no accelerated second phase (Figure 10Aii). The residual shape change and aggregation responses in the double-knockout mouse was inhibited by the Src-family kinase inhibitor PP1 (10 μmol/L, not shown). Shape change and aggregation offyn−/−lyn−/− platelets in response to thrombin were similar to control responses (Figure10B).
Aggregation stimulated by CRP in wild-type andfyn−/−lyn−/−platelets.
(Ai) Shape change of wild-type andfyn−/−lyn−/−platelets was stimulated by CRP (1.0-3.0 μg/mL). (Aii) Aggregation of wild-type andfyn−/−lyn−/−platelets was induced by a higher concentration of CRP (10.0 μg/mL) in the presence of added 1 mmol/L CaCl2. (B) Aggregation of wild-type andfyn−/−lyn−/−platelets was stimulated with 0.1 U/mL thrombin. Results are representative of 3 experiments.
Aggregation stimulated by CRP in wild-type andfyn−/−lyn−/−platelets.
(Ai) Shape change of wild-type andfyn−/−lyn−/−platelets was stimulated by CRP (1.0-3.0 μg/mL). (Aii) Aggregation of wild-type andfyn−/−lyn−/−platelets was induced by a higher concentration of CRP (10.0 μg/mL) in the presence of added 1 mmol/L CaCl2. (B) Aggregation of wild-type andfyn−/−lyn−/−platelets was stimulated with 0.1 U/mL thrombin. Results are representative of 3 experiments.
Discussion
Previous reports, based on a biochemical approach and use of inhibitors, have suggested that the Src kinases Fyn and Lyn are involved in regulating collagen-induced platelet activation.10 11 However, these studies were unable to dissect the individual functions of Fyn and Lyn because of the lack of selectivity of available inhibitors, such as PP1 and PD17036. The current study has addressed this through a genetic approach usingfyn−/− and lyn−/−platelets. Our findings confirm that Fyn and Lyn regulate FcRγ chain tyrosine phosphorylation on ligation of GPVI by CRP.
Fyn−/− platelets have reduced Fcγ chain tyrosine phosphorylation, resulting in the partial loss of phosphorylation of downstream proteins including the tyrosine kinase Syk and the adapters SLP-76 and LAT. Although tyrosine phosphorylation of PLCγ2 does not appear to be affected by Fyn deficiency, its activity is significantly reduced. The mismatch between the tyrosine phosphorylation of PLCγ2 and proteins involved in its regulation is unclear. It could, for example, reflect a role of a Fyn-regulated protein phosphatase in the regulation of PLCγ2 phosphorylation. The inhibition of PLCγ2 activity, despite the normal level of phosphorylation, is likely to be due to the decrease in phosphorylation of adapter proteins such as LAT (thereby reducing the recruitment of PLCγ2 to the membrane) or possibly phosphorylation at novel sites in the phospholipase. In response to CRP, lyn−/−platelets have a delay 30 to 45 seconds in the onset of aggregation, tyrosine phosphorylation, and PLCγ2 activation, followed by either recovery or potentiation of response. The initial delay in activation observed in the Lyn-deficient platelets, in comparison to the reduction observed in the fyn−/− cells, suggests that Lyn may have the more important role in initiating phosphorylation of the ITAM.fyn−/−lyn−/− platelets exhibit a severe inhibition and a marked delay in activation. There is some recovery in the response offyn−/−lyn−/− platelets with higher concentrations of CRP, but potentiation is not seen. Residual activation of fyn−/−lyn−/−platelets is blocked by the inhibitor of Src kinases, PP1, suggesting that an additional Src-family kinase may underlie this response. This kinase is likely to have a relatively minor role relative to that of Fyn and Lyn in platelet activation by GPVI.
Fyn and Lyn have been shown to mediate phosphorylation of the ITAM in the Fcγ chain on stimulation of other Fc receptors, including FcεRI receptor.21 The role of Fyn and Lyn in mediating this event has been attributed to their presence in glycolipid-enriched membrane domains (GEMs), an association that is dependent on palmitoylation of both Src family kinases. In contrast, Src, which is not palmitoylated and therefore is not present in GEMs, is unable to induce phosphorylation of the Fcγ chain.21 This mechanism is likely to account for the role of Fyn and Lyn in mediating phosphorylation of the Fcγ chain in platelets rather than Src, which is expressed at a much greater level.
The delayed potentiation in lyn−/− platelets suggests that Lyn is also involved in regulating a novel inhibitory pathway. This pathway appears to be mediated at least partly through an increase in PLCγ2 activity. In lyn−/−platelets, there was an initial delay in the activation of PLCγ2 that recovered to control levels by 180 seconds, whereas infyn−/− platelets, PLCγ2 activity was inhibited at all times. The increase in PLCγ2 activity is consistent with the sustained increase in Ca++ in thelyn−/− platelets. It seems likely, however, that an additional mechanism contributes to the increase in aggregation and α-granule secretion observed in lyn−/−platelets. The potentiation of aggregation in response to GPVI precedes the recovery of PLC activity and Ca++ responses. Moreover, the potentiation of aggregation also occurs in thrombin-stimulated platelets in the absence of changes in PLC activity and Ca++.
It is of interest that B cells from lyn−/−mice also manifest exaggerated proliferative responses, Ca++ mobilization, and activation of the ERK pathway after stimulation through the B-cell antigen receptor.16,22 The hyperresponsive phenotype of lyn−/− B cells is, in part, mediated through the loss of inhibitory signaling by the immune tyrosine-based inhibition motif (ITIM)–containing coreceptor, CD22.22,23 After the activation of B cells through the antigen receptor, tyrosine phosphorylation of the ITIM on CD22 increases dramatically, resulting in increased association with SHP-1 and down-modulation of the B-cell response. Activation-induced CD22 phosphorylation and association with SHP-1 does not occur inlyn−/− B cells. It is notable that SHP-1 undergoes tyrosine phosphorylation and associates with Syk and Lyn in CRP-stimulated platelets. However, we have shown that platelets derived from mev/mev mice are hyporesponsive to CRP, suggesting an activatory role in GPVI signaling for SHP-1.24
Inositol polyphosphate 5′-phosphatase (SHIP), which metabolizes 5′-phosphorylated phosphoinositides such as PI3,4,5P3, is also a potential mediator of the Lyn-sensitive pathway of inhibition. SHIP is an important effector of FcγRIIB, a negative regulator of B-cell activation. Tyrosine phosphorylation of the ITIM in FcγRIIB by Src-family kinases results in its association with SHIP,25a response that is reduced in lyn−/− B cells (C. A. Lowell, unpublished data, June 2000). SHIP has also been shown to play an important role in regulating platelet activation by inhibiting PI3,4,5P3-potentiated Ca++ influx pathways.26
The importance of Src family kinases in GPVI signaling is yet another facet of the close relation between the platelet collagen receptor and immune cell receptors. Significantly, this study not only shows that Fyn and Lyn are important for collagen signaling through GPVI, it also identifies Lyn as an important regulator of inhibitory signaling in platelets, possibly through the regulation of an unidentified ITIM-containing protein. The pivotal roles of both Fyn and Lyn in controlling the mechanisms that promote and limit the activation of platelets by collagen are a step forward for our understanding of prothrombic and antithrombotic signaling.
Acknowledgment
We thank James Major for help with the preparation of figures.
Supported by the British Heart Foundation, the Wellcome Trust, and National Institutes of Health grants DK50267 and HL54467 (C.A.L.). L.S.Q. held a British Heart Foundation studentship. S.P.W. is a British Heart Foundation Senior Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steve P. Watson, Department of Pharmacology, University of Oxford, Mansfield Rd, Oxford, OX1 3QT, United Kingdom; e-mail: steve.watson@pharm.ox.ac.uk.


![Fig. 4. PLC activation in fyn−/−platelets. / Pleckstrin phosphorylation (an indicator of PKC activation) and phosphatidic acid (PA) production (a metabolite of diacylglycerol) were measured in [32P]-labeled platelets in the presence of 1 mmol/L EGTA and 10 μmol/L indomethacin. (A) The autoradiographs show pleckstrin phosphorylation stimulated by a range of concentrations of CRP (0.3-3.0 μg/mL) and thrombin (0.1-1.0 U/mL) in wild-type andfyn−/− platelets. The results are representative of 3 to 5 experiments. (B) Autoradiographs show phosphatidic acid production stimulated by a range of concentrations of CRP (0.3-3.0 μ/mL) and thrombin (0.1-1.0 U/mL) in wild-type andfyn−/− platelets. The results are representative of 3 to 5 experiments. Wild-type andfyn−/− samples were run in parallel, and the same exposure times are shown. A lighter exposure of the autoradiogram from representative samples of the more powerful agonist thrombin is shown to enable differentiation between PA levels stimulated by different concentrations of thrombin. (C) Ca2+ mobilization was measured in Fura-2–labeled murine platelets in the presence of 1 mmol/L EGTA. Wild-type and fyn−/− platelets (i) and wild-type and lyn−/− platelets (ii) were stimulated by 0.3 μg/mL CRP over 200 seconds. The arrow indicates the point of addition of an agonist. Results are representative of 5 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/13/10.1182_blood.v96.13.4246/5/m_h82400511004.jpeg?Expires=1769195494&Signature=jFUp3sXoGSV~if4i0TLhuuJEXnFtsxPFShootjFFOCWQS5A8LGxqChj9zHJ7t7688runTv51TMvH3Dym7~FM7VM2~ddKhjY0cTR34kvHuonntioirErF9YDjkX12aC3pri5a7qAo77Go6-TMyOmtI1hH1tRt9zcfxi5Yhyu4n08g4JpKHeJ9gf8qKawxpa1S0T2oQ9mq2rreORyg62TGH2cH9Jif8pIDWBX8cAqC992EAPWNOCxN-s6vMdoieWrYI9KdpgIIcSwY0myb6HRLbJjAkBi1sjhnTtsb4DG5pIo79kMx74-dgKFZGmYv19Ac~zmd~IuHjKPQ-9BmnkJibA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal